Cirrhosis Case Study (45 min)
Watch More! Unlock the full videos with a FREE trial

Included In This Lesson
Study tools.
Access More! View the full outline and transcript with a FREE trial
Mr. Garcia is a 43-year-old male who presented to the ED complaining of nausea and vomiting x 3 days. The nurse notes a large, distended abdomen and yellowing of the patient’s skin and eyes. The patient reports a history of alcoholic cirrhosis.
What initial nursing assessments should be performed?
- Full abdominal assessment, including assessing for ascites
- Heart and lung sounds
- Skin assessment – color, turgor, etc.
- Full set of vital signs
- Neurological assessment
What diagnostic testing do you anticipate for Mr. Garcia?
- LFT’s, CBC, BMP
Mr. Garcia’s vitals are stable, BP 100/58, bowel sounds are active but distant, and the nurse notes a positive fluid wave test on his abdomen. The patient denies itching but is constantly scratching at his chest. He is oriented to person only and his brother at the bedside reports he hasn’t been himself today. He keeps trying to get out of bed
Which finding is most concerning and needs to be reported to the provider? Why?
- Confusion, disorientation – this could indicate hepatic encephalopathy, which could lead to seizures and death if left untreated
What further diagnostic and lab tests should be ordered to determine Mr. Garcia’s priority problems?
- Abdominal X-ray and/or Abdominal ultrasound to visualize liver and whether the distended abdomen is related to ascites or other sources
- Ammonia level to determine if hepatic encephalopathy is the source of Mr. Garcia’s altered mental status.
The provider places orders for the following:
Keep SpO 2 > 92%
Keep HOB > 30 degrees
Insert 2 large bore PIV’s
500 mL NS IV bolus STAT
100 mL/hr NS IV continuous infusion
Hydrocodone/Acetaminophen 5-500 mg 1-2 tabs q4h PRN moderate to severe pain
Diphenhydramine 25 mg PO q8h PRN itching
Ondansetron 4 mg IV q6h PRN nausea
Lactulose 20 mg PO q6h
Mr. Garcia’s LFT’s and Ammonia level are elevated. He is extremely confused and agitated and appears somewhat short of breath. The patient’s current vital signs are as follows:
HR 82 RR 22
BP 94/56 SpO 2 93%
Temp 98.9°F
Which order should be implemented first? Why?
- Insert two large-bore IV’s. The patient requires IV fluids and has other IV meds ordered and will likely need labs drawn. This needs to be a priority.
- You could also say elevate the HOB to 30 degrees or higher, if there was indication that he was lying flat
- His SpO2 is >92%, so no intervention is required there.
- Lactulose should be the next priority intervention – to get the ammonia levels down – but it may take a bit for pharmacy to profile it, send it to the unit, etc.
Which order should be questioned? Why?
- Hydrocodone/Acetaminophen – Acetaminophen can be toxic for patients with liver disease. The way this order is written, this patient could receive anywhere from 3 g – 6 g of Acetaminophen in a 24-hour period. The max for a healthy person is 4 g, but for liver patients, it is 2 g max.
- Either the dose and frequency should be lowered significantly, or the medication should be changed altogether
The order is changed to Fentanyl 25 mcg IV q4h PRN moderate to severe pain. The provider notes somewhat shallow breathing and severe ascites and requests for you to set up for paracentesis. At this time, you express your concern that the patient is extremely confused and agitated and trying to get out of bed. You do not feel that he will be still enough for the procedure. The provider agrees and plans to postpone the paracentesis for now, but orders for you to report any signs of respiratory depression or hypoxia.
Why is Mr. Garcia so confused and agitated?
- His ammonia levels are elevated due to his liver failure – this causes hepatic encephalopathy – damage to the brain cells
- This causes altered mental status, agitation, confusion, and can eventually lead to seizures and death if left untreated
What is the rationale for performing a paracentesis for Mr. Garcia?
- The excess fluid in Mr. Garcia’s belly is compressing his thoracic cavity, causing him to feel short of breath and to only take shallow breaths. Draining this fluid will not only relieve some discomfort, but it can also help improve Mr. Garcia’s breathing
After 6 doses of lactulose, Mr. Garcia is much more calm and cooperative. He is oriented times 2-3 most times. The provider performs the paracentesis and is able to remove 1.5 L of fluid. The patient’s shortness of breath is relieved, and his breathing is less shallow. Ultrasound of the liver showed severe scarring on the liver. Mr. Garcia’s condition continues to improve, and the plan is to discharge him home tomorrow.
What discharge teaching should be included for Mr. Garcia, including nutrition?
- Mr. Garcia should not be eating a high protein diet as this can contribute to the increased ammonia levels and development of hepatic encephalopathy.
- Mr. Garcia should avoid drinking alcohol at all times
- Medication instructions for any new or changed medications
- Especially the importance of taking Lactulose regularly as ordered
- Signs to report to the provider of exacerbation or encephalopathy
View the FULL Outline
When you start a FREE trial you gain access to the full outline as well as:
- SIMCLEX (NCLEX Simulator)
- 6,500+ Practice NCLEX Questions
- 2,000+ HD Videos
- 300+ Nursing Cheatsheets
“Would suggest to all nursing students . . . Guaranteed to ease the stress!”
Nursing Case Studies

This nursing case study course is designed to help nursing students build critical thinking. Each case study was written by experienced nurses with first hand knowledge of the “real-world” disease process. To help you increase your nursing clinical judgement (critical thinking), each unfolding nursing case study includes answers laid out by Blooms Taxonomy to help you see that you are progressing to clinical analysis.We encourage you to read the case study and really through the “critical thinking checks” as this is where the real learning occurs. If you get tripped up by a specific question, no worries, just dig into an associated lesson on the topic and reinforce your understanding. In the end, that is what nursing case studies are all about – growing in your clinical judgement.
Nursing Case Studies Introduction
Cardiac nursing case studies.
- 6 Questions
- 7 Questions
- 5 Questions
- 4 Questions
GI/GU Nursing Case Studies
- 2 Questions
- 8 Questions
Obstetrics Nursing Case Studies
Respiratory nursing case studies.
- 10 Questions
Pediatrics Nursing Case Studies
- 3 Questions
- 12 Questions
Neuro Nursing Case Studies
Mental health nursing case studies.
- 9 Questions
Metabolic/Endocrine Nursing Case Studies
Other nursing case studies.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 28 March 2023
Global epidemiology of cirrhosis — aetiology, trends and predictions
- Daniel Q. Huang ORCID: orcid.org/0000-0002-5165-5061 1 , 2 , 3 ,
- Norah A. Terrault 4 ,
- Frank Tacke ORCID: orcid.org/0000-0001-6206-0226 5 ,
- Lise Lotte Gluud 6 , 7 ,
- Marco Arrese 8 , 9 ,
- Elisabetta Bugianesi 10 &
- Rohit Loomba ORCID: orcid.org/0000-0002-4845-9991 1 , 11
Nature Reviews Gastroenterology & Hepatology volume 20 , pages 388–398 ( 2023 ) Cite this article
111 Citations
116 Altmetric
Metrics details
- Epidemiology
- Liver cirrhosis
Cirrhosis is an important cause of morbidity and mortality in people with chronic liver disease worldwide. In 2019, cirrhosis was associated with 2.4% of global deaths. Owing to the rising prevalence of obesity and increased alcohol consumption on the one hand, and improvements in the management of hepatitis B virus and hepatitis C virus infections on the other, the epidemiology and burden of cirrhosis are changing. In this Review, we highlight global trends in the epidemiology of cirrhosis, discuss the contributions of various aetiologies of liver disease, examine projections for the burden of cirrhosis, and suggest future directions to tackle this condition. Although viral hepatitis remains the leading cause of cirrhosis worldwide, the prevalence of non-alcoholic fatty liver disease (NAFLD) and alcohol-associated cirrhosis are rising in several regions of the world. The global number of deaths from cirrhosis increased between 2012 and 2017, but age-standardized death rates (ASDRs) declined. However, the ASDR for NAFLD-associated cirrhosis increased over this period, whereas ASDRs for other aetiologies of cirrhosis declined. The number of deaths from cirrhosis is projected to increase in the next decade. For these reasons, greater efforts are required to facilitate primary prevention, early detection and treatment of liver disease, and to improve access to care.
Hepatitis C virus (HCV) infection remains the leading cause of global deaths related to cirrhosis, followed by alcohol-associated liver disease.
The global burden of cirrhosis associated with non-alcoholic fatty liver disease (NAFLD) has increased substantially in the past decade.
In the Americas, the dominant cause of cirrhosis is shifting from viral hepatitis to NAFLD and alcohol-associated liver disease.
The COVID-19 pandemic has set back progress in the elimination of HCV and hepatitis B virus, and most countries are not on track to meet the WHO viral hepatitis elimination targets.
The focus of care should be shifted upstream towards primary prevention and early detection of liver disease to reduce the global burden of cirrhosis.
Similar content being viewed by others
The incidence trends of liver cirrhosis caused by nonalcoholic steatohepatitis via the GBD study 2017
Global epidemiology of alcohol-associated cirrhosis and HCC: trends, projections and risk factors
Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention
Introduction.
Cirrhosis is an important cause of morbidity and mortality among patients with chronic liver disease 1 . Cirrhosis can lead to hepatocellular carcinoma (HCC) and hepatic decompensation, including ascites, hepatic encephalopathy and variceal bleeding 1 , 2 , 3 , 4 , 5 , 6 , 7 , and is a leading cause of death worldwide — it was associated with 2.4% of global deaths in 2019 (ref. 8 ). The major aetiologies of cirrhosis are hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, alcohol-associated liver disease and non-alcoholic fatty liver disease (NAFLD) 9 , 10 . However, the past decade has seen major changes in the aetiology and burden of liver disease 11 , 12 , 13 , 14 , 15 , 16 .
Increasing HBV vaccination coverage and improved availability of effective antivirals against HBV have contributed to a reduction in the global age-standardized death rates (ASDRs) for HBV-associated cirrhosis 12 , 17 , 18 , 19 , 20 . Similarly, since 2015, safe and effective directly acting antivirals (DAAs) have revolutionized treatment of HCV infection, although the full impact of DAAs on the global burden of HCV-associated cirrhosis is unclear 12 , 21 , 22 , 23 . Despite the rising consumption of alcohol 24 , 25 , ASDRs for alcohol-associated cirrhosis have decreased, although under-reporting and under-diagnosis are a concern 26 . By contrast, the obesity and diabetes epidemic has led to a rapid rise in the prevalence of NAFLD, and ASDRs for NAFLD-associated cirrhosis have increased 26 .
Estimates of the global burden of cirrhosis and of the contributions made by various causes of liver disease are important for practitioners, researchers and health-care policymakers to inform clinical practice, provide directions for research and guide the use of resources, respectively. In this Review, we highlight global trends in the epidemiology of cirrhosis, discuss the contributions of various aetiologies of liver disease, examine projections for the burden of cirrhosis, and suggest future directions for reducing the burden of cirrhosis.
Prevalence of cirrhosis
The Global Burden of Disease (GBD) Study provides a comprehensive overview of the estimated global burden of cirrhosis and chronic liver diseases (which are collectively referred to as cirrhosis in the GBD Study) 27 , 28 . In the GBD Study 2017, the estimated number of people with compensated cirrhosis was 112 million worldwide, corresponding to an age-standardized global prevalence of compensated cirrhosis of 1,395 cases per 100,000 population 12 .
The data used in the GBD Study depended on the quality of each country’s registry 27 , and where data were not available, modelling was used to extrapolate from previous trends. Such extrapolation could have introduced bias, which would have reduced the accuracy of these prevalence estimates. In regions with lower standards of health care, cirrhosis is likely to be under-reported owing to a lack of disease awareness and access to care. In light of these limitations, interpretation of data from the GBD Study requires caution, but the study remains an important source of information about the burden of cirrhosis.
Several country-specific, population-based studies conducted in Europe have examined the prevalence of cirrhosis on the basis of non-invasive tests. Estimates of prevalence in these studies have ranged from 0.3% to 0.8% 29 . However, such data on the global prevalence of cirrhosis are limited.
Aetiologies of liver disease
The prevalence of HBV and HCV infection among individuals with cirrhosis was estimated in a large systematic review and meta-analysis of 520 studies (including 1,376,503 individuals from 86 countries or territories) published between 1993 and 2021 (ref. 30 ). Although the primary aim of this study was to evaluate the prevalence of HBV and HCV infection, data on heavy alcohol consumption and NAFLD were also reported where available. The analysis included only studies of unselected patients with cirrhosis, who were assumed to be representative of the population at each centre.
Globally, among individuals with cirrhosis, 42% had HBV infection and 21% had HCV infection. By WHO region, the prevalence of HBV infection among patients with cirrhosis was highest in the Western Pacific region (59%) and lowest in the Americas (5%), whereas the highest prevalence of HCV infection among patients with cirrhosis was in the Eastern Mediterranean region (70%) and the lowest was in Africa and the Western Pacific (both 13%). The proportion of patients with cirrhosis and heavy alcohol use was high in Europe (16–78%) and the Americas (17–52%) and was generally lower in Asia (0–41%). Data on the prevalence of NAFLD among patients with cirrhosis in this study were more limited, but estimates ranged from 2% in South Korea and Brazil to 18% in Canada (when considering estimates based on at least three studies). The dominant reported cause of cirrhosis varied by country (Fig. 1 ).
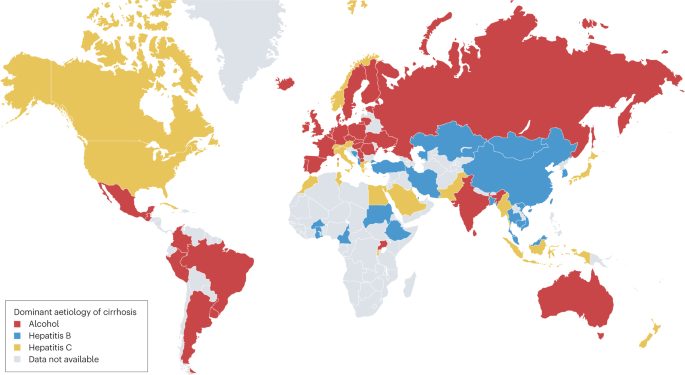
Data were obtained from a systematic review of cirrhosis that included studies published during the period 1993–2021 (ref. 30 ).
Interpretation of the data from this large meta-analysis requires caution. The primary aim of the meta-analysis was to evaluate the prevalence of viral hepatitis, rather than heavy alcohol use or NAFLD, in cirrhosis, so studies relevant to alcohol use and NAFLD might have been omitted. In addition, many of the included studies did not account for multiple aetiologies of cirrhosis. We speculate that cirrhosis has more than one cause in a substantial proportion of patients, particularly considering the growing prevalence of obesity and increasing alcohol consumption.
In addition, the concept of metabolic-associated fatty liver disease (MAFLD) is likely to alter the apparent aetiology of cirrhosis. MAFLD is defined — on the basis of recently proposed criteria 31 , 32 — as hepatic steatosis with obesity, type 2 diabetes mellitus or other factors associated with metabolic dysfunction without the need to exclude alternative causes of chronic liver disease, such as viral hepatitis or alcohol consumption 32 . Data on the global burden of MAFLD-associated cirrhosis are limited, but we speculate that the burden of MAFLD-associated cirrhosis will increase over time owing to an increasing proportion of individuals with more than one cause of liver disease, such as concomitant alcohol consumption and NAFLD, or concomitant NAFLD and viral hepatitis. Finally, as the studies included in this meta-analysis spanned nearly 30 years, the data might not reflect the trends in the past decade.
Trends in the aetiology of cirrhosis
Studies from across the world provide insights into trends in the aetiology of cirrhosis (Supplementary Table 1 ); these trends are discussed by WHO region in the sections that follow. Many of the larger studies conducted in North America, Europe and the Western Pacific were based on data from administrative disease registries and relied on International Classification of Diseases (ICD) codes (Box 1 ) to identify cases of cirrhosis; this approach is susceptible to bias related to incomplete records or incorrect coding. By contrast, studies in which clinical criteria and chart review were used to identify cases of cirrhosis are less susceptible to bias, but many such studies included modest sample sizes and, consequently, might not fully reflect trends in aetiology.
Similarly, the definitions of NAFLD-associated cirrhosis varied across studies, so interpretation of data based on these definitions requires caution. Criteria for NAFLD as a cause of cirrhosis have been proposed to enable consistent enrolment into clinical trials 33 . These criteria enable categorization of the likelihood that NAFLD is the cause of cirrhosis (definite, probable or possible) on the basis of histological evidence and metabolic risk factors 33 . However, these criteria have not been uniformly adopted and might require further validation.
Box 1 International Classification of Diseases codes for cirrhosis
Multiple studies have been conducted in North America, Europe and Australia to examined the use of International Classification of Diseases 9 (ICD-9) and ICD-10 codes to identify cirrhosis 36 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 . These studies showed that ICD codes used to identify cirrhosis generally have a relatively high positive predictive value but, when used in isolation, they have only modest sensitivity for identifying cirrhosis 36 , 113 , 115 .
Algorithms that use a combination of ICD codes, or a combination of ICD codes and laboratory results, could improve diagnostic accuracy 36 , 113 , 115 , 122 . One systematic review has identified nine ICD-10 codes that have been used to identify cirrhosis most frequently in the literature 118 . When validated in Europe and North America, this set of nine ICD-10 codes had greater sensitivity than the code set most frequently used in the literature 121 , and maintained a high positive predictive value (83–89%). This consensus code set could, therefore, be a useful tool for identification of cirrhosis in large databases and health records, and should be further validated in regions outside North America and Europe 118 .
The Americas
Multiple studies conducted in the Americas indicate a shift in the dominant cause of cirrhosis over the past 10–20 years. In a population-based study conducted in Indiana, USA, analysis of the incident cases of cirrhosis from 2004 to 2014 ( n = 9,261) 16 identified increases in the proportion of patients with alcohol-associated cirrhosis (0.8% per year) and NAFLD-associated cirrhosis (0.6% per year), whereas the proportion with viral hepatitis decreased by 1.4% per year 16 . In a prospective study in 1,717 people with cirrhosis at five hospitals in Texas, USA, between 2016 and 2019, the major causes of cirrhosis were cured HCV infection (33%), alcohol consumption (31%) and NAFLD (23%), highlighting the shift away from active viral hepatitis as the dominant cause of cirrhosis 34 . Similarly, in an analysis of population-based administrative health-care data from 159,549 people with cirrhosis in Ontario, Canada, the most common causes of incident cirrhosis between 2000 and 2017 were NAFLD (53%) and alcohol consumption (24%) 35 . However, this study differed from other studies conducted in Canada, in which the leading cause of cirrhosis was HCV infection — some degree of misclassification bias might have contributed to the high estimated proportion of cirrhosis caused by NAFLD 36 , 37 .
Changes in the aetiology of cirrhosis over time in Mexico were assessed in a study including 4,584 people who were diagnosed with incident cirrhosis in six tertiary hospitals between 2000 and 2019 (ref. 38 ). In this study, diagnostic criteria for MAFLD 31 , 32 , rather than NAFLD, were used. MAFLD was identified as the third most common cause of incident cirrhosis in 2000 (14%) but had become the leading cause of incident cirrhosis (36%) in 2019. By contrast, HCV infection was the leading cause of cirrhosis in 2000 (45%) but had declined by 2019 (11%). The proportion of cirrhosis cases due to alcohol consumption increased from 28% to 33% during the study period. However, the way in which patients who fulfilled the criteria for MAFLD and had concomitant viral hepatitis or high alcohol consumption were classified was not clear 38 . Finally, a study of the United Network for Organ Sharing database from 2014 to 2019 determined that NAFLD and alcohol consumption have become the most common causes of liver disease among people without HCC waiting for a liver transplant 39 .
Taken together, these data suggest that the aetiology of cirrhosis in the Americas is shifting from active HBV and HCV infection towards resolved or treated viral hepatitis, alcohol consumption and NAFLD. These data are in line with increases in obesity and alcohol consumption in the Americas 40 , 41 , 42 .
As in the Americas, studies in Europe indicate a change in the dominant aetiology of cirrhosis. Analysis of data from all hospital admissions in Germany from 2005 to 2018 revealed that the prevalence of NAFLD-associated cirrhosis increased fourfold during the study period 43 . Nevertheless, alcohol consumption remained the dominant cause of cirrhosis in Germany in 2018, accounting for 52% of cirrhosis cases; NAFLD and NASH accounted for only 3% and 1%, respectively. In Sweden, in a cohort study in patients with cirrhosis who visited a tertiary hospital, the proportion of patients with cirrhosis due to viral hepatitis declined from 43% in the first 5 years (2004–2008) of the study to 31% in the final 4 years (2014–2017), whereas the proportion due to NAFLD increased from 6% to 15% in the same period 44 . Similarly, in Italy, in a multicentre study in patients with cirrhosis ( n = 832) at 16 hospitals 45 , the proportions of people with cirrhosis due to alcohol consumption and HCV infection decreased, whereas the proportion of people with cirrhosis due to NAFLD increased when compared with a historical cohort (2001) 46 .
Collectively, these data indicate that the prevalence of NAFLD-associated cirrhosis is increasing in Europe, whereas the prevalence of alcohol-associated cirrhosis seems to be decreasing, possibly in response to public health measures such as the enforcement of a minimum price for alcohol and increased alcohol taxation 41 , 47 , 48 . The data also suggest that the prevalence of HCV-associated and HBV-associated cirrhosis is declining in Europe.
Africa, the Eastern Mediterranean and Southeast Asia
Limited data are available on trends in the aetiology of cirrhosis in Africa, the Eastern Mediterranean and Southeast Asia, as many studies conducted in these regions have included modest sample sizes 30 . More data are required from these regions to accurately determine trends in the aetiology of cirrhosis and to identify and address the gaps in linkage to care.
In Africa, research on liver diseases is a major unmet need. Estimates from the GBD Study determined that most cases of cirrhosis in Africa were related to HBV infection, alcohol consumption and HCV infection, but no other up-to-date population-based data exist for the aetiologies of cirrhosis 12 , 49 , 50 . Data from larger studies of HCC in Africa can provide some indication of the relative contributions that each aetiology of liver disease makes to the prevalence of cirrhosis, although some people with HCC do not have cirrhosis, so caution is required in interpreting the data. In one study, analysis of 1,251 people with HCC (all of whom had cirrhosis) from Egypt determined that the dominant aetiology was HCV infection (84%), followed by other or unknown causes (12%), HBV–HCV co-infection (2%) and HBV infection (1%) 51 . In the same study, analysis of 1,315 people with HCC (66% of whom had cirrhosis) from other African countries (Nigeria, Ghana, the Ivory Coast, Cameroon, Sudan, Ethiopia, Tanzania and Uganda) determined that the most common aetiology was HBV infection (55%), followed by other or unknown causes (22%), alcohol consumption (13%) and HCV infection (6%) 51 .
In the Eastern Mediterranean region, single-centre studies suggest that viral hepatitis remains the dominant cause of cirrhosis. For example, in one study of 953 Jewish and 95 Arab Bedouin individuals with cirrhosis at a tertiary hospital in Israel determined that the most common cause of cirrhosis was HCV infection (39%) among Jewish individuals and NAFLD (21%) among Bedouin individuals 52 . In Qatar, study of 109 individuals with cirrhosis who were admitted to an intensive care unit showed that the most common aetiology of cirrhosis was HCV infection (34%), followed by alcohol consumption (26%), cryptogenic liver disease (24%) and HBV infection (21%) 53 . However, population-based data from this region are lacking and are needed to get a more accurate understanding of aetiology.
Data from the Southeast Asia region are similarly limited. In one study conducted in India, the most common aetiology among 4,413 patients with cirrhosis from 11 hospitals was alcohol consumption (34%), followed by other causes (29%), HBV infection (18%), HCV infection (17%) and NAFLD (2%) 54 . However, among 192 people with cirrhosis who underwent endoscopic band ligation in a hospital in Pakistan, cirrhosis was attributed to HCV infection in 63% and to HBV infection in 19% 55 .
The Western Pacific
Studies in the Western Pacific region have shown that NAFLD-associated and alcohol-associated cirrhosis are increasing in this region, but viral hepatitis remains the dominant cause of cirrhosis 30 . These trends were demonstrated in a large study in 48,621 individuals with cirrhosis identified on the basis of clinical criteria at 79 hospitals in Japan 56 . Comparison of aetiology in 2007 with that in 2014–2016 demonstrated an increase in NAFLD-associated cirrhosis (2% to 9%) and alcohol-associated cirrhosis (14% to 25%). The proportion of cirrhosis cases due to HCV and HBV infection declined (from 59% to 40% and from 14% to 9%, respectively), although these remained the largest contributors. In another study in 15,716 patients with cirrhosis at five university hospitals in South Korea between 2000 and 2014, the proportion of cirrhosis cases due to NAFLD, alcohol consumption and HCV infection increased over the study period 57 . However, the study periods of both of these studies were before the widespread availability of DAA therapy for HCV infection, which is likely to reduce the proportion of cirrhosis cases that are due to HCV infection over time.
Also in the Western Pacific region, analysis of data from 1,582 individuals with a new diagnosis of cirrhosis at a hospital in China determined that the proportion of NAFLD-associated cirrhosis cases was higher (3%) in the last 2 years of the study (2012–2013) than the average (2%) over the entire study period (2003–2013) 58 . In Taiwan, analysis of data from 18,423 individuals with HCC who were diagnosed between 1981 and 2001 showed that the percentage of HBV-associated HCC decreased from 82% to 66% among men and from 67% to 41% among women over the study period owing to an increase in HCV-associated HCC 59 . Despite the continued dominance of viral hepatitis as a cause of cirrhosis in the Western Pacific region, tracking changes in the aetiology of cirrhosis over time remains important, as the rates of obesity and alcohol consumption continue to rise in parallel with increasing economic wealth 24 , 41 , 60 , 61 , 62 , 63 .
Decompensated cirrhosis
Hepatic decompensation is potentially preventable with antiviral therapy (in HBV-associated and HCV-associated cirrhosis) and lifestyle changes (in alcohol-associated and NAFLD-associated cirrhosis), but epidemiological data suggest that decompensated cirrhosis is increasing in prevalence. In prior studies in patients with compensated cirrhosis, transition from a compensated state to a decompensated state (including ascites, hepatic encephalopathy and variceal bleeding) occurred at a rate of 5–12% per year 64 , 65 , 66 . Data from the GBD Study 2017 indicate that the global number of prevalent cases of decompensated cirrhosis increased from 5.2 million in 1990 to 10.6 million in 2017, corresponding to an increase in the estimated age-standardized prevalence of decompensated cirrhosis from 110.6 per 100,000 population in 1990 to 132.5 per 100,000 population in 2017 (ref. 12 ). In 2017, the proportion of decompensated cirrhosis associated with HBV and HCV infection, alcohol consumption, NAFLD and other causes was 28%, 25%, 23%, 9% and 16%, respectively 12 .
Acute-on-chronic liver failure (ACLF) is characterized by acute decompensation of chronic liver disease that is associated with organ failure and carries a high risk of mortality 67 , 68 . Several definitions exist for ACLF, including criteria from the Asia Pacific Association for the Study of the Liver 69 , the European Association for the Study of the Liver–Chronic Liver Failure (EASL–CLIF) 70 , and the North American Consortium for the Study of End-Stage Liver 71 . ACLF has been reviewed in detail elsewhere 67 , 72 . A meta-analysis of 30 studies determined that the global prevalence of ACLF (defined by the EASL–CLIF criteria) among patients admitted with decompensated cirrhosis was 35% 73 . Alcohol consumption was the underlying cause of chronic liver disease in 45% of people with ACLF globally; regional pooled estimates ranged from 24% in North America to 55% in Europe 73 . The global 90-day mortality associated with ACLF was 58% and ranged from 41% in North America to 68% in Southeast Asia 73 .
In combination, these data highlight the need for increased efforts to identify liver disease at an earlier stage. Earlier identification would enable the use of preventive measures, thereby reducing the burden of decompensation 74 .
Deaths associated with cirrhosis
The GBD Study 2019 provides a comprehensive global overview of the estimated mortality associated with cirrhosis (and chronic liver diseases) 27 . Although deaths attributed to liver cancer were excluded from this analysis, the proportions of liver cancer due to the various aetiologies of liver disease were used as co-variates in determining the proportion of cirrhosis deaths due to the various aetiologies of liver disease. The analysis provided global and regional estimates for the number of deaths and ASDRs associated with cirrhosis in 2019 (Table 1 ).
The estimated number of deaths associated with cirrhosis worldwide in 2019 was 1,472,000 (ref. 27 ). This number had increased by 10% from 2010 (ref. 27 ). The absolute number of deaths associated with cirrhosis was lowest in the Eastern Mediterranean region (146,000) and highest in Southeast Asia (443,000). The estimated global ASDR for cirrhosis in 2019 was 18 deaths per 100,000 population 27 (Table 1 ). By region, the lowest estimated ASDR was in the Western Pacific (9.6 deaths per 100,000 population) and the highest was in the Eastern Mediterranean region (36.2 deaths per 100,000 population). The disconnect between the number of cirrhosis-related deaths and the ASDR in the Eastern Mediterranean region results from the relatively small and young population in that region 75 . The estimated ASDR in different countries (Fig. 2 ) ranged from 3.3 deaths per 100,000 population in Singapore to 126.7 deaths per 100,000 population in Egypt.
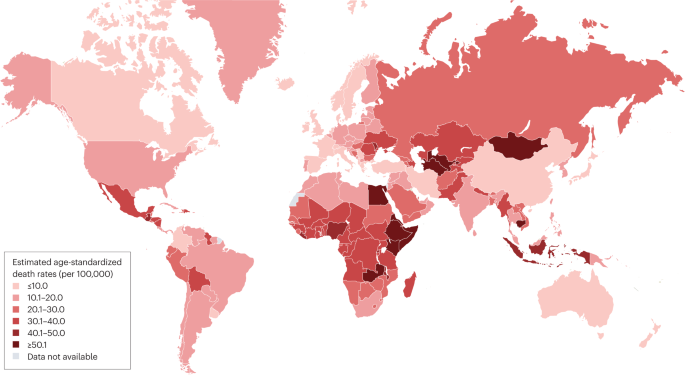
Data for the age-standardized death rate in 2019 were estimated in the Global Burden of Disease Study 2019 and these data were obtained from the GBD Results Tool 27 . Where data for countries or regions were unavailable, the Global Burden of Disease Study 2019 results depended on modelling and past trends, potentially resulting in discrepancies in the accuracy of the data.
The number of deaths associated with cirrhosis with different aetiologies (Table 1 ) ranged from 134,241 deaths for NAFLD-associated cirrhosis to 395,022 for HCV-associated cirrhosis. The corresponding estimated ASDRs ranged from 1.7 deaths per 100,000 population for NAFLD-associated cirrhosis to 4.8 deaths per 100,000 population for HCV-associated cirrhosis 27 . However, a substantial proportion of cases of cirrhosis that were categorized as having ‘other causes’ in the GBD Study might have been due to NAFLD or occult HBV infection, which would mean that the figures for NAFLD-associated and HBV-associated cirrhosis are underestimates.
Data from the GBD Study 2017 has provided insight into temporal trends in deaths associated with cirrhosis. Between 2012 and 2017, the number of deaths associated with cirrhosis increased by 9%, although the global ASDR for cirrhosis declined from 17.1 to 16.5 deaths per 100,000 population (ref. 26 ). The disconnect between the number of deaths and ASDR is due to population growth and ageing. The ASDRs for HBV-associated cirrhosis, HCV-associated cirrhosis and alcohol-associated cirrhosis also decreased by 1.4%, 0.5% and 0.4%, respectively, between 2012 and 2017. By contrast, the ASDR for NAFLD-associated cirrhosis increased by 0.3% over the same period 26 . However, cardiovascular diseases are the leading cause of death among patients with NAFLD, highlighting the need for a multidisciplinary approach to this condition 76 , 77 , 78 .
Predictions
Several modelling studies been done to project the burden of various aetiologies of cirrhosis over the next decade 22 , 35 , 79 , 80 , 81 , 82 . However, these studies should be interpreted with caution. The input data were largely derived from administrative databases, which are susceptible to bias, including under-reporting and misclassification bias. In addition, fibrosis can regress after antiviral treatment or lifestyle modification but few of these modelling studies accounted for the possibility of such regression 83 , 84 , 85 , 86 . Despite these limitations, the resulting data serve as an important reference to guide public health policymakers and researchers.
HCV-associated cirrhosis
Integration of a literature review, a Delphi process and Markov modelling to forecast the burden of incident decompensated HCV-associated cirrhosis in 2030 led to an estimated increase from 148,000 cases in 2020 to 174,000 in 2030 worldwide 22 . However, the rate of cases per 100,000 population was projected to remain relatively stable. The same modelling study also provided an estimate of the prevalence of viraemic HCV infection, which indicated that the global hepatitis elimination target for 2030 is unlikely to be attained should current trends persist 87 .
Caution must be exercised when interpreting the estimates from this study, as sufficient data for model generation were available for fewer than half of the countries. In addition, treatment rates were uncertain, especially for countries in which a substantial proportion of treatments were exported for overseas use. Nevertheless, the COVID-19 pandemic has set back the progress made in HCV elimination 88 , 89 (Box 2 ), and these data serve as a call to action for an increased emphasis on HCV elimination.
Box 2 Impacts of the COVID-19 pandemic on cirrhosis
Population-based analyses conducted in the USA determined that mortality due to cirrhosis increased markedly during the COVID-19 pandemic 123 . Evidence from multiple countries has demonstrated that alcohol consumption increased substantially during the pandemic 97 , 124 , 125 , 126 , 127 , 128 , and the observed increase in mortality was mainly related to alcohol-associated cirrhosis 123 .
Among patients with COVID-19 and chronic liver disease in the US National COVID Cohort Collaborative, the presence of cirrhosis was associated with an increased risk of mortality (adjusted HR 3.3) 129 .
In the early phase of the COVID-19 pandemic, cirrhosis referrals, hospitalizations and clinic visits declined substantially, which might have contributed to lower quality of care, delayed presentation and loss of follow-up 95 , 96 , 130 , 131 .
A global study conducted at 44 international centres determined that consultations, testing and treatment rates for hepatitis B virus and hepatitis C virus infection decreased substantially between January 2019 and December 2020, and this decrease was related to the COVID-19 pandemic 89 .
Modelling of a 1-year delay to the hepatitis C elimination programmes of 110 countries owing to the COVID-19 pandemic indicated that such a delay would result in 72,300 excess liver-related deaths (likely to be related to HCV-associated cirrhosis in the majority of these cases) over the next 10 years relative to the situation with no delay 132 .
HBV-associated cirrhosis
Data on the projected global burden of HBV-associated cirrhosis are limited. Projections in one study suggest that the incidence of HBV infection will fall by 2030 but that HBV-related deaths will increase by 39% between 2015 and 2030 (ref. 81 ). Data from the GBD Study 2019 showed that only four countries had attained the WHO Global Health Sector Strategy on Viral Hepatitis 2020 interim impact target of a 10% reduction in deaths between 2015 and 2019 (ref. 90 ). Furthermore, despite the availability of vaccines and life-saving antiviral therapy, HBV infection remains severely under-diagnosed, so a minority of treatment-eligible patients receive antiviral treatment 91 , 92 , and the COVID-19 pandemic has hindered HBV elimination efforts globally 89 (Box 2 ). Together, these observations highlight that HBV is likely to remain a major threat to public health in the next decade, and increased political will and resources are required to eliminate HBV, which remains a major cause of cirrhosis and HCC worldwide.
Alcohol-associated cirrhosis
In a study in the USA, data from the National Vital Statistics System, the National Institute on Alcohol Abuse and Alcoholism, the National Death Index, the National Epidemiologic Survey on Alcohol and Related Conditions, and other published data were used to predict outcomes from alcohol-associated liver disease in the USA between 2019 and 2040 (ref. 82 ). The result was a predicted increase of 77% in the age-standardized incidence of decompensated alcohol-associated cirrhosis, from 9.9 cases per 100,000 patient-years in 2019 to 17.5 cases per 100,000 patient-years in 2040, should current trends be left unchecked 82 .
In a study conducted in Canada, cirrhosis incidence rates were calculated from the crude rates observed in Ontario between 2000 and 2017, and projections of incidence were calculated on the basis of the estimated population of Ontario from 2018 to 2040 (ref. 35 ). The incidence of cirrhosis (of all aetiologies) was projected to increase by 9% between 2018 and 2040, with a steady increase in the age-standardized incidence rate for alcohol-associated cirrhosis.
The projected burden of alcohol-associated cirrhosis in these studies might be underestimated owing to under-reporting. In addition, neither study accounted for comorbid diseases, such as concomitant HCV infection, which could increase the rate of fibrosis progression. Nevertheless, these data indicate that the burden of alcohol-associated cirrhosis in North America is likely to increase further unless alcohol-related policies are instituted or specific therapies become available for alcohol-associated liver disease. Data for the projected burden of alcohol-associated cirrhosis outside North America are limited.
One additional point to consider is that the COVID-19 pandemic resulted in an increase in alcohol consumption in many countries (Box 2 ). This increase in alcohol consumption could further increase the global burden of alcohol-associated cirrhosis in the coming years 93 , 94 , 95 , 96 , 97 , 98 .
NAFLD-associated cirrhosis
Several studies have generated predictions about the burden of NAFLD-associated cirrhosis by 2030. In one study, the prevalence of NAFLD in China, France, Germany, Italy, Japan, Spain, the UK and the USA was predicted on the basis of published data, expert consensus and country-level prevalence of obesity and type 2 diabetes mellitus 80 . Markov modelling was then used to project the burden of NAFLD-associated cirrhosis in 2030 (ref. 80 ). The smallest projected increase in the prevalence of compensated NAFLD-associated cirrhosis cases was 64% in Japan and the largest projected increase was 156% in France. The smallest projected increase in the prevalence of decompensated NAFLD-associated cirrhosis cases was 75% in Japan and the largest projected increase was 187% in France.
A similar methodology was used in another study to forecast the burden of NAFLD-associated cirrhosis in 2030 in Hong Kong, Singapore, South Korea and Taiwan 79 . From 2019 to 2030, incident decompensated NAFLD-associated cirrhosis was projected to increase by 65% in Hong Kong, 85% in South Korea and 100% in Singapore and Taiwan. In both studies, definitions of NAFLD varied between the input sources, contributing to variation in the projected prevalence of NAFLD.
In the Canadian study of cirrhosis discussed above 36 , projections indicated that NAFLD-associated cirrhosis would account for 75% of incident cases of cirrhosis in Ontario by 2040. These data require cautious interpretation, as the proportion of historical cirrhosis cases due to NAFLD was much higher than estimates in other Canadian studies 36 , 37 . Taken together, however, these data highlight the growing burden of NAFLD-associated cirrhosis and the need for urgent measures to control the underlying metabolic risk factors at a global and regional level.
Future directions
Liver disease is often under-diagnosed, and many individuals present late with decompensated cirrhosis 99 , 100 . Disadvantaged and under-served communities are often disproportionately affected by liver disease but often lack timely access to appropriate care 91 , 101 , 102 . Despite advances in the diagnosis, treatment and management of cirrhosis, medical interventions often have limited benefit on long-term survival, necessitating the use of resource-heavy treatments such as liver transplantation 7 , 103 , 104 , 105 , 106 . Therefore, the focus of care should be shifted upstream, from the management of complications to prevention and early treatment 74 .
As an example, consumption of alcohol per capita (alcohol consumption within one calendar year in litres of pure alcohol in people aged ≥15 years) in Europe has declined over time (12.3 l in 2005 to 9.8 l in 2016), and this decline is related to policies that enforce a minimum price for alcohol and increased alcohol taxation 26 , 41 , 47 , 48 . The strong political will required to implement these policies has contributed to a reduction in mortality from alcohol-associated cirrhosis in Europe over the past decade 26 , 41 , 47 , 48 . By contrast, in the Americas, Western Pacific and Southeast Asia, ASDRs due to alcohol-associated cirrhosis increased during the same time period 26 . A consensus statement published in 2021 highlighted the fact that most countries in the world lack a national strategy for NAFLD, reflecting the low priority of this disease in national health agendas 107 . Greater efforts are required at national and regional levels to implement public health policies that target the metabolic risk factors for liver diseases, such as high-sugar foods, lack of physical activity and heavy alcohol consumption 108 .
A case-finding approach could help to detect patients with early cirrhosis or advanced fibrosis, and thereby facilitate treatment. In a population-based, prospective cohort study conducted in Germany, a structured screening programme (a combination of routine serum tests including aspartate aminotransferase, alanine aminotransferase and platelet levels) of individuals participating in health check-ups was associated with 59% higher odds of detecting early cirrhosis compared with routine care, after excluding individuals with decompensated cirrhosis 109 . The American Gastroenterological Association has proposed a clinical care pathway to facilitate risk stratification and management of individuals with NAFLD in primary care, endocrinology and obesity medicine, which could strengthen linkage to tertiary care but requires prospective validation 110 .
Outreach efforts to improve screening, treatment and linkage to care for viral hepatitis are effective but are not widely adopted 22 , 111 . The Polaris Observatory estimated that only 1% of prevalent cases of HCV infection worldwide were treated in 2020, and <10% of treatment-eligible individuals with HBV infection received antiviral treatment 22 , 81 . These sobering figures highlight the uphill task faced by the global hepatology community, and underscore the need for stronger multidisciplinary collaboration between primary care physicians, preventive care specialists, nurse practitioners, infectious disease specialists, hepatologists, patient representatives and policymakers.
Conclusions
The aetiology of cirrhosis is changing, and the global burden of NAFLD-associated cirrhosis is steadily rising in parallel with the epidemic of obesity and type 2 diabetes mellitus. Global alcohol consumption continues to rise, and national policies are required to reduce the burden of alcohol-associated cirrhosis. Despite the availability of effective antiviral therapies for HCV and HBV infection, most countries are not on track to meet the WHO viral hepatitis elimination targets. The burden of cirrhosis remains substantial owing to under-diagnosis and under-treatment of chronic liver disease, and the number of deaths and cases of decompensated cirrhosis are projected to rise in the next decade. More resources should be directed towards primary prevention, early detection of liver disease and linkage to care to reduce the global burden of cirrhosis.
Ginès, P. et al. Liver cirrhosis. Lancet 398 , 1359–1376 (2021).
Article PubMed Google Scholar
Tapper, E. B., Ufere, N. N., Huang, D. Q. & Loomba, R. Review article: current and emerging therapies for the management of cirrhosis and its complications. Aliment. Pharmacol. Ther. 55 , 1099–1115 (2022).
Article PubMed PubMed Central Google Scholar
Tapper, E. B. & Parikh, N. D. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. BMJ 362 , k2817 (2018).
Huang, D. Q. et al. Hepatocellular carcinoma incidence in alcohol-associated cirrhosis: systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. https://doi.org/10.1016/j.cgh.2022.06.032 (2022).
Tan, D. J. H. et al. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol. 23 , 521–530 (2022).
Tan, D. J. H. et al. Global burden of liver cancer in males and females: changing etiological basis and the growing contribution of NASH. Hepatology https://doi.org/10.1002/hep.32758 (2022).
Ajmera, V. et al. Liver stiffness on magnetic resonance elastography and the MEFIB index and liver-related outcomes in nonalcoholic fatty liver disease: a systematic review and meta-analysis of individual participants. Gastroenterology 163 , 1079–1089.e5 (2022).
The Global Health Observatory. Global health estimates: Leading causes of death. WHO https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death . (2023).
Moon, A. M., Singal, A. G. & Tapper, E. B. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin. Gastroenterol. Hepatol. 18 , 2650–2666 (2020).
Loomba, R., Friedman, S. L. & Shulman, G. I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 184 , 2537–2564 (2021).
Article CAS PubMed Google Scholar
Asrani, S. K., Devarbhavi, H., Eaton, J. & Kamath, P. S. Burden of liver diseases in the world. J. Hepatol. 70 , 151–171 (2019).
GBD 2017 Cirrhosis Collaborators.The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 5 , 245–266 (2020).
Article Google Scholar
Huang, D. Q. et al. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab. 34 , 969–977.e2 (2022).
Article CAS PubMed PubMed Central Google Scholar
Huang, D. Q., El-Serag, H. B. & Loomba, R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 18 , 223–238 (2021).
Golabi, P. et al. Burden of non-alcoholic fatty liver disease in Asia, the Middle East and North Africa: Data from Global Burden of Disease 2009-2019. J. Hepatol. 75 , 795–809 (2021).
Orman, E. S. et al. Trends in characteristics, mortality, and other outcomes of patients with newly diagnosed cirrhosis. JAMA Netw. Open. 2 , e196412 (2019).
Chen, V. L. et al. Anti-viral therapy is associated with improved survival but is underutilised in patients with hepatitis B virus-related hepatocellular carcinoma: real-world east and west experience. Aliment. Pharmacol. Ther. 48 , 44–54 (2018).
Terrault, N. A. et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 67 , 1560–1599 (2018).
European Association for the Study of the Liver EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 67 , 370–398 (2017).
Sarin, S. K. et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol. Int. 10 , 1–98 (2016).
Ghany, M. G. & Morgan, T. R. AASLD-IDSA Hepatitis C Guidance Panel Hepatitis C guidance 2019 update: American Association for the Study of Liver Diseases–Infectious Diseases Society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology 71 , 686–721 (2020).
Polaris Observatory HCV Collaborators.Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol. Hepatol. 7 , 396–415 (2022).
European Association for the Study of the Liver EASL recommendations on treatment of hepatitis C: final update of the series. J. Hepatol. 73 , 1170–1218 (2020).
Manthey, J. et al. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. Lancet 393 , 2493–2502 (2019).
Rehm, J. & Shield, K. D. Global burden of alcohol use disorders and alcohol liver disease. Biomedicines 7 , 99 (2019).
Paik, J. et al. The global burden of liver cancer (LC) and chronic liver diseases (CLD) is driven by non-alcoholic steatohepatitis (NASH) and alcohol liver disease (ALD) [abstract GS008]. J. Hepatol. 77 (Suppl. 1), S5–S7 (2022).
GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396 , 1204–1222 (2020).
GBD 2017 Disease and Injury Incidence and Prevalence Collaborators.Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392 , 1789–1858 (2018).
Ginès, P. et al. Population screening for liver fibrosis: toward early diagnosis and intervention for chronic liver diseases. Hepatology 75 , 219–228 (2022).
Alberts, C. J. et al. Worldwide prevalence of hepatitis B virus and hepatitis C virus among patients with cirrhosis at country, region, and global levels: a systematic review. Lancet Gastroenterol. Hepatol. 7 , 724–735 (2022).
Eslam, M. et al. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 158 , 1999–2014.e1 (2020).
Ng, C. H., Huang, D. Q. & Nguyen, M. H. NAFLD versus MAFLD: prevalence, outcomes and implications of a change in name. Clin. Mol. Hepatol. https://doi.org/10.3350/cmh.2022.0070 (2022).
Noureddin, M. et al. Attribution of nonalcoholic steatohepatitis as an etiology of cirrhosis for clinical trials eligibility: recommendations from the multi-stakeholder liver forum. Gastroenterology 159 , 422–427.e1 (2020).
El-Serag, H. B. et al. Risk factors for cirrhosis in contemporary hepatology practices–findings from the Texas Hepatocellular Carcinoma Consortium Cohort. Gastroenterology 159 , 376–377 (2020).
Flemming, J. A., Djerboua, M., Groome, P. A., Booth, C. M. & Terrault, N. A. NAFLD and alcohol-associated liver disease will be responsible for almost all new diagnoses of cirrhosis in Canada by 2040. Hepatology 74 , 3330–3344 (2021).
Philip, G., Djerboua, M., Carlone, D. & Flemming, J. A. Validation of a hierarchical algorithm to define chronic liver disease and cirrhosis etiology in administrative healthcare data. PLoS ONE 15 , e0229218 (2020).
Sharma, S. A. et al. Toronto HCC risk index: a validated scoring system to predict 10-year risk of HCC in patients with cirrhosis. J. Hepatol. https://doi.org/10.1016/j.jhep.2017.07.033 (2017).
& Gonzalez-Chagolla, A. et al. Cirrhosis etiology trends in developing countries: transition from infectious to metabolic conditions. Report from a multicentric cohort in central Mexico. Lancet Reg. Health Am. 7 , 100151 (2022).
PubMed Google Scholar
Wong, R. J. & Singal, A. K. Trends in liver disease etiology among adults awaiting liver transplantation in the United States, 2014-2019. JAMA Netw. Open. 3 , e1920294 (2020).
Ward, Z. J. et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N. Engl. J. Med. 381 , 2440–2450 (2019).
World Health Organization. Global status report on alcohol and health 2018 (WHO, 2018).
Díaz, L. A. et al. Liver diseases in Latin America: current status, unmet needs, and opportunities for improvement. Curr. Treat. Options Gastroenterol. 20 , 261–278 (2022).
Gu, W. et al. Trends and the course of liver cirrhosis and its complications in Germany: Nationwide population-based study (2005 to 2018). Lancet Reg. Health Eur. 12 , 100240 (2022).
Hagström, H. et al. Etiologies and outcomes of cirrhosis in a large contemporary cohort. Scand. J. Gastroenterol. 56 , 727–732 (2021).
Stroffolini, T. et al. Characteristics of liver cirrhosis in Italy: evidence for a decreasing role of HCV aetiology. Eur. J. Intern. Med. 38 , 68–72 (2017).
Stroffolini, T. et al. Characteristics of liver cirrhosis in Italy: results from a multicenter national study. Dig. Liver Dis. 36 , 56–60 (2004).
World Health Organization. Equity, social determinants and public health programmes (WHO, 2010).
World Health Organization. European action plan to reduce the harmful use of alcohol 2012–2020 (WHO, 2012).
Vento, S., Dzudzor, B., Cainelli, F. & Tachi, K. Liver cirrhosis in sub-Saharan Africa: neglected, yet important. Lancet Glob. Health 6 , e1060–e1061 (2018).
Mokdad, A. A. et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med 12 , 145 (2014).
Yang, J. D. et al. Characteristics, management, and outcomes of patients with hepatocellular carcinoma in Africa: a multicountry observational study from the Africa Liver Cancer Consortium. Lancet Gastroenterol. Hepatol. 2 , 103–111 (2017).
Tailakh, M. A. et al. Liver cirrhosis, etiology and clinical characteristics disparities among minority population. J. Immigr. Minor. Health 24 , 1122–1128 (2022).
Elzouki, A. N. et al. Predicting mortality of patients with cirrhosis admitted to medical intensive care unit: an experience of a single tertiary center. Arab. J. Gastroenterol. 17 , 159–163 (2016).
Mukherjee, P. S. et al. Etiology and mode of presentation of chronic liver diseases in India: a multi centric study. PLoS ONE 12 , e0187033 (2017).
Alvi, H., Zuberi, B. F., Rasheed, T. & Ibrahim, M. A. Evaluation of endoscopic variceal band ligation sessions in obliteration of esophageal varices. Pak. J. Med. Sci. 36 , 37–41 (2020).
PubMed PubMed Central Google Scholar
Enomoto, H. et al. Transition in the etiology of liver cirrhosis in Japan: a nationwide survey. J. Gastroenterol. 55 , 353–362 (2020).
Jang, W. Y. et al. Changes in characteristics of patients with liver cirrhosis visiting a tertiary hospital over 15 years: a retrospective multi-center study in Korea. J. Korean Med. Sci. 35 , e233 (2020).
Xiong, J. et al. Non-alcoholic steatohepatitis-related liver cirrhosis is increasing in China: a ten-year retrospective study. Clinics 70 , 563–568 (2015).
Lu, S.-N. et al. Secular trends and geographic variations of hepatitis B virus and hepatitis C virus-associated hepatocellular carcinoma in Taiwan. Int. J. Cancer 119 , 1946–1952 (2006).
Schmidt, L. A. & Room, R. Alcohol and inequity in the process of development: contributions from ethnographic research. Int. J. Alcohol. Drug. Res. 1 , 41–55 (2013).
Wang, H., Ma, L., Yin, Q., Zhang, X. & Zhang, C. Prevalence of alcoholic liver disease and its association with socioeconomic status in north-eastern China. Alcohol. Clin. Exp. Res. 38 , 1035–1041 (2014).
Charatcharoenwitthaya, P., Liangpunsakul, S. & Piratvisuth, T. Alcohol-associated liver disease: East versus West. Clin. Liver Dis. 16 , 231–235 (2020).
Fan, J. G., Kim, S. U. & Wong, V. W. New trends on obesity and NAFLD in Asia. J. Hepatol. 67 , 862–873 (2017).
D’Amico, G., Garcia-Tsao, G. & Pagliaro, L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J. Hepatol. 44 , 217–231 (2006).
Jepsen, P., Ott, P., Andersen, P. K., Sørensen, H. T. & Vilstrup, H. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology 51 , 1675–1682 (2010).
Fleming, K. M., Aithal, G. P., Card, T. R. & West, J. The rate of decompensation and clinical progression of disease in people with cirrhosis: a cohort study. Aliment. Pharmacol. Ther. 32 , 1343–1350 (2010).
Arroyo, V., Moreau, R. & Jalan, R. Acute-on-chronic liver failure. N. Engl. J. Med. 382 , 2137–2145 (2020).
Hernaez, R., Solà, E., Moreau, R. & Ginès, P. Acute-on-chronic liver failure: an update. Gut 66 , 541–553 (2017).
Sarin, S. K. et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol. Int. 13 , 353–390 (2019).
Moreau, R. et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 144 , 1426–1437.e9 (2013).
O’Leary, J. G. et al. NACSELD acute-on-chronic liver failure (NACSELD-ACLF) score predicts 30-day survival in hospitalized patients with cirrhosis. Hepatology 67 , 2367–2374 (2018).
Arroyo, V. et al. Acute-on-chronic liver failure in cirrhosis. Nat. Rev. Dis. Prim. 2 , 16041 (2016).
Mezzano, G. et al. Global burden of disease: acute-on-chronic liver failure, a systematic review and meta-analysis. Gut 71 , 148–155 (2022).
Karlsen, T. H. et al. The EASL-Lancet Liver Commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet 399 , 61–116 (2022).
GBD 2019 Demographics Collaborators.Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950–2019: a comprehensive demographic analysis for the Global Burden of Disease Study 2019. Lancet 396 , 1160–1203 (2020).
Adams, L. A. et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 129 , 113–121 (2005).
Huang, D. et al. Shared mechanisms between cardiovascular disease and NAFLD. Semin. Liver Dis. https://doi.org/10.1055/a-1930-6658 (2022).
Younossi, Z. M. et al. Nonalcoholic steatofibrosis independently predicts mortality in nonalcoholic fatty liver disease. Hepatol. Commun. 1 , 421–428 (2017).
Estes, C. et al. Modelling NAFLD disease burden in four Asian regions – 2019-2030. Aliment. Pharmacol. Ther. 51 , 801–811 (2020).
Estes, C. et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J. Hepatol. 69 , 896–904 (2018).
Razavi-Shearer, D. et al. The disease burden of hepatitis B and hepatitis C from 2015 to 2030: the long and winding road [abstract OS050]. J. Hepatol. 77 (Suppl. 1), S43 (2022).
Julien, J., Ayer, T., Bethea, E. D., Tapper, E. B. & Chhatwal, J. Projected prevalence and mortality associated with alcohol-related liver disease in the USA, 2019-40: a modelling study. Lancet Public. Health 5 , e316–e323 (2020).
Hsu, W. F. et al. Hepatitis C virus eradication decreases the risks of liver cirrhosis and cirrhosis-related complications (Taiwanese chronic hepatitis C cohort). J. Gastroenterol. Hepatol. 36 , 2884–2892 (2021).
Sanyal, A. J. et al. Cirrhosis regression is associated with improved clinical outcomes in patients with nonalcoholic steatohepatitis. Hepatology https://doi.org/10.1002/hep.32204 (2021).
Xie, Y. D., Feng, B., Gao, Y. & Wei, L. Effect of abstinence from alcohol on survival of patients with alcoholic cirrhosis: A systematic review and meta-analysis. Hepatol. Res. 44 , 436–449 (2014).
Chang, T. T. et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology 52 , 886–893 (2010).
Cox, A. L. et al. Progress towards elimination goals for viral hepatitis. Nat. Rev. Gastroenterol. Hepatol. 17 , 533–542 (2020).
Tergast, T. L. et al. Updated epidemiology of hepatitis C virus infections and implications for hepatitis C virus elimination in Germany. J. Viral Hepat. 29 , 536–542 (2022).
Kondili, L. A. et al. Impact of the COVID-19 pandemic on hepatitis B and C elimination: an EASL survey. JHEP Rep. 4 , 100531 (2022).
GBD 2019 Hepatiitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 7 , 796–829 (2022).
Ye, Q. et al. Substantial gaps in evaluation and treatment of patients with hepatitis B in the US. J. Hepatol. 76 , 63–74 (2022).
Le, M. H. et al. Chronic hepatitis B prevalence among foreign-born and U.S.-born adults in the United States, 1999-2016. Hepatology 71 , 431–443 (2020).
Julien, J. et al. Effect of increased alcohol consumption during COVID-19 pandemic on alcohol-associated liver disease: a modeling study. Hepatology 75 , 1480–1490 (2022).
Bittermann, T., Mahmud, N. & Abt, P. Trends in liver transplantation for acute alcohol-associated hepatitis during the COVID-19 pandemic in the US. JAMA Netw. Open. 4 , e2118713 (2021).
Toyoda, H., Huang, D. Q., Le, M. H. & Nguyen, M. H. Liver care and surveillance: the global impact of the COVID-19 pandemic. Hepatol. Commun. 4 , 1751–1757 (2020).
Tan, E. X.-X. Impact of COVID-19 on liver transplantation in Hong Kong and Singapore: a modelling study. Lancet Reg. Health West. Pac. 16 , 100262 (2021).
Lee, B. P., Dodge, J. L., Leventhal, A. & Terrault, N. A. Retail alcohol and tobacco sales during COVID-19. Ann. Intern. Med. 174 , 1027–1029 (2021).
White, A. M., Castle, I.-J. P., Powell, P. A., Hingson, R. W. & Koob, G. F. Alcohol-related deaths during the COVID-19 pandemic. JAMA https://doi.org/10.1001/jama.2022.4308 (2022).
Hussain, A. et al. Decompensated cirrhosis is the commonest presentation for NAFLD patients undergoing liver transplant assessment. Clin. Med. 20 , 313–318 (2020).
Trebicka, J. et al. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J. Hepatol. 73 , 842–854 (2020).
Lee, B. P., Dodge, J. L. & Terrault, N. A. Geographic density of gastroenterologists is associated with decreased mortality from alcohol-associated liver disease. Clin. Gastroenterol. Hepatol. https://doi.org/10.1016/j.cgh.2022.07.020 (2022).
Oluyomi, A. O., El-Serag, H. B., Olayode, A. & Thrift, A. P. Neighborhood-level factors contribute to disparities in hepatocellular carcinoma incidence in Texas. Clin. Gastroenterol. Hepatol. https://doi.org/10.1016/j.cgh.2022.06.031 (2022).
de Franchis, R., Bosch, J., Garcia-Tsao, G., Reiberger, T. & Ripoll, C. Baveno VII – Renewing consensus in portal hypertension. J. Hepatol. 76 , 959–974 (2022).
European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J. Hepatol. 64 , 433–485 (2016).
Lucey, M. R. et al. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 19 , 3–26 (2013).
Sharpton, S. R. et al. Gut metagenome-derived signature predicts hepatic decompensation and mortality in NAFLD-related cirrhosis. Aliment. Pharmacol. Ther. 56 , 1475–1485 (2022).
Lazarus, J. V. et al. Advancing the global public health agenda for NAFLD: a consensus statement. Nat. Rev. Gastroenterol. Hepatol. https://doi.org/10.1038/s41575-021-00523-4 (2021).
Díaz, L. A. et al. The establishment of public health policies and the burden of non-alcoholic fatty liver disease in the Americas. Lancet Gastroenterol. Hepatol. 7 , 552–559 (2022).
Labenz, C. et al. Structured early detection of asymptomatic liver cirrhosis: results of the population-based liver screening program SEAL. J. Hepatol. 77 , 695–701 (2022).
Kanwal, F. et al. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology 161 , 1657–1669 (2021).
Rozenberg-Ben-Dror, K. et al. Improving quality of hepatitis B care in the Veteran’s Health Administration. Clin. liver Dis. 19 , 213–218 (2022).
Paik, J. M., Golabi, P., Younossi, Y., Mishra, A. & Younossi, Z. M. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatology 72 , 1605–1616 (2020).
Nehra, M. S. et al. Use of administrative claims data for identifying patients with cirrhosis. J. Clin. Gastroenterol. 47 , e50–e54 (2013).
Hayward, K. L. et al. ICD-10-AM codes for cirrhosis and related complications: key performance considerations for population and healthcare studies. BMJ Open Gastroenterol. 7 , e000485 (2020).
Ramrakhiani, N. S. et al. Validity of international classification of diseases, tenth revision, codes for cirrhosis. Dig. Dis. 39 , 243–246 (2021).
Mapakshi, S., Kramer, J. R., Richardson, P., El-Serag, H. B. & Kanwal, F. Positive predictive value of international classification of diseases, 10th revision, codes for cirrhosis and its related complications. Clin. Gastroenterol. Hepatol. 16 , 1677–1678 (2018).
Bengtsson, B., Askling, J., Ludvigsson, J. F. & Hagström, H. Validity of administrative codes associated with cirrhosis in Sweden. Scand. J. Gastroenterol. 55 , 1205–1210 (2020).
Shearer, J. E. et al. Systematic review: development of a consensus code set to identify cirrhosis in electronic health records. Aliment. Pharmacol. Ther. 55 , 645–657 (2022).
Ratib, S., West, J., Crooks, C. J. & Fleming, K. M. Diagnosis of liver cirrhosis in England, a cohort study, 1998-2009: a comparison with cancer. Am. J. Gastroenterol. 109 , 190–198 (2014).
Thygesen, S. K., Christiansen, C. F., Christensen, S., Lash, T. L. & Sørensen, H. T. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med. Res. Methodol. 11 , 83 (2011).
Kramer, J. R. et al. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs Administrative databases. Aliment. Pharmacol. Ther. 27 , 274–282 (2008).
Goldberg, D., Lewis, J., Halpern, S., Weiner, M. & Lo Re, V. 3rd Validation of three coding algorithms to identify patients with end-stage liver disease in an administrative database. Pharmacoepidemiol. Drug. Saf. 21 , 765–769 (2012).
Kim, D., Alshuwaykh, O., Dennis, B. B., Cholankeril, G. & Ahmed, A. Trends in etiology-based mortality from chronic liver disease before and during COVID-19 pandemic in the United States. Clin. Gastroenterol. Hepatol. 20 , 2307–2316.e3 (2022).
Pollard, M. S., Tucker, J. S. & Green, H. D. Jr. Changes in adult alcohol use and consequences during the COVID-19 pandemic in the US. JAMA Netw. Open. 3 , e2022942 (2020).
The Lancet Gastroenterology Hepatology. Drinking alone: COVID-19, lockdown, and alcohol-related harm. Lancet Gastroenterol. Hepatol. 5 , 625 (2020).
NANOS Research. COVID-19 and increased alcohol consumption: NANOS poll summary report (Canadian Centre on Substance Use and Addiction, 2020).
Vanderbruggen, N. et al. Self-reported alcohol, tobacco, and cannabis use during COVID-19 lockdown measures: results from a web-based survey. Eur. Addict. Res. 26 , 309–315 (2020).
Sidor, A. & Rzymski, P. Dietary choices and habits during COVID-19 lockdown: experience from Poland. Nutrients 12 , 1657 (2020).
Ge, J., Pletcher, M. J. & Lai, J. C. Outcomes of SARS-CoV-2 infection in patients with chronic liver disease and cirrhosis: a national COVID cohort collaborative study. Gastroenterology 161 , 1487–1501.e5 (2021).
Mahmud, N., Hubbard, R. A., Kaplan, D. E. & Serper, M. Declining cirrhosis hospitalizations in the wake of the COVID-19 pandemic: a national cohort study. Gastroenterology 159 , 1134–1136.e3 (2020).
Tapper, E. B. & Asrani, S. K. The COVID-19 pandemic will have a long-lasting impact on the quality of cirrhosis care. J. Hepatol. 73 , 441–445 (2020).
Blach, S. et al. Impact of COVID-19 on global HCV elimination efforts. J. Hepatol. 74 , 31–36 (2021).
Download references
Acknowledgements
R.L. receives funding support from NCATS (5UL1TR001442), NIDDK (U01DK061734, U01DK130190, R01DK106419, R01DK121378, R01DK124318, P30DK120515), NHLBI (P01HL147835) and NIAAA (U01AA029019). D.Q.H. receives funding support from the Singapore Ministry of Health’s NMRC Research Training Fellowship (MOH-000595-01). M.A. acknowledges partial support from the Chilean government through the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT grant 1191145).
Author information
Authors and affiliations.
NAFLD Research Center, Division of Gastroenterology, University of California at San Diego, San Diego, CA, USA
Daniel Q. Huang & Rohit Loomba
Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
Daniel Q. Huang
Division of Gastroenterology and Hepatology, Department of Medicine, National University Health System, Singapore, Singapore
Division of Gastrointestinal and Liver Diseases, University of Southern California, Los Angeles, CA, USA
Norah A. Terrault
Department of Hepatology & Gastroenterology, Campus Virchow-Klinikum and Campus Charité Mitte, Charité Universitätsmedizin Berlin, Berlin, Germany
Frank Tacke
Gastro Unit, Copenhagen University Hospital, Hvidovre, Denmark
Lise Lotte Gluud
Department of Clinical Medicine, Faculty of Health Sciences, University of Copenhagen, Copenhagen, Denmark
Departamento de Gastroenterologia, Escuela de Medicina, Pontificia Universidad Catolica de Chile, Santiago, Chile
Marco Arrese
Centro de Envejecimiento Y Regeneración (CARE), Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile
Division of Gastroenterology and Hepatology, Department of Medical Sciences, University of Turin, Turin, Italy
Elisabetta Bugianesi
Division of Epidemiology, Department of Family Medicine and Public Health, University of California at San Diego, San Diego, CA, USA
Rohit Loomba
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to all aspects of the manuscript.
Corresponding author
Correspondence to Rohit Loomba .
Ethics declarations
Competing interests.
D.Q.H. has served as an advisory board member for Eisai and Gilead. N.A.T. receives institutional grant support from DURECT Corporation, Eiger Pharmaceuticals, Gilead Sciences, Glaxo-Smith-Kline, Helio Health and Roche-Genentech. F.T. serves as a consultant to Abbvie, Alnylam, Boehringer-Ingelheim, CSL Behring, Falk, Gilead, Intercept, Inventiva, Ionis, Novartis, Novo Nordisk and Pfizer. His institute has received research grants from Allergan, Bristol Myers Squibb, Gilead and Inventiva. L.L.G. serves as a consultant to Novo Nordisk and Pfizer, and her institutes have received research grants from Alexion, Gilead, Novo Nordisk, Sobi Int. and Vingmed. M.A. receives support from the Chilean government through the Fondo Nacional De Ciencia y Tecnología de Chile (FONDECYT no. 1191145) and the Comisión Nacional de Investigación, Ciencia y Tecnología (CONICYT, AFB170005, CARE, Chile, UC). E.B. serves as a consultant to AstraZeneca, Boehringer-Ingelheim, Eli Lilly, Gilead, Intercept, Inventiva, Novartis, Novo Nordisk, Merck, MSD and Pfizer. Her institute has received a research grant from Gilead. R.L. serves as a consultant to 89 Bio, Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol Myers Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen, Madrigal, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, Terns Pharmaceuticals and Viking Therapeutics. In addition his institutes have received research grants from Arrowhead Pharmaceuticals, Astrazeneca, Boehringer-Ingelheim, Bristol Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes and Terns Pharmaceuticals. He is also a co-founder of LipoNexus.
Peer review
Peer review information.
Nature Reviews Gastroenterology & Hepatology thanks M.-L. Yu, who co-reviewed with P.-Y. Hsu, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria PubMed was searched from inception to June 2022 using the terms ‘cirrhosis’, ‘end-stage liver disease’ and ‘liver cirrhosis’ without language restrictions. Guidelines, original articles and reviews were evaluated. Data for the global and regional number and age-adjusted rate of deaths estimated in the Global Burden of Disease (GBD) Study 2019 were obtained from the GBD Results Tool 27 . The GBD Study was used to identify trends in the mortality rates of cirrhosis 112 , but not temporal trends in the prevalence of the aetiologies of cirrhosis, as limited data on these trends were available within the GBD framework. Individual country-specific and region-specific studies were, therefore, selected to provide data from diverse geographical locations on temporal trends in the prevalence of the aetiologies of cirrhosis. When multiple studies originating from the same country were available, studies that provided data for temporal trends in aetiologies of cirrhosis were preferentially selected.
Supplementary information
Supplementary information, rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Huang, D.Q., Terrault, N.A., Tacke, F. et al. Global epidemiology of cirrhosis — aetiology, trends and predictions. Nat Rev Gastroenterol Hepatol 20 , 388–398 (2023). https://doi.org/10.1038/s41575-023-00759-2
Download citation
Accepted : 22 February 2023
Published : 28 March 2023
Issue Date : June 2023
DOI : https://doi.org/10.1038/s41575-023-00759-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
A novel network pharmacology strategy to decode mechanism of wuling powder in treating liver cirrhosis.
Chinese Medicine (2024)
Usefulness of a questionnaire for assessing the relationship between eating behavior and steatotic liver disease among Japanese male young adults
- Satoko Tajirika
- Mayumi Yamamoto
Scientific Reports (2024)
Prediction of early recovery of graft function after living donor liver transplantation in children
- Bingqian Tan
- Chenyu Yang
- Mingman Zhang
Screening for liver fibrosis: lessons from colorectal and lung cancer screening
- Maja Thiele
- Patrick S. Kamath
Nature Reviews Gastroenterology & Hepatology (2024)
Hesperetin derivative 2a inhibits lipopolysaccharide-induced acute liver injury in mice via downregulation of circDcbld2
- Li-jiao Sun
Acta Pharmacologica Sinica (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

Introduction
Case report, disclosure statement, a 25-year-old woman with type 2 diabetes and liver disease.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Anders Ellekær Junker , Lise Lotte Gluud , Jens Pedersen , Jill Levin Langhoff , Jens Juul Holst , Filip Krag Knop , Tina Vilsbøll; A 25-Year-Old Woman with Type 2 Diabetes and Liver Disease. Case Rep Gastroenterol 1 December 2014; 8 (3): 398–403. https://doi.org/10.1159/000369968
Download citation file:
- Ris (Zotero)
- Reference Manager
A 25-year-old female nurse was referred to our diabetes outpatient clinic with poorly controlled type 2 diabetes, obesity and elevated liver function tests (LFTs). Following a liver biopsy she was diagnosed with non-alcoholic steatohepatitis (NASH) and liver fibrosis. Treatment with subcutaneous injections of the glucagon-like peptide-1 receptor (GLP-1R) agonist liraglutide was initiated. After 46 weeks of treatment the patient had lost 16 kg, glycemic control was excellent and LFTs had normalized. Repeat liver biopsy and ultrasound showed reduction in hepatic fat content and inflammatory cells. The biopsy no longer fulfilled the criteria for NASH. The liver biopsies did not express hepatic GLP-1Rs using quantitative polymerase chain reaction. Our case suggests that liraglutide may benefit patients with NASH.
Elevated liver function tests (LFTs) in obese patients either with or without diabetes are a common clinical problem. The most frequent cause is non-alcoholic fatty liver disease (NAFLD), which represents both a diagnostic and therapeutic challenge. NAFLD is defined as a total liver fat content of more than 5%. The diagnostic gold standard is histology. The diagnostic criteria also include absence of significant alcohol intake (women: >20 g per day; men: >30 g per day) and use of steatogenic drugs (e.g. amiodarone and glucocorticoids) [ 1 ]. NAFLD is associated with an increase in mortality due to cardiovascular disease, irrespective of diabetes [ 2 ]. The prevalence is closely associated with obesity and type 2 diabetes. Up to 70% of patients with type 2 diabetes have NAFLD. The underlying pathophysiology of the development from simple steatosis to non-alcoholic steatohepatitis (NASH) is not fully understood. Excess hepatic fat infiltration seems to cause lipid-induced mitochondrial dysfunction and oxidative stress (lipotoxicity), which result in inflammation and fibrosis [ 3 ]. Studies have shown that NASH is associated with cirrhosis and hepatocellular carcinoma and is predicted to be the leading cause of liver transplantation by 2020 in the US [ 1 ].
Weight loss is an effective treatment of NASH, but is difficult to maintain for a majority of patients [ 2 ]. Current medical interventions are limited and seem to be associated with risk of side-effects. We present a case of severe liver disease in a young woman with type 2 diabetes. She was treated with the glucagon-like peptide-1 receptor (GLP-1R) agonist liraglutide with remarkable results on glycemic control, LFTs and liver histology.
A 25-year-old female nurse was referred to our diabetes outpatient clinic with poorly controlled type 2 diabetes, obesity and elevated LFTs. Her general practitioner had initiated treatment with metformin (1,000 mg twice-daily) and simvastatin (40 mg once-daily), but compliance was limited. At her first visit to our outpatient clinic her body weight was 90 kg and her body mass index 32.6 kg/m 2 . She was asymptomatic and clinical examination, fundus photography, filament testing and albumin/creatinine ratio revealed no signs of complications related to type 2 diabetes. Blood samples showed a glycated hemoglobin A 1c (HbA 1c ) of 8.9% (74 mmol/mol) and a fasting plasma glucose of 7.3 mmol/l. LFTs showed an alanine aminotransferase (ALT) of 196 U/l (reference range 10-45 U/l), an aspartate aminotransferase (AST) of 132 U/l (reference range 15-35 U/l) and an alkaline phosphatase (ALP) of 127 U/l (reference range 35-105 U/l). Lipids were elevated: total cholesterol 4.5 mmol/l, high-density lipoprotein 0.84 mmol/l, low-density lipoprotein 2.4 mmol/l and triglycerides 2.86 mmol/l. Bilirubin, international normalized ratio and albumin were within normal ranges. The patient had no history of alcohol abuse nor did she take any herbal drug. Abdominal ultrasound revealed increased echogenicity and poor visualization of the intrahepatic vessel walls, suggesting diffuse hepatic steatosis.
Statins were discontinued and the patient was strongly encouraged to be compliant with her metformin treatment (1,000 mg twice-daily). In addition neutral protamine Hagedorn insulin was initiated. The daily dose of basal insulin was gradually increased to 30 IU once daily.
After 8 weeks on metformin and insulin, HbA 1c had markedly improved to 6.3%, but LFTs except for ALP remained elevated (ALT 133 U/l, AST 76 U/l, ALP 69 U/l). The patient was then scheduled for a liver biopsy, which showed hepatic fat infiltration involving more than 66% of hepatocytes, ballooned hepatocytes, lobular inflammation as well as pericellular and periportal fibrosis. The histological diagnosis was NASH with a NAFLD activity score of 5 (score range 0-8) and a fibrosis score of 2 (score range 0-4) (fig. 1 a). Treatment with subcutaneous injections of the GLP-1R agonist liraglutide was initiated. The initial dose was 0.6 mg once daily subcutaneously. The dose was increased with weekly increments of 0.6-1.8 mg once daily during the following weeks. Liraglutide was well tolerated with no side effects such as nausea or vomiting. Insulin was gradually reduced and discontinued after 7 weeks.

a Microscopic view of liver tissue (hematoxylin-eosin, ×100). Liver tissue with hepatic fat infiltration including >66% of hepatocytes, ballooning cells and lobular inflammation (NAFLD activity score 5) consistent with NASH. Pericellular and periportal fibrosis (fibrosis score 2). b Microscopic view of liver tissue (hematoxylin-eosin, ×100). Liver tissue with hepatic fat infiltration including 40-50% of hepatocytes, sparse lobular inflammation and no ballooning cells (NAFLD activity score 2) consistent with simple steatosis. Pericellular and periportal fibrosis (fibrosis score 2).
After 46 weeks of treatment with liraglutide, total weight loss was 16 kg, LFTs were in the lower normal range (ALT 29 U/l, AST 25 U/l, ALP 67 U/l), glycemic control was excellent (HbA 1c 5.6%) and the lipid profile was normalized without statin treatment. Repeat abdominal ultrasound showed diminished echogenicity, suggesting an overall reduction in steatosis. A repeated liver biopsy confirmed decreased hepatic fat infiltration (involving 40-50% of hepatocytes), no ballooned hepatocytes and only distinct lobular inflammation (fig. 1 b). The histology was no longer consistent with NASH, but pericellular and periportal fibrosis were still present (NAFLD activity score 3, fibrosis score 2).
To investigate the expression of hepatic GLP-1Rs in the present case, the baseline and the post-treatment liver biopsy were compared to liver tissue from another patient with type 2 diabetes but without steatosis, using quantitative polymerase chain reaction. No biopsies showed expression of GLP-1Rs.
The prevalence of obesity and type 2 diabetes is increasing, and obese patients with type 2 diabetes and elevated LFTs are regularly referred to outpatient clinics. This case illustrates the necessity for both diabetologists and hepatologist to be aware, and together take part in the diagnosis and treatment of NAFLD.
Abdominal ultrasound is recommended as a first-line evaluation if LFTs are elevated, but has high inter-observer variability and hepatic fat infiltration fat must exceed 20-30% to be detected. Magnetic resonance spectroscopy is expensive and not widely available, but can identify >5.5% hepatic fat infiltration. Transient elastography enhanced with controlled attenuation parameter can quantify liver steatosis, but has not been validated in large trials [ 4 ]. Imaging techniques cannot distinguish between simple steatosis and NASH. The final diagnosis requires histological assessment of a representative liver biopsy. The NAFLD fibrosis score can help identify patients with a high risk of NASH and fibrosis and, thus, eligible for liver biopsy. The score is based on body mass index, age, presence of diabetes and blood levels of ALT, AST, platelets and albumin [ 5 ]. If coexisting liver diseases to NASH are suspected, liver biopsy should always be considered [ 2 ].
The distinction between simple steatosis and NASH is of both prognostic and therapeutic value. The natural history of simple steatosis is benign from a ‘liver standpoint' and should be managed by treating comorbidities such as obesity, hyperlipidemia and insulin resistance. On the other hand, NASH per se increases the risk of liver-related death [ 6 ] and management should include liver specific treatment(s). In patients with NASH the primary goal is to reverse oxidative stress and reduce hepatic fat infiltration, thereby reducing insulin resistance and further reverse hepatic inflammation [ 2 ]. Weight loss and glycemic control are effective measures in NASH, but can be difficult to achieve and maintain for a majority of patients. Nevertheless, if weight loss can be obtained it improves insulin sensitivity and reduces LFTs. A weight loss of 3-5% improves steatosis and a 10% decrease in body weight reduces hepatic inflammation. In line with these considerations, bariatric surgery should be considered in patients who are overweight and have type 2 diabetes as well as NASH [ 7 ].
Metformin increases hepatic and muscular insulin sensitivity, but does not improve LFTs or liver histology in NASH. Pioglitazones reduce hepatic steatosis but are rarely used due to adverse events including heart failure, bladder cancer and loss of bone density [ 2 ]. One trial suggests that vitamin E 800 IU per day improves LFTs, steatosis, ballooning and inflammation, and vitamin E is recommended for NASH in non-diabetic subjects. However, emerging evidence suggests that vitamin E may increase mortality [ 2 ].
The use of GLP-1R agonists for patients with type 2 diabetes is increasing. These drugs are based on the incretin hormone GLP-1, which is released from enteroendocrine cells in response to food ingestion. Native GLP-1 acts via the GLP-1R (a G protein-coupled receptor expressed in several tissues) and plays an essential role in the maintenance of normal glucose homeostasis and regulation of appetite and food intake. The effects of the native hormone have been exploited through the development of stable GLP-1R agonists. These drugs have sustained effects on glucose levels, increase insulin secretion and reduce glucagon secretion, satiety, food intake and body weight [ 8 ]. Furthermore, a recent post hoc analysis concluded that liraglutide was well tolerated and safe to use in patients with type 2 diabetes and elevated LFTs [ 9 ]. In rodents, GLP-1R agonists reduce hepatic steatosis by suppressing enzymes involved in hepatic lipogenesis through activation of the 5′ adenosine monophosphate-activated protein kinase, and have been suggested to reduce hepatic expression of pro-inflammatory mediators [ 10 ]. Nevertheless, initial observations of the GLP-1R on human hepatocytes [ 11 ] have not been confirmed in subsequent studies [ 12,13 ]. As previously described, no liver biopsies from this case had expression of GLP-1R.
The patient in this report showed remarkable results after 46 weeks of treatment with a GLP-1R agonist. She lost 16 kg of body weight (from a baseline of 90 kg) and achieved normalized LFTs and lipid profile. Her hepatic fat infiltration was reduced by ∼30%, a decreased number of inflammatory cells were observed and, thus, she no longer fulfilled the criteria for NASH. Since no GLP-1Rs were found in the liver tissue, these improvements may be explained by indirect results of glucose metabolic improvement and weight loss induced by treatment with the GLP-1R agonist. In spite of the fact that we were unable to identify GLP-1Rs in liver tissue in the present case, some evidence suggests that GLP-1R agonists may have direct effects on hepatic steatosis (in vitro models) [ 10,11 ]. Furthermore, one other paper has investigated the effect of GLP-1R agonist on liver histology in NAFLD. Kenny et al. [ 14 ] presented a case series with eight biopsy-proven NAFLD patients who received exenatide 10 mg twice daily for 26 weeks. Repeat liver biopsies showed improved NAFLD activity scores of 1-2 in four patients, but no changed in fibrosis score. The patients also lost body weight and glycemic control improved. Cuthbertson et al. [ 15 ] treated 19 patients with exenatide 10 mg twice-daily and 6 patients with liraglutide 1.2 mg once daily for 6 month. Patients had a 42% relative reduction in intrahepatic lipid content assessed by magnetic resonance spectroscopy, independent of body weight loss but in correlation with a decrease in HbA 1c . However, the study was open-labeled, without a control group, and did not asses liver histology. Thus, there is some evidence suggesting that there may be a direct effect of GLP-1R agonists on hepatocytes, but this case does not allow for any conclusion regarding the potential direct or indirect effect of GLP-1 on the liver.
Options for the treatment of NASH are limited and hold risk of severe side effects. In this case, the GLP-1R agonist liraglutide was well tolerated and markedly improved LFTs and histology in this patient with type 2 diabetes and NASH. Randomized controlled trials are needed to evaluate whether the present findings hold promise for the treatment of NASH.
The authors declare no conflict of interest regarding this paper. There were no funding sources.

Email alerts
Citing articles via, suggested reading.
- Online ISSN 1662-0631
INFORMATION
- Contact & Support
- Information & Downloads
- Rights & Permissions
- Terms & Conditions
- Catalogue & Pricing
- Policies & Information
- People & Organization
- Stay Up-to-Date
- Regional Offices
- Community Voice
SERVICES FOR
- Researchers
- Healthcare Professionals
- Patients & Supporters
- Health Sciences Industry
- Medical Societies
- Agents & Booksellers
Karger International
- S. Karger AG
- P.O Box, CH-4009 Basel (Switzerland)
- Allschwilerstrasse 10, CH-4055 Basel
- Tel: +41 61 306 11 11
- Fax: +41 61 306 12 34
- Contact: Front Office
- Experience Blog
- Privacy Policy
- Terms of Use
This Feature Is Available To Subscribers Only
Sign In or Create an Account

- Nutrition counsellor
- Food Allergy
- gastroenterology
- Heptologist In Delhi
- Liver transplant
- Alcoholic Liver Cirrhosis
Alcoholic liver cirrhosis is a late stage of fibrosis of the liver caused by many forms of liver diseases and conditions, such as chronic alcoholism. A person diagnosed with an alcoholic liver case may start from having fatty liver disease, then alcoholic hepatitis, and ultimately develop alcoholic cirrhosis. Hence, alcoholic liver cirrhosis stages in three levels. The diagnosed liver cirrhosis can be of two types :
- Compensated cirrhosis – when symptoms are not noticeable
- Uncompensated cirrhosis- when the symptoms can be noticed
The most common alcoholic liver causes are:
- Chronic alcohol consumption
- Chronic hepatitis
- Fatty liver disease
- Iron buildup in the body or hemochromatosis
- Copper accumulated in the liver (Wilson’s disease)
- Cystic fibrosis
- Biliary Atresia
- Inherited sugar metabolism or digestive disorders
- Infection like syphilis
Many other factors like the destruction of bile ducts(Primary Biliary Cirrhosis) or leaky gut also called, increased intestinal permeability are cofactors for the development of alcoholic liver cirrhosis.
Now, let us have a look at alcoholic liver cirrhosis symptoms:
- Food pipe problems
- Portal hypertension
- Swelling in legs (oedema) and abdomen (ascites)
- Bleeding in mouth
- Confusion, poor memory, loss of appetite
- Patchy red skin on palms (erythema)
Food pipe problem is also known as esophageal varices. Kidney failure and hypersplenism are other complications that happen due to this medical condition. If the symptoms are not taken seriously, then this deficient liver may arise a life-threatening situation. Hence, a person needs to keep a track of these indicators as if these signs are caught early and treated, it may slow down the progression of the disease.
How to treat alcoholic liver cirrhosis:
The first and foremost step in treatments is to help the patient to cease alcohol consumption. Medications like corticosteroids, calcium channel blockers, insulin can also be prescribed by the doctor as per the alcoholic liver care plan. Hepatologists may advise the patient to follow an alcoholic liver disease diet inclusive of fiber and protein. If the condition of the patient gets worsened, then the hepatologist may have to suggest a liver transplant surgery.
Dr. Nivedita Pandey is one of the best liver specialist doctors in Patna, Bihar. She is a well-renowned liver specialist doctor in Delhi, the best stomach doctor in Patna, the best gastroenterologist in Jammu, and a notable stomach doctor in Faridabad. Now, you can sway off all your gastroenterological worries online by booking an online gastroenterologist consultation with one of the best hepatologists in India. She is a liver specialist in Delhi NCR, one of the finest gastroenterologists in Jhansi and Jammu, and an acidity specialist doctor in Patna. Dr. Pandey’s gastroenterologist live chat has also helped people in several ways.
Case Review
This alcoholic liver case study presents a patient with liver cirrhosis. A 43-year-old man was brought into the hospital with a complaints of loss of appetite, abdominal distention, and arrhythmia. He also experienced itchy skin and blood in the stool. The patient’s family rushed him into the hospital, and he was in a half-conscious state. The patient was taken to the emergency room for evaluation. As told by the family, he had a past medical history and was a heavy alcohol consumer. This alcoholic liver case history consisted of various medical ailments like fatty liver, asthma, tuberculosis, malnutrition, hypertension, and hepatitis C. The patient had a heart attack three years back and stented for the same. Due to his health conditions, he was on several medications. In the emergency room, when the patient was under observation by a stomach specialist doctor in Patna and her team, they were able to diagnose from his symptoms that it was alcoholic liver cirrhosis. The patient went for a few scans including, a liver function test, liver ultrasound, and endoscopy along with CT, blood test, and urine tests.
Case Discussion
Though most of the liver cirrhosis causes remain unknown, with the help of her team, the best liver doctor and specialist in Patna was able to find out the reason for this one. The liver cirrhosis caused in this case was due to the medical history of the patient. His scans came out to be reasonably sound, and a liver biopsy was conducted to confirm the severity and type of liver disease. There were problems in his blood and urine culture, and they were taken care of by the team. His liver appeared swollen in the reports. There were certain other problems seen in his ultrasound and endoscopy. The crew decided to start with the treatment while keeping him under observation for the next 72 hours.
Clinical Symptoms
He was initially confused and was not able to respond or hear properly. According to the condition reported by his family i.e.- appetite loss, memory loss, and confusion were some other clinical symptoms of alcoholic liver cirrhosis. When the doctor talked to the family of the patient, she was able to get a clearer picture. The patient complained about acute abdominal pain. When Dr. Pandey, one of the best doctors in Patna for the stomach, observed the patient and talked to him, she noticed bleeding in his mouth. This further helped doctors to eliminate all doubts, and after looking at the lab results, they made out it was alcoholic liver cirrhosis.
The first and foremost management required when treating the alcoholic liver cirrhosis case is calming the patient down. The liver specialist with the help of her fellow doctors was able to counsel the patient and explain his medical condition to the family. After a complete diagnosis, the patient was taken to the ICU as he was under observation. The doctor prescribed him antioxidant drugs and insulin to control any future problems while treating the present one. The doctor is the best gastroenterologist in Faridabad, Delhi, and Patna, and she handled the situation well before any further complications. The patient got his discharge in due time and was sent back home in a healthy and sound condition.
1. Which Group of People are More Likely to get diagnosed with alcoholic liver cirrhosis?
A person who has drunk heavily for a long time is more prone to acquire this disease. Women are also at risk for this medical disease due to the absence of many enzymes which break down alcohol particles.
Consider consulting the best gastroenterologist in India , Dr. Nivedita Pandey who is also well known for her nutritional counselling services and teleconsultation services. She is also famous for her care from afar service. You can also find her as the best liver specialist doctor in Patna, Bihar or hepatologist in Patna or the best doctor for hepatitis b in Patna , a gastroenterologist in Faridabad , the best gastro doctor in Delhi, NCR , a gastroenterologist In Uttarakhand , a liver specialist in Jhansi , best gastroenterologist in Jammu take advantage of the online gastroenterology consultation to gastroenterologist live chat and receive the best treatment that your body deserves!
2. Is liver cirrhosis cancer?
No, liver cirrhosis is not a type of cancer. If a person has alcoholic liver cirrhosis, he/she has an increased risk of liver cancer.
3. Is liver cirrhosis a hereditary disease?
Negative, alcoholic liver cirrhosis is not a hereditary disease, rather it is a type of an acquired disease.
Privacy Preference Center
Privacy preferences, nutrition counselling.
Nutrition counseling is the assessment of an individual’s dietary intake after which, they are helped set achievable goals and taught various ways of maintaining these goals. The nutrition counselor provides information, educational materials, support and follow-up care to help an individual make and maintain the needed dietary changes for problems like obesity.
Obesity/ Food allergy
I assist people dealing with weight-related health problems by evaluating the health risks and help in obesity management. I also help patients manage various food allergies.
As a hepatologist, I specialize in the treatment of liver disorders, pancreas, gallbladder, hepatitis C, jaundice and the biliary tree. I also see patients suffering from pancreatitis, liver cancers alcoholic cirrhosis and drug induced liver disease(DILI), which has affected the liver.
Gastroenterology
As a gastroenterologist, my primary focus is the overall health of the digestive system. I treat everything from acid reflux to ulcers, IBS, IBD: Crohns disease and ulcerative colitis, and colon cancer.
Endoscopy is a nonsurgical procedure to examine a person’s digestive tract. It is carried out with an endoscope, a flexible tube with a light and camera attached to it so that the doctor can see pictures of the digestive tract on a color TV monitor.
Medical Gastroenterology
Gastroenterology is a specialty that evaluates the entire alimentary tract from the mouth to anus and involves studying the diseases of the pancreas.
Liver Transplant Services
A liver transplant is a surgical procedure that removes a patient’s non-functioning liver and replaces it with a healthy liver from a deceased donor or a portion of healthy liver from a living donor. It is reserved as a treatment option for people who have significant complications due to end-stage chronic liver disease or in case of sudden failure of a previously healthy liver.
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

CASE STUDY OF PATIENT WITH LIVER CIRRHOSIS

Livercirrhosisisadiseaseinwhichnormaltissueofliverreplacedwithscartissue,livercirrhosisisthe12thleadingcauseofdeathsbydiseaseintheworld.Livercirrhosisiscausedbyanyfactorthatcandamagelivertissues,mostlyfattyliverandchronicliverdiseasesarethemajorcauseoflivercirrhosis. WearepresentingherethecaseofanAsianmanwhowasthevictimoflivercirrhosisthatwascomplicatedbyuntreatedhepatitisc.Hewasexperiencinggeneralizedbodyweakness,brownishtintintheurine,andsuddenweightgainof8-kgswithinaperiodofthreeweeks.Bloodpressurecountwas100/60,pulseratefoundtobe76beats/min,temperature98F,andenlargedumbilical.Laboratorytestsincludingcompletebloodcount,liverfunctiontests,andureatestscameouttobesignificantlyabnormal,complicatingthecase.,ultrasoundreportrevealedthathisliverwasenlarged,urinarybladderpartiallyfilled,umbilicalherniagapereported,ascitespresent(retentionoffluidintheabdomen),Cirrhosisandsplenomegalywerereported.Hisliverwasdamagedandliverfunctioningtestswerenotreturningtonormal,hepatologistrecommendedlivertransplantationasthelastresortforhim. Thereistheneedofgoodclinicalevaluationbyaqualifiedtherapistanduseofappropriateinvestigativestudiestosecurepatientfromsuchacriticalhealthhazard. Keywords:chronicliverdisease,livertransplantation,
Related Papers
Journal of Pharmaceutical Research International
Jaya Khandar
Liver is the second largest organ in human body, more than 5,000 separate bodily functions .including helping blood to clot, cleansing the blood of toxins to converting food into nutrients to control hormone levels, fighting infections and illness, regenerating back after injury and metabolizing cholesterol, glucose, iron and controlling their levels. A 56- years old patient was admitted in AVBRH on date 9/12/2020 in ICU with the chief complaint of abdominal distension, breathlessness on exertion, pedal edema, fever since 8 days. After admitted in hospital all investigation was done including blood test, ECG, fluid cytology, peripheral smear, ultrasonography, etc. All investigation conducted and then final diagnosis confirmed as cirrhosis of liver. Patient was not having any history of communicable disease or any hereditary disease but he has history of hypertension and type II Diabetes mellitus for 12 years. Patient was COVID-19 negative and admitted in intensive care unit. Patient...
The Journal of medical research
Amrendra Yadav
The liver is the multi-functional and vital organ of the body. It is found in the upper abdomen region of the vertebrates. Due to long-term damage, liver stops functioning properly which may lead to cirrhosis. This long-term damage occurred when scar tissue replaces the normal tissue of the liver. This disease develops slowly and has no early symptoms, but when it develops and become worse, then it leads to tiredness, itchiness, weakness, yellow skin, swelling in the lower legs, spider-like blood vessels and an easy bruise on the skin with fluid in the abdomen. The severe complications like bleeding dilated veins in esophagus or stomach, hepatic encephalopathy leading to confusion and unconsciousness and liver cancer may occur in the body. This review article is focusing on the effect of liver damage in the
Asian Australasian Neuro and Health Science Journal (AANHS-J)
Hanun Mahyuddin
Liver cirrhosis is a chronic disease characterized by the presence of fibrosis and regeneration of nodules in the liver, the consequence of which is the development of portal hypertension and liver failure. Usually associated with infectious infectious diseases such as viral hepatitis, alcohol consumption, metabolic syndrome, autoimmune processes, storage diseases, toxic substances and drugs. Major complications include gastrointestinal variceal bleeding, ascites, spontaneous bacterial peritonitis infection, hepatorenal syndrome, hepatic encephalopathy, and hepatocellular carcinoma. A 23-year-old woman comes to the ER, dr. Soegiri Lamongan with complaints of vomiting blood. The patient also complained of black bloody stools. Referred patient from Intan Medika Hospital with the initial complaint of vomiting blood more than 5 times (± equivalent to one medium drinking bottle) four days ago. On examination also found anemic conjunctiva and found splenomegaly. On abdominal ultrasound ex...
Fortune Journals , Umair Akram
Cirrhosis results from chronic liver disease, and is characterized by advanced fibrosis, scarring, and formation of regenerative nodules leading to architectural distortion. The main objective of the study is to find the cirrhosis and its complication, other clinical complications except ACLF and critical illness. This descriptive study was conducted at DHQ hospital, Vehari, Pakistan during June 2022 to January 2023. A comprehensive literature search of the published data was performed in regard with the spectrum, diagnosis, and management of cirrhosis and its complications. Data was also collected from OPD of the hospital record. We include all patients suffering from liver cirrhosis and its complication. It is concluded that patients with cirrhosis have progressive disease and suffer from multiple complications like ascites, HE, variceal bleeding, hepatorenal syndrome, cirrhotic cardiomyopathy, pulmonary syndromes, sarcopenia, frailty, and HCC. The prevention, early diagnosis, treatment, and palliation of these complications are essential in comprehensive clinical care plans.
The Egyptian Journal of Hospital Medicine
ibrahim al rajeh
Clinical Chemistry
Corinne Fantz
Clinical Transplantation
Cyrille Feray
Asian J Pharm Clin Res
Dr Bibhu Prasad Behera
Objective: Efforts can be made to normalize the hematological parameters so that the morbidity and mortality in these patients could be effectively reduced. Methods: This observational study was carried out among 69 cirrhosis patients that fulfills the inclusion and exclusion criteria, attended the medicine outpatient department, and admitted in medicine ward Results: In our study, we had 59 male and 10 female patients with an average age of 49.8±13.19 years. About 92.75% of the patients were alcoholic. Abdominal distension (92.75%) and ascites (84.06%) were the most common presenting complaints. Pallor was present in 42 (60.87%) cases. Splenomegaly was present in 35 (50.72%) cirrhotic patients. Renal dysfunction was present in 23 (33.33%) cases. Sixty-six (95.65%) patients had anemia and 47 (68.12%) patients had thrombocytopenia. Conclusions: From this study, we can conclude that, in cirrhosis of liver patients, various hematological changes are very common which need to be identified and corrected early to reduce morbidity and mortality.
Faridpur Medical College Journal
Shahin Ul Islam
Liver cirrhosis is an important cause of death and disability globally. This cross-sectional study was carried out in Faridpur Medical College Hospital from November 2018 to April 2019 to see the patterns of clinical presentations and associated factors among admitted liver cirrhosis patients. A total of 89 patients were included. Data were collected by detailed history from patients or their relatives followed by thorough physical examination as well as diagnostic evaluation; then those were checked, verified for consistency and edited for result. Among total respondents, the majority were male (69.7%) with a male to female ratio of 1:0.44. Age of patients ranged between 22-106 years with mean age of 52.33 year. The patients predominantly presented with ascites (49.4%), gastrointestinal bleeding (27%), peripheral edema (24.7%), and encephalopathy (21.3%). The in-hospital case fatality rate was 11.2% and the patients presented with decreased urinary output, peripheral edema and ence...

RELATED PAPERS
Yerling Aguilera
2020 IEEE International Solid- State Circuits Conference - (ISSCC)
BAIBHAB CHATTERJEE
Micromachines
Osman Ahmed
Brazilian Journal of Health Review
Brunella Petronetto
BMC Family Practice
Marcello Sarini
Archive of Applied Mechanics
Journal of Iranian Medical Council
SeyedAhmad SeyedAlinaghi
University of Chitral Journal of Linguistics and Literature
Syed Hanif Rasool
仿制查尔斯达尔文大学毕业证 cdu毕业证文凭毕业证文凭学历认证原版一模一样
Turkish Neurosurgery
Bahadir Öztürk
Plant Methods
Mariana da Silva Azevedo
jual kitchen set
mans role in history
Agha H Amin
Journal of Imaging
Dmitri Alekseevsky
Influenza and other respiratory viruses
Md Ariful Islam
Maria Regina Bernardo
Health in emergencies and disasters quarterly
MOJ applied bionics and biomechanics
Chinmaya padhy
Annales UMCS, Informatica
Marcin Piękoś
abdal chaqil harimi
MGG Molecular & General Genetics
Bruno Jarry
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
Liver disease case studies: Case study level 1 – Alcoholic cirrhosis; alcohol withdrawal
In this chapter Case studies levels 1–3 explore the management of a patient with alcoholic liver disease. The patient has alcoholic liver cirrhosis and first presents with alcohol withdrawal (Case study level 1), then the patient’s risk of bleeding and treatment for the maintenance of alcohol abstinence are considered (Case study level 2). The patient then goes on to develop encephalopathy (Case study level 3). Case studies levels Ma and Mb consider two patients: one presents with TB and the other liver failure.
Case study level 1 – Alcoholic cirrhosis; alcohol withdrawal
Learning outcomes Level 1 case study: You will be able to:
- describe the risk factors
- describe the disease
- describe the pharmacology of the drug
- outline the formulation, including drug molecule, excipients, etc. for the medicines
- summarise basic social pharmacy issues (e.g. opening containers, large labels).
Mrs MW, 59 years old, is divorced and unemployed. She was admitted to an acute medical ward at the hospital presenting with general malaise, a grossly distended abdomen, swollen ankles, and jaundice. It was also noted that she smelt of alcohol and was showing signs of alcohol withdrawal.
1. What is cirrhosis of the liver?
2. List possible causes of cirrhosis.
3. What other clinical signs and symptoms may Mrs MW present with?
4. What drug treatment, including dose, would you recommend for Mrs MW’s alcohol withdrawal? What recommendations would you make if the patient was unable to take the medication orally?
1 What is cirrhosis of the liver?
Cirrhosis is defined as the histological development of regenerative nodules surrounded by fibrous bands in response to chronic liver injury. It is an advanced stage of liver fibrosis that is accompanied by distortion of the hepatic vasculature.
2 What are the risk factors for developing primary dysmenorrhoea?
Causes of cirrhosis can usually be identified by the patient’s history combined with serological and histological investigation. Alcoholic liver disease and hepatitis C and B are the most common causes of cirrhosis.
The association of excessive alcohol consumption with liver disease has been recognized for centuries. After the identification of the hepatitis C virus and of non-alcoholic steatohepatitis in obese patients with diabetes, the diagnosis of cirrhosis without an apparent cause (cryptogenic cirrhosis) is rarely made. Genetic causes of cirrhosis include hemochromatosis and Wilson’s disease.
Epidemiological studies have identified a number of factors that contribute to the risk of developing cirrhosis. Regular (moderate) alcohol consumption, age older than 50 years, and male gender are examples that increase cirrhosis risk in chronic hepatitis C infection, and older age, obesity, insulin resistance or type 2 diabetes, hypertension and hyperlipidemia in non-alcoholic steatohepatitis.
3 What other clinical signs and symptoms may Mrs MW present with?
Cirrhosis is often asymptomatic until complications of liver disease are present. Mrs MW may present with itching, jaundice, dark urine, pale fatty stools, abdominal pain, nausea, fatigue, bleeding – such as nosebleeds, hepatic encephalopathy, hepatomegaly, ascites, distended abdominal veins, spider angiomata, palmar erythema and asterixis. She may also present with the signs and symptoms of alcohol withdrawal, which include irritability, anxiety, tachycardia, tremor, sweating, confusion, and hallucinations.
4 What drug treatment, including dose, would you recommend for Mrs MW’s alcohol withdrawal? What recommendations would you make if the patient was unable to take the medication orally?
Long-acting benzodiazepines (e.g. diazepam and chlordiazepoxide) are used to attenuate alcohol withdrawal symptoms but they also have a dependence potential. To minimize the risk of dependence, administration should be for a limited period only (e.g. chlordiazepoxide 20 mg 4 times daily, gradually reducing to zero over 7–14 days). Mild alcohol withdrawal symptoms may be treated with a lower starting dose, such as 15 mg four times a day. In all cases, the patient should be counseled about the proposed length of the treatment course . Benzodiazepines should not be prescribed if the patient is likely to continue drinking alcohol.
In patients unable to take medication by the oral route, diazepam may be administered by intramuscular or slow intravenous injection (into a large vein, at a rate of not more than 5 mg/min), at a dose of 10 mg, repeated if necessary after not less than 4 hours. Alternatively, diazepam may be administered via the rectal route as a rectal solution or suppository. The intramuscular route should only be used when both the oral and intravenous routes are not possible.
General references
- Schuppan D and Afdhal NH (2008) Liver cirrhosis. Lancet 371: 838–851.
- Heidelbaugh JJ and Sherbondy M (2006) Cirrhosis and chronic liver failure: Part II. Complications and treatment. American Family Physician 74: 767–776.
- Joint Formulary Committee (2008) British National Formulary 55. London: British Medical Association and Royal Pharmaceutical Society of Great Britain, March.
- Vincent WR, Smith KM, Winstead PS and Lewis DA (2007) Review of alcohol withdrawal in the hospitalized patient: management . Orthopedics 30: 446–449.
Author: Caron Weeks [BPharm (Hons), MRPharmS, DipPharmPrac. Lead pharmacist – Medicine, Southampton University Hospitals NHS Trust] and Mark Tomlin [BPharm, MSc, MRPharmS (IPresc) Consultant Pharmacist, Critical Care, Southampton General Hospital]
- Alcohol withdrawal
- Case study for pharmacist
- liver cirrhosis
- liver disease
- Pharmacy case study
You might be interested in

How to be kind to yourself when giving up alcohol

4 Ways to Support Loved Ones During Substance Abuse Recovery

Drug and Alcohol Testing: Types, Accuracy, and Getting Help
Leave a reply cancel reply.
Your email address will not be published.
{{#message}}{{{message}}}{{/message}}{{^message}}Your submission failed. The server responded with {{status_text}} (code {{status_code}}). Please contact the developer of this form processor to improve this message. Learn More {{/message}}
{{#message}}{{{message}}}{{/message}}{{^message}}It appears your submission was successful. Even though the server responded OK, it is possible the submission was not processed. Please contact the developer of this form processor to improve this message. Learn More {{/message}}
Submitting…
This site uses Akismet to reduce spam. Learn how your comment data is processed .
Cardiovascular Case Studies: Case study level Mb – Myocardial infarction

Noakhali Science and Technology University (NSTU)
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.14(11); 2022 Nov

Cirrhosis of the Liver: A Case Report and Literature Review of a Rare Case Presentation of Autoimmune Hepatitis With Systemic Sclerosis
Shashank banait.
1 Department of Ophthalmology, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences, (Deemed to be University), Wardha, IND
Chetan Burriwar
2 Department of Medicine, Mahatma Gandhi Institute of Medical Sciences, Wardha, IND
Priti G Verma
3 Department of Obstetrics and Gynaecology, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences, (Deemed to be University), Wardha, IND
Tanvi Banait
4 Department of Medicine, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences, (Deemed to be University), Wardha, IND
Madhura Joshi
Systemic sclerosis (SSc) is a chronic systemic disease that affects the skin, heart, lungs, kidneys, gastrointestinal tract, and musculoskeletal system. Although gastrointestinal involvement has been reported in approximately 90% of scleroderma patients, liver involvement is uncommon. A 51-year-old female was admitted to the hospital due to abdominal distension and pedal edema. She had a history of Raynaud's syndrome and multiple hypopigmented and hyperpigmented patches over her body for the last year. Her ascetic fluid analysis was transudative with a serum ascites albumin gradient >1.1, and the abdomen and pelvis ultrasonography reported liver cirrhosis with splenomegaly with perisplenic varices. Her antinuclear antibody and anti-centromere antibody were positive. Skin thickening was visible. Her alanine aminotransferase (ALT), aspartate aminotransferase (AST), and serum globulin were raised. Viral serology was negative. We managed her with diuretics, beta-blockers, prednisolone (30 mg/day administered orally), angiotensin-converting enzyme inhibitors, and calcium channel blockers. Edema and abdominal distension decreased with this management, and no Raynaud's phenomenon was observed during the hospital stay.
Introduction
Systemic sclerosis (SSc) is a rare chronic disorder of unknown etiology, characterized by diffuse fibrosis and generalized vascular abnormalities in the skin, joints, and internal organs, leading to the failure of these organs. The etiology of SSc is multifactorial, with multiple genetic, endogenous, and exogenous factors appearing to interact with disease development. The pathogenesis of fibrosis is complex and is due to the interaction between immunological events and vascular changes, which activate fibrogenic fibroblasts [ 1 ]. Autoimmune hepatitis is a chronic inflammation of the liver characterized by the presence of autoantibodies and raised serum globulin levels. It is predominantly a disease that affects women and can present at any age. The disease may begin as acute hepatitis, which may progress to cirrhosis [ 2 ]. It is commonly associated with various autoimmune diseases, like autoimmune thyroiditis, type 1 diabetes mellitus, glomerulonephritis, ulcerative colitis, autoimmune hemolytic anemia, and autoimmune thrombocytopenia. However, its association with systemic connective tissue disorders like SSc, systemic lupus erythematosus, and mixed connective tissue disorders is infrequent [ 3 , 4 ]. Here, we report the case of a patient presenting with cirrhosis of the liver due to autoimmune hepatitis along with systemic sclerosis.
Case presentation
A 51-year-old non-obese female with no history of alcoholism was admitted with complaints of gradually progressive abdominal distension and swelling over both lower limbs for the last month. She had difficulty swallowing 15 days before the presentation, which was gradual and worse with solid food. She had a history of multiple small hypopigmented and hyperpigmented patches around the neck, over the back, forearms, and legs for one year. She also presented with Raynaud's phenomenon, which was characterized by bluish discoloration followed by diffuse pain in her fingers since last year, especially during contact with water. However, there was no history of joint pain or deformity, morning stiffness, fever, pain in the abdomen, diarrhea, constipation, chest pain, or paroxysmal nocturnal dyspnoea or orthopnoea. There was no past history of any neurological deficit like dysarthria, gait abnormalities, dystonia, tremors, diabetes, hypertension, dyslipidemia, metabolic syndrome, rheumatic heart disease, or ischemic heart disease. A family history of liver cirrhosis or any chronic liver disease was obtained to rule out hereditary hemochromatosis, as well as red skin lesions and epistaxis for hereditary hemorrhagic telangiectasia, which were all negative.
On general examination, she was conscious, afebrile, and pale, with a pulse rate of 100/min and blood pressure of 110/80 mmHg. She was anicteric, and bilateral pitting pedal edema up to the knee was present. Salt and pepper pigmentation around the neck, over the back, forearms, and legs was present (Figure (Figure1 1 ).

Skin distal to the metacarpophalangeal joint in the hands and at the elbow was tight, but there were no ulcers, swollen fingertips, calcinosis, sclerodactyly, or telangiectasia (Figure (Figure2 2 ).

On systemic examination, the abdomen was distended with full flanks, the umbilicus was everted, fluid thrill and shifting dullness were present, and the liver was not palpable, but splenomegaly was present. The rest of the systemic (cardiovascular, respiratory, and central nervous system) examination did not reveal any abnormalities. On slit-lamp examination of the anterior chamber of the eyes, the Kayser-Fleischer (KF) ring was absent. Her hemogram revealed a hemoglobin of 9.6 g/dl, anisopoikilocytosis, microcytic hypochromic red blood corpuscles with a mean corpuscular volume of 71, a white blood corpuscle count of 3.88 with 77.1% granulocytes, and reduced platelets of 0.68 lac/ul. Her liver function tests showed hypoalbuminemia (2.44 gm/dl), hypergammaglobulinemia (4.08 gm/dl), and raised alanine aminotransferase (ALT) of 127 IU/L and aspartate aminotransferase (AST) of 276 IU/L with normal bilirubin and alkaline phosphatase (73 IU/L). Other parameters like blood sugar, serum electrolytes, lipid profile, kidney function tests, and electrocardiogram were normal. Her ascitic fluid was transudative in nature with a serum ascites albumin gradient of 1.35. Her blood, urine, and ascitic fluid cultures did not show the growth of any bacteria. The presence of bilateral pitting pedal edema, ascites, splenomegaly, hypoalbuminemia, and a serum ascites albumin gradient of 1.35 led to the diagnosis of liver cirrhosis with portal hypertension [ 5 , 6 ].
Ultrasonography of the abdomen showed a small-sized (9.7 cm) shrunken liver with coarsened echotexture of parenchyma with irregular margins, suggestive of liver cirrhosis with gross ascites, periportal fibrosis, and splenomegaly (14.1 cm), a dilated portal vein of 12 mm with the normal color flow on Doppler imaging, a dilated splenic vein of 15 mm, and a partially distended gall bladder. The common bile duct and intra-hepatic biliary radicals were normal (Figure (Figure3 3 ).

Her fibroscan of the liver could not be done due to the unavailability of this facility at our rural hospital. Her viral serology, hepatitis B surface antigen (HBsAg) for hepatitis B, antibody to hepatitis C virus (anti-HCV), and quantitative hepatitis C virus ribonucleic acid (HCV RNA) by polymerase chain reaction (PCR) and human immunodeficiency virus (HIV) infection test by enzyme-linked immunosorbent assay (ELISA) method were negative. Her serum ferritin of 69 ng/ml, serum ceruloplasmin of 30 mg/dL, and 24-hour urinary copper of 20 µg/day were normal. Her anti-mitochondrial antibodies were negative. Antinuclear antibody 17 profile blot (ANA17B) and anti-centromere antibody with a class index of four were positive (Figure (Figure4 4 ).

On the American College of Rheumatology (ACR)/European Alliance of Associations for Rheumatology (EULAR) criteria for the classification of SSc (2013), her total score was 15. (nine for skin thickening and three each for Raynaud's phenomenon and positive anti-centromere antibody [ 7 ]. A liver biopsy could not be performed as the patient did not give consent.
The provisional diagnosis of SSc with autoimmune hepatitis leading to cirrhosis of the liver was considered as per the international autoimmune hepatitis group diagnostic criteria [ 2 ] (Table 1 ).
ALT: alanine aminotransferase; ANA: antinuclear antibody; Anti - LKM - 1: Anti – Liver kidney microsomal – 1 antibody; ASMA: anti-smooth muscle antibody; AST: aspartate aminotransferase.
She was managed with diuretics, beta-blockers, angiotensin-converting enzyme inhibitors, calcium channel blockers, and steroids. She showed significant improvement in her symptoms; her edema and abdominal distension decreased, and no Raynaud's phenomenon was observed during the hospital stay.
Systemic sclerosis is a multisystem disorder that primarily affects the skin, heart, lungs, gastrointestinal tract, musculoskeletal system, and kidneys. The disease is characterized by tissue fibrosis, vasculopathy, and an autoimmune response associated with specific autoantibodies. Gastrointestinal involvement is common, up to 95% [ 8 ]. However, the liver is rarely involved in this disease. As shown in the review, eight (1.1%) of 727 patients with scleroderma had involvement of the liver [ 9 ]. Primary biliary cirrhosis is the most commonly found hepatic disease in patients with SSc, while autoimmune hepatitis is rare [ 10 , 11 , 12 ].
Although the pathogenesis of the development of autoimmune hepatitis in patients with SSc is still unclear, it may be due to a dysregulated response of the cellular and humoral immune systems. According to the literature, anti-centromere antibodies are found in 3-13% of patients with autoimmune hepatitis [ 1 , 9 ]. Our patient was diagnosed with autoimmune hepatitis according to the criteria proposed by the international autoimmune hepatitis group, and she fulfilled all the diagnostic criteria as mentioned in Table Table1 1 [ 2 , 13 ].
A few cases of autoimmune hepatitis without primary biliary cirrhosis in SSc patients have been reported. In three cases, SSc was diagnosed before the development of autoimmune hepatitis, while in one case, both conditions were diagnosed simultaneously [ 14 - 17 ]. The clinical characteristics of five patients and their laboratory profiles, along with the patients described in the current report, are summarized in Table Table2 2 .
ALT: alanine aminotransferase; anti-centromere: anti-centromere antibody; AST: aspartate aminotransferase; CREST: calcinosis, Raynaud’s phenomenon, oesophageal dysmotility, sclerodactyly, and telangiectasia syndrome; CRST: calcinosis cutis, Raynaud’s phenomenon, sclerodactyly, telangiectasia syndrome; FANA: fluorescent anti-nuclear antibody; g/dL: grams/deciliter; g/L: Grams/liter; IgG: immunoglobulin G; IU/L: international units /liter; lacs/ul: lacs/microliter; mg/day: milligrams/day; mg/dl: milligrams/deciliter; SSc: systemic sclerosis.
In our case, cirrhosis of the liver due to autoimmune hepatitis and SSc was diagnosed during the current admission. However, her fibroscan of the liver could not be done due to the unavailability of this facility at our rural hospital, and a liver biopsy could not be performed as the patient did not give consent. In the present case, liver involvement was advanced compared to the previous four cases. These previous case reports indicate that patients with SSc are at an increased risk of developing autoimmune hepatitis (Table (Table2 2 ).
This case was diffuse SSc in which the patient had Raynaud's phenomenon, skin thickening in the hands, feet, and elbows, and salt and pepper pigmentation skin over the back and trunk also, but there were no calcinosis cutis, sclerodactyly, or telangiectasia. High-dose oral corticosteroids and azathioprine are the drugs of choice for treatment as they effectively alleviate symptoms and the outcome of autoimmune hepatitis [ 18 ].
Managing autoimmune hepatitis is difficult with diffuse cutaneous SSc patients, as these patients may develop a renal crisis with high-dose steroid therapy [ 19 , 20 ]. Future studies are required to determine the treatment outcome and complications of high-dose steroid therapy for combined autoimmune hepatitis and SSc.
Conclusions
Patients with SSc and autoimmune hepatitis should be observed for the development of other autoimmune diseases. A high degree of observation is necessary to diagnose autoimmune hepatitis in patients with SSc as symptoms are non-specific and may appear gradually over years. Patients with SSc should be monitored for liver function tests regularly as early diagnosis and treatment of autoimmune hepatitis will help achieve a reasonable remission rate.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. Institutional Ethics Committee, Mahatma Gandhi Institute of Medical Sciences, Sevagram. Reg. No. ECR/47/Inst/MH/2013/RR-19 issued approval MGIMS/IEC/MED/336/2022. Approved.
- Open access
- Published: 26 May 2024
Bacterial profile, drug resistance pattern, clinical and laboratory predictors of ascites infection in cirrhosis patients
- Abubeker Shemsu Helil 1 ,
- Shambel Araya Haile 1 , 6 ,
- Yohannis Birhanu 2 ,
- Hailemichael Desalegn 3 ,
- Daniel Melese Desalegn 1 ,
- Rozina Ambachew Geremew 5 ,
- Zenebe Gebreyohannes 5 ,
- Awad Mohammed 4 ,
- Daniel Dejene Wondimagegnehu 4 ,
- Gonfa Ayana 4 ,
- Anteneh Mehari Tizazu 5 &
- Kassu Desta 1
BMC Infectious Diseases volume 24 , Article number: 528 ( 2024 ) Cite this article
Metrics details
Ascites is a pathological collection of free fluid in the peritoneal cavity, which is a common complication in patients with cirrhosis, an advanced liver disease. Bacterial infection increases the mortality rate of hospitalized patients with cirrhosis, irrespective of the severity of the liver disease. Around 60% of patients with compensated cirrhosis developed ascites within 10 years during the course of their disease. The in-hospital mortality rate due to spontaneous bacterial peritonitis (SBP) could exceed 90%, but with early diagnosis and prompt antibiotic therapy, this rate has been shown to decrease to 20%. Here, we enrolled adult (age ≥ 18) patients with liver disease with evidence of cirrhosis who developed ascites and assessed the presence of spontaneous ascites fluid infection (SAFI) in these patients. Of the total 218 patients, 22.9% (50/218) develop ascites infection. The liver organ function tests like alanine aminotransferase, aspartate aminotransferase, total bilirubin, and direct bilirubin were found to be significantly ( P < 0.05) higher in patients with ascites fluid infection compared to patients with non-ascites fluid infection. Of the gram-negative bacteria, K. pneumonia and E. coli were isolated and found to be 100% resistant to amoxicillin and clavulanate. From the gram-positive bacterial isolates, S. aureus was only resistant to penicillin, whereas Str. viridans was resistant to ceftriaxone, cefotaxime, cefepime, and penicillin. On the other hand, clinical features such as a history of jaundice, low arterial blood pressure, and ultrasound results such as a shrunken liver and enlarged spleen were also independent predictors of spontaneous bacterial peritonitis. In conclusion, given the high probability of death following SAFI, early detection, and treatment, as well as knowledge of the microbial agent, resistance profile, and predictive markers in various contexts, are essential for the timely diagnosis and management of SAFI in these patients.
Peer Review reports
Ascites is a pathological collection of free fluid in the peritoneal cavity, which is a common complication in patients with cirrhosis, an advanced liver disease [ 1 ]. Deregulation of the gut-liver immune axis in patients with cirrhosis puts them at risk of developing infection. Bacterial infection increases the mortality rate of hospitalized patients with cirrhosis, irrespective of the severity of the liver disease [ 2 ]. Bacterial infections, including spontaneous bacterial peritonitis (SBP), are common in patients with ascites and cirrhosis, and they are associated with increased morbidity and mortality. In these patients, infectious bacterial pathogens frequently arise from the commensal intestinal microbiota [ 3 ]. The most common forms of ascitic fluid infection are SBP and culture-negative neutrocytic ascites (CNNA); the other forms include secondary bacterial peritonitis, monomicrobial non-neutrocytic bacterascites (MNB), and polymicrobial bacterascites [ 4 ].
Around 60% of patients with compensated cirrhosis develop ascites within 10 years of the disease. Alongside physical examination, ultrasound evaluation and ascites fluid analysis help to rule out ascites cases caused by other than cirrhosis. Furthermore, an ascites fluid polymorphonuclear neutrophil (PMN) count greater than 250/mm3 is used to diagnose the presence of SBP in these patients [ 5 ]. A systematic analysis of the global burden of SBP among cirrhotic liver patients revealed a prevalence of 17.12%, of which the prevalence in Africa was the highest at 68.5%, whereas the prevalence in North America was the lowest at 10.81%. Similarly, the level of drug resistance among isolated pathogens was also high, at 11.77% [ 6 ]. Other studies have also shown that the prevalence of SBP is high, around 25% among patients with cirrhotic ascites [ 7 ].
It has been demonstrated that early diagnosis and fast antibiotic therapy can reduce the in-hospital death rate from over 90–20%, highlighting the criticality of early intervention [ 8 ]. Since the Enterobacterales group of bacteria is the common cause of SBP, third-generation cephalosporins (TGC) are utilized as an empirical antibiotic treatment. The emergency and spread of multidrug-resistant (MDR) and TGC-resistant organisms, as well as the increased incidence of ascites infection due to gram-positive bacteria, have created concern about the effective treatment of patients [ 9 ]. Thus, the local epidemiological pattern of microbial resistance should be considered while treating SBP patients empirically [ 10 ]. Furthermore, ruling out the source of infection (nosocomial vs. community-acquired) also has implications for the treatment of SBP infection in patients with cirrhosis [ 11 ]. This implies that in order to assist direct the practical therapy of ascites SBP in patients with hepatic cirrhosis, a continuous evaluation of common bacterial infections and their antibiograms is necessary.Clinical and laboratory parameters, as well as the bacteriological profile of ascites infection among cirrhosis patients, are lacking in Ethiopia. Here, we enrolled cirrhosis patients with ascites and assessed the presence of ascites fluid infection, the causative bacterial pathogens, and the drug susceptibility pattern of the organisms. Furthermore, we assessed different clinical, imaging, and laboratory test parameters in predicting ascites fluid infection in these patients.
Materials and methods
A hospital-based cross-sectional study was conducted at St. Paul’s Hospital Millennium Medical College (SPHMMC) and Yekatit 12 Hospital Medical College in Addis Ababa, participants were selected through purposive sampling method.
All patients attending the internal medicine department as outpatients and inpatients in the selected hospitals for chronic liver disease with ascites comprised the source population.
Inclusion and Exclusion Criteria: Patients aged 18 and above with clinical and ultrasound evidence of cirrhosis and ascites were included in the study while study subjects with evidence of secondary peritonitis were excluded from the study.
Study Variables: The study assessed the prevalence of Spontaneous Bacterial Peritonitis (SBP), bacterial isolates from ascitic fluid culture, and the antimicrobial sensitivity pattern of bacterial isolates were considered as dependent variables. Moreover, Socio-demographic information (e.g., age and sex), clinical features (e.g., arterial blood pressure, Body Mass Index [BMI], body temperature, history of upper gastrointestinal bleeding), and laboratory features (e.g., liver function tests [LFT], complete blood count [CBC], prothrombin time/international normalized ratio [PT/INR], and hepatitis serology) served as independent variables in the study.
Measurement and data Collection
Sample size determination.
As far as our knowledge goes there is no previous study conducted on the prevalence of SBP among adult cirrhosis patients in Ethiopia. Therefore, we used a study done at Korle-Bu Teaching Hospital in Ghana that reported the Patterns of Ascites fluid infection among adult patients with ascites to be 21.4% to calculate our sample size.
Z = Standard score corresponding to 95% confidence level. d = the margin of error (precision) = 5%. n = the required sample size.
About 85% of the determined sample size was achieved for this research.
Sampling method
The study sites were selected by purposive sampling method. A convenient sampling method was used to select each study subject. Cirrhosis patients with ascites whether symptomatic or not for SBP who presented to the internal medicine department, particularly the Gastroenterology and Hepatology unit were consecutively recruited.
Data collection procedure
Patients’ medical records were meticulously reviewed to gather relevant history, including alcohol use, and physical characteristics indicative of liver cirrhosis. Clinical features such as ascites, hepatomegaly, splenomegaly, abdominal pain, and the presence of collateral veins were examined. Ascites diagnosis was based on specific criteria, including abdominal distention, the presence of shifting dullness, positive fluid thrill, and confirmation through diagnostic paracentesis or abdominal ultrasound scan [ 12 , 13 , 14 ].
Patient recruitment and questionnaire
After explaining the objective of the study to patients, those providing informed consent were recruited. A questionnaire covering socio-demographic data and the clinical history of patients was administered. The questionnaire encompassed information on socio-demographics, clinical signs and symptoms, laboratory investigations, ultrasound results, and other relevant aspects related to the study.
Laboratory analysis
Ascitic fluid sample collection.
Abdominal paracentesis was performed with an aseptic technique at the right or left iliac fossa, 3 cm above and 3 cm medial to the anterior superior iliac spine. Exactly 20 ml of ascites fluid was collected using a sterile syringe by a senior gastroenterologist or resident doctor. Subsequently, 10 ml was inoculated into a blood culture bottle at the bedside. Additionally, ascitic fluid analysis, including cell count, differential, ascitic fluid albumin, and total protein, was conducted as part of clinical utility, with data obtained from the patient card.
Isolation of bacterial pathogen
After the sample was inoculated into a blood culture bottle (broth) (BHI and TSB), the culture medium was incubated at 37 o C for 24 h using an incubator. After 24 h the culture medium was observed for possible microbial growth. For those who show microbial growth, a portion of the sample was transferred to a blood agar plate, chocolate agar plate, and MacConckey Agar. Mannitol salt agar was also used to isolate staphylococcus species.
Identification of bacterial pathogen
Bacterial identification was made using biochemical tests, including, indole, citrate, oxidase, H 2 S production, lysine decarboxylase, lactose fermentation, urea hydrolysis, gas production, catalase, and mannitol fermentation from the pre-collected and stored samples.
Antimicrobial sensitivity testing (AST)
Whenever growth was detected on the culture medium, antimicrobial sensitivity testing was done based on the identified bacterial pathogen for antibiotic disc choice. Antimicrobial sensitivity of the bacterial isolates was done by the Kirby-Bauer disc diffusion method. In the procedure, fresh sub-cultures of bacterial isolates were used after overnight growth on Muller Hinton Agar. The inoculums were prepared by suspending several of the colonies in sterile phosphate-buffered saline (pH 7.2) to achieve a turbidity of 0.5 McFarland standards. This resulted in a suspension containing approximately 1–2 × 10 8 CFU/ml. A sterile cotton swab was dipped into the bacterial suspension, elevated above the liquid, and rotated several times against the inside wall of the tube to remove excess of the inoculum. The swabs were then streaked evenly in three different directions onto the Muller Hinton Agar. Susceptibility Testing was done by discs of choice using the Kirby-Baur disk diffusion method and the interpretation of results was made following the CLSI’s guidelines, January 2020 (30th Edition) for Sensitive, Intermediate, and Resistance Zones. Throughout the experiment Pre-analytical, analytical and post-analytical qualities were maintained. All of the results were collected using the appropriate data collection sheet.
Ultrasound scan
All patients underwent an abdominal ultrasound scan after overnight fasting and the following details were obtained from patients’ cards: maximum vertical span of the liver; nodularity of liver surface; spleen size (length of its longest axis); presence of collateral vessels, portal vein dimension and presence of ascites.
Blood analysis and serology
Normally all cirrhosis patients undergoing laboratory investigation for hemoglobin (HB), white blood cell count (WBC), platelet (PLT) count, international normalized ratio (INR), and serum concentrations of total protein (TP) and direct bilirubin (DB), total bilirubin (TB), serum total protein, albumin, alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Alkaline phosphatase (ALP), serum sodium (Na+), potassium (K+), urea, and creatinine as well as testing for hepatitis B surface antigen (HBsAg) and antibodies to hepatitis C virus (anti-HCV-Ab) as part of clinical utility and other data were collected from patient’s cards.
Data analysis and interpretation
Descriptive statistics were performed for all continuous variables and data was presented in appropriate graphs and tables. The prevalence of spontaneous bacteria peritonitis was determined. Further analysis was done to determine if there were any associations between spontaneous bacterial peritonitis and the clinical or laboratory test parameters. The chi-square test was used to determine the level of association. Binomial and multinomial logistic regression analyses were conducted using SPSS version 23 for possible association.The multicollinearity between the independent variables was checked using a Variance Inflation Factor (VIF), and a VIF of less than 3 was used for logistic regression. P value ˂ 0.05 was taken as a significant association for clinical or laboratory.
Operational definitions
Cirrhosis patients.
Patients with liver cirrhosis, diagnosis established by using clinical, biological, and imagistic criteria.
Classical spontaneous bacterial peritonitis
is defined as ascitic fluid polymorph nuclear count ≥ 250/mm3 and positive ascitic fluid culture.
Culture-negative neutrocytic ascites (CNNA)
are defined as ascitic fluid polymorph nuclear count ≥ 250/mm3 and negative ascitic fluid culture.
Non-neutrocytic bacterascites (NNBA)
is defined as ascitic fluid neutrophil count ≤ 250/mm3 with positive ascitic fluid culture.
Socio-demographic characteristics and clinical features
A total of 218 cirrhosis patients with ascites were recruited for this study with a mean age of 38.67 ± 12.0 years (age range 19 to 76 years), with the majority of the age group between 18 and 40 years. Of the total patients, 145 (67%) were males, with a male-to-female ratio of 2.03:1. Whereas 135 (62.8%) were in the 18 years–40 years age group and 56 (25.7%) participants were single (Table 1 ). The clinical presentation of patients showed that 64 (29.4%) presented with upper GI bleeding, 110 (50.5%) with abdominal pain, 69 (31.7%) with jaundice, 86 (39.4%) with sleeping disturbance, 91 (41.75%) with pedal edema, 92 (42.2%) with fever, and 84 (38.5%) with chills (Table 1 ).
Cirrhosis patients with ascitic fluid infection show different organ function tests
Of the total 218 patients, 22.9% (50/218) develop ascitic infection. Organ function tests are crucial in monitoring cirrhotic patients, for instance, renal dysfunction has been identified as a robust predictor of mortality in cirrhotic patients with SBP [ 15 ]. Similarly, in our case, the liver organ function tests showed a significant difference between patients with ascitic fluid infections and those without. Thus, the median levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, and direct bilirubin were found to be 37 U/L (24.1 U/L – 63.1 U/L), 53 U/L (23.9 U/L – 87.7 U/L). 1.41 mg/dL (0.625 mg/dL − 2.53 mg/dL) and 0.58 mg/dL (0.2 mg/dL − 1.62 mg/dL) respectively, which were significantly higher ( P < 0.05) compared to patients without ascitic fluid infections, which was 25.2 U/L (17.9 U/L − 36.6 U/L), 33.5 U/L (21.1 U/L − 60 U/L), 0.67 mg/dL (0.45 mg/dL − 1.09 mg/dL) and 0.2 mg/dL (0.12 mg/dL − 0.35 mg/dL) respectively (Table 2 ). Likewise, a significantly ( P < 0.05) higher level of WBC count and a significantly ( P < 0.05) lower level of Hgb concentration were observed in patients with ascites fluid infection (Table 2 ). However, we observed no significant ( P > 0.05) difference in the levels of alkaline phosphatase, urea, creatinine, and the ion concentrations of chloride, sodium, and potassium between the two groups (Table 2 ). Previous reports have shown that simple laboratory parameters were able to predict different chronic and non-chronic disease [ 16 , 17 ] which indicate the relevance of these parameters in identifying infection among cirrhosis patients.
Ascitic fluid analysis in cirrhosis patients show differences in protein levels
The fluid analysis for chronic liver disease patients ( n = 218) demonstrated that 98% (50/51) of patients with ascitic fluid had an ascitic fluid neutrophil counts greater than 250 and 96.1% (49/51) of patients with SAFI had an ascites fluid albumin levels less than 0.5 g/dL. The ascitic fluid total protein showed an increased levels in non-infected individuals compared to patients with ascites infection (Table 3 ).
Culture-negative neurocytic ascites and HBV were common in cirrhosis liver patients
SBP was present in 23.39% (51/218) -liver cirrhotic patients. Of the 51 patients who developed SBP, culture-positive SBP was present in 22% (11/50), and CNNA was found in 78.4% (40/50). The prevalence of MNB was 1.96% (1/51) in this study (Fig. 1 a ) . Among patients with culture-positive SBP, 4 (36.36%) isolates were E. coli , and 3 (27.27%) isolates were Klebsiella spp. Of the gram-positive bacteria, 1 (9.09%) isolate was a Staphylococcus aureus , 1 (9.09%) isolate was Streptococcus viridian , and 2 (18.18%) isolates were found to be CoNs (coagulase-negative staphylococcus species) (Fig. 1 b ) . We also assessed the prevalence of different hepatitis virus infections in these patients. Hepatitis markers were tested for 218 CLD patients, and about 61% (133/218) were positive for hepatitis B, 10.1% (22/218) were positive for hepatitis C virus, and 1% (2/218) were positive for hepatitis B and C virus, and 28.4% (62/218) were tested negative for both hepatitis B and C viruses (Fig. 1 c).

- Prevalence of SPB, bacterial isolates, and seroprevalence of hepatitis Band C virus in Cirrhosis Patients. (A) The prevalence of different types of spontaneous ascites fluid infection, (B) the different gram-positive and gram-negative bacterial isolates, (C) the Sero-positivity of HBV and HCV among study participants
Antimicrobial-resistant profile of isolated bacterial isolates
We tested different antibiotics being employed to treat bacterial infections using the Kirby-Bauer disc diffusion method, as described in detail in the material and method part of this paper. The gram-negative isolate K. pneumonia showed 100% (3/3) sensitivity to Cefazolin, Cefepime, Cefotaxime, Cefotetan, Cefuroxime, Gentamicin, Meropenem, and Levofloxacin, whereas it showed 100% (3/3) resistance to amoxicillin/clavulanate (Table 4 ). On the other hand, E. coli showed 100% (4/4) sensitivity to Meropenem and Levofloxacin, whereas it showed 100% (4/4) resistance to amoxicillin/clavulanate, Ampicillin, and Cefazolin (Table 4 ). Both K. pneumonia and E. coli were 100% (7/7) resistant to amoxicillin/clavulanate and were 100% (7/7) sensitive to meropenem and levofloxacin (Table 4 ). From the gram-positive results, S. aureus was only resistant to penicillin, whereas Str. viridian was resistant to ceftriaxone, cefotaxime, cefepime, and penicillin. The isolated S. aureus was susceptible to Azitromycin, erythromycin, doxycycline, oxacillin, Gentamycin, and vancomycin, whereas Str. viridian was susceptible only to vancomycin ( Table 4 ).
Clinical and laboratory parameters were able to predict spontaneous bacterial peritonitis
A logistic regression was performed to appreciate the effect of age, gender, marital status, and clinical, and laboratory parameters on the likelihood of predicting SBP in cirrhosis patients. The logistic regression model showed that the variables age, gender, and marital status were statistically insignificant ( P > 0.05) in predicting SBP. However, the clinical features of a history of jaundice, low arterial blood pressure on admission, and fever were found to be independent predictors ( P < 0.05) of spontaneous bacterial peritonitis. Ultrasound studies depicted a shrunken livers and enlarged spleens, which were also found as independent predictors ( P < 0.05) of spontaneous bacterial peritonitis (Table 5 ).
Similarly laboratory parameters were also able to significantly predict the presence of spontaneous bacterial peritonitis (SBP). Increased level of ALT (AOR = 1.02, P = 0.039, 95% CI (0.99–1.12), increased level of ALP (AOR = 1.05, P = 0.013, 95% CI (0.98–1.13), decreased level of serum albumin (AOR = 0.141, P = 0.03, 95% CI (0.034–0.58), increased number of total WBC count (AOR = 1.35, P = 0.0001, 95% CI (1.23–1.64)), and platlate count below 150,000/uL (OR = 0.67, P = 0.002, 95% CI (0.41–1.07)) were able to predict SBP (Table 6 ) .
Ascites is one of the most common complications in patients with cirrhosis [ 18 ]. One of the most common etiologies of cirrhosis has been related to hepatitis B infection, which is also common in our case [ 19 ]. Around 10–30% of patients with ascites develop spontaneous bacterial peritonitis (SBP), which is linked with high morbidity and mortality [ 20 , 21 , 22 ]. In our case, the prevalence of SBP was 23.34%, which is comparable to previous reports [ 23 ] and lower compared to a study conducted in Germany, which was 33.9%, and in Vietnam, which was 29.3% [ 24 , 25 ]. Timely antimicrobial therapy includes a third-generation cephalosporin for community-acquired infection; nosocomial infections should be treated empirically with a carbapenem or with piperacillin-tazobactam, or based on local susceptibility testing. Patients with gastrointestinal (GI) hemorrhage should receive ceftriaxone prophylactically for GI hemorrhage [ 26 ]. Other studies have shown that follow-up of infected patients shows that 30% of patients die within 1 month after infection and another 30% die within 1 year [ 27 ]. Similarly, the odds (OR 2.522, 95% CI 1.044–6.091, p = 0.040) of mortality rate among cirrhosis patients acquiring infection during hospitalization are much higher compared to non-infected individuals [ 24 ]. This indicates that preventing infection in cirrhosis patients is crucial, as it decreases the likelihood of poor clinical outcomes in these patients. Early predictive parameters indicating infection among patients with cirrhosis could be important in preventing mortality in these patients.
On the other hand, the rate of culture-negative neutrocytic ascites was 78.43%, which is higher compared to previous reports, which reported around 27–60% [ 20 , 28 , 29 , 30 ], and compared to other reports, which identified 64% of SBP as culture-negative [ 9 ]. Geographic factors and laboratory methods could contribute to this differences, but in our case and other reports, culture-negative neutrocytic ascites is the most common form of ascitic fluid infection. The most frequent bacterial isolate turned out to be gram-negative enteric bacteria, which is similar with other studies [ 31 ]. The common bacterial isolate was E. coli (36.3%, n = 4), which agrees with other reports [ 7 , 32 , 33 , 34 ]. Of the gram-positive bacterial isolates, coagulase-negative staphylococcus species were common, accounting for 18.18% and aligning with other studies [ 9 ]. Although the frequency of the bacterial isolates from our study was small, we observed a pattern of resistance. The isolated gram-negative bacteria showed a full resistance to amoxicillin/clavulanic [ 35 ].
However, isolated gram-positive bacteria showed resistance to penicillin. The outcome of patients with SBP is poor since chronic liver failure and death occur in 60% and 40% of the patients, respectively, and early antibiotic treatment for these patients is crucial. However, the increase in microbiological resistance makes current management more challenging [ 36 ]. We found that antibiotics like Meropenem and levofloxacin were effective against gram-negative bacteria, and vancomycin was effective against gram-positive bacteria and can be used in treating SBP patients. As the standard treatment for SBP mainly depends on prompt broad-spectrum antibiotic administration, isolating the causative agents and profiling the resistance pattern in different areas is important [ 37 ].
One of the most important findings of this study was the role of clinical and laboratory parameters as predictors of SBP. We found that clinical data like maximum liver span, spleen size, fever, and jaundice were important predictors of SBP. For instance, other studies showed that fever was one of the predictors of SBP in children with cirrhosis [ 38 ]. Likewise, laboratory parameters including platelet count, WBC count, and ascitic fluid levels of albumin, were important predictors of SBP in cirrhosis patients. Studies showed that platelet count predict SBP [ 39 , 40 ], which is complimentary with our finding, whereas others found age, sex, diabetes [ 41 ], the value of INR [ 7 ] and neutrophil-to-lymphocyte ratio (NLR) [ 42 ] as positive predictors of SBP. In other studies, the presence of inflammatory markers like IL-6 in the blood was correlated with disease severity in patients with ascites [ 43 ]. Likewise, simple immunological measurements like lymphocyte-to-monocyte ratio were found to be the best predictors of bacterial infection in patients with liver cirrhosis [ 44 ]. Overall, the measured parameters in our study and others can be easily performed, used as a simple indicator of SBP, and help to initiate early medical intervention.
To conclude, as the mortality rate after SBP is high [ 22 ], prompt diagnosis and treatment, understanding the microbial agent, resistant profile, and predictive parameters in different settings are crucial. Here, we conducted the first study in Ethiopia on ascites infections among cirrhosis patients that have developed ascites and found that ascites fluid infection is common in these patients. The combination of clinical data and laboratory parameters can be used for rapid diagnosis or exclusion of SBP and to initiate evidence-based treatment for these patients. Furthermore, the most common form of SBP was culture-negative neutrocytic ascites, and even if the amount of bacterial isolate was low, a pattern of drug resistance was still evident. One limitation of this study was that the bacterial isolates were too small to fully elucidate the pattern of drug resistance among the isolated pathogens; thus, we suggest further study in other hospitals focusing on culture-positive SBP to further strengthen our finding.
Data availability
All data supporting the findings of this study are available within the paper, incorporated in table form and the attached its Supplementary Information.
Abbreviations
Alkaline phosphatase
Alanine aminotransferine
American Type Culture Collection
Chronic Liver Disease
Clinical Laboratory Standardization Institute
Culture negative neutrocytic ascites
Coagulase negative Staphylococus
Hepatitis B Virus
Hepatitis C Virus
International Normalization ratio
Neutrophil to lymphocyte ratio
Non neutrocytic bacteriascites
Spontaneous ascites fluid infection
Spontaneous bacterial infection
Statistical package for social science
Zhang G, Jazwinski Faust A. Spontaneous Bacterial Peritonitis JAMA. 2021;325:1118–1118.
PubMed Google Scholar
Díaz-Hernández HA, Vázquez-Anaya G, Miranda-Zazueta G, Castro-Narro GE. The impact of different infectious complications on mortality of hospitalized patients with liver cirrhosis. Ann Hepatol. 2020;19:427–36.
Article PubMed Google Scholar
Tranah TH, Edwards LA, Schnabl B, Shawcross DL. Targeting the gut-liver-immune axis to treat cirrhosis. Gut. 2021;70:982–94.
Article CAS PubMed Google Scholar
Alam Sarker J, Shahinul Alam M, Khan M, Mahtab M-A, Shahed Ashraf M, Ahmad Khondaker F. Variant of Ascitic Fluid bacterial infections in patients of liver cirrhosis. Euroasian J Hepatogastroenterol. 2015;5:131–3.
EASL clinical practice guidelines on the management of. Ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417.
Article Google Scholar
Tay PWL, Xiao J, Tan DJH, Ng C, Lye YN, Lim WH et al. An epidemiological Meta-analysis on the Worldwide Prevalence, Resistance, and outcomes of spontaneous bacterial peritonitis in cirrhosis. Front Med. 2021;8.
Duah A, Nkrumah KN. Prevalence and predictors for spontaneous bacterial peritonitis in cirrhotic patients with ascites admitted at medical block in Korle-Bu Teaching Hospital, Ghana. Pan Afr Med J. 2019;33:35.
Article PubMed PubMed Central Google Scholar
Garcia–Tsao G. Current management of the complications of cirrhosis and portal hypertension: Variceal hemorrhage, ascites, and spontaneous bacterial peritonitis. Gastroenterology. 2001;120:726–48.
Ratnasekera IU, Johnson A, Powell EE, Henderson A, Irvine KM, Valery PC. Epidemiology of ascites fluid infections in patients with cirrhosis in Queensland, Australia from 2008 to 2017: a population-based study. Medicine. 2022;101:e29217.
Article CAS PubMed PubMed Central Google Scholar
Alaniz C, Regal RE. Spontaneous bacterial peritonitis. P T. 2009;34:204–10.
PubMed PubMed Central Google Scholar
Chon YE, Kim SU, Lee CK, Park JY, Kim DY, Han K-H, et al. Community-acquired vs. nosocomial spontaneous bacterial peritonitis in patients with liver cirrhosis. Hepatogastroenterology. 2014;61:2283–90.
CAS PubMed Google Scholar
Hou W, Sanyal AJ. Ascites: diagnosis and management. Med Clin North Am. 2009;93:801–17. vii.
Huang L-L, Xia HH-X, Zhu S-L. Ascitic Fluid Analysis in the Differential diagnosis of ascites: Focus on Cirrhotic Ascites. J Clin Transl Hepatol. 2014;2:58–64.
Tsochatzis EA, Gerbes AL. Diagnosis and treatment of ascites. J Hepatol. 2017;67:184–5.
Tandon P, Garcia–Tsao G. Renal dysfunction is the most important independent predictor of mortality in cirrhotic patients with spontaneous bacterial peritonitis. Clin Gastroenterol Hepatol. 2011;9:260–5.
Ostrowska M, Ostrowski A, Łuczak M, Jaguszewski M, Adamski P, Bellwon J, et al. Basic laboratory parameters as predictors of in-hospital death in patients with acute decompensated heart failure: data from a large single-centre cohort. Kardiologia Polska 2017;75(2):157–163. https://doi.org/10.5603/KP.a2016.0147 .
Gize A, Belete Y, Kassa M, Tsegaye W, Hundie GB, Belete BM, et al. Baseline and early changes in laboratory parameters predict disease severity and fatal outcomes in COVID-19 patients. Front Public Health. 2023;11:1252358. https://doi.org/10.3389/fpubh.2023.1252358 . PMID: 38152668; PMCID: PMC10751315.
D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–31.
Wong YJ, Kalki RC, Lin KW, Kumar R, Tan J, Teo EK, et al. Short- and long-term predictors of spontaneous bacterial peritonitis in Singapore. Singap Med J. 2020;61:419–25.
Rimola A, García-Tsao G, Navasa M, Piddock LJ, Planas R, Bernard B, et al. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. Int Ascites Club J Hepatol. 2000;32:142–53.
CAS Google Scholar
Niu B, Kim B, Limketkai BN, Sun J, Li Z, Woreta T, et al. Mortality from spontaneous bacterial peritonitis among hospitalized patients in the USA. Dig Dis Sci. 2018;63:1327–33.
Lim KHJ, Potts JR, Chetwood J, Goubet S, Verma S. Long-term outcomes after hospitalization with spontaneous bacterial peritonitis. J Dig Dis. 2015;16:228–40.
Bibi S, Ahmed W, Arif A, Khan F, Alam SE. Clinical, laboratory and bacterial profile of spontaneous bacterial peritonitis in Chronic Liver Disease patients. J Coll Physicians Surg Pak. 2015;25:95–9.
Kremer WM, Gairing SJ, Kaps L, Ismail E, Kalampoka V, Hilscher M, et al. Characteristics of bacterial infections and prevalence of multidrug-resistant bacteria in hospitalized patients with liver cirrhosis in Germany. Ann Hepatol. 2022;27:100719.
Nguyen LC, Lo TT-B, La HD, Doan HT-N, Le NT. Clinical, Laboratory and Bacterial Profile of spontaneous bacterial peritonitis in Vietnamese patients with liver cirrhosis. Hepat Med. 2022;14:101–9.
MacIntosh T. Emergency Management of spontaneous bacterial peritonitis – a clinical review. Cureus J Med Sci. 2018;10.
Arvaniti V, D’Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–56. 1256.e1-5.
Borzio M, Salerno F, Piantoni L, Cazzaniga M, Angeli P, Bissoli F, et al. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Digestive and liver disease: official journal of the Italian Society of Gastroenterology. Volume 33. and the Italian Association for the Study of the Liver; 2001.
Bystrianska N, Skladany L, Adamcova Selcanova S, Vnencakova J, Janceková D, Koller T. Infection in patients hospitalised with advanced chronic liver disease (cirrhosis) – single-centre experience. Gastroenterologie Hepatologie. 2020;74:1–5.
Google Scholar
Al-Ghamdi H, Al-Harbi N, Mokhtar H, Daffallah M, Memon Y, Aljumah AA, et al. Changes in the patterns and microbiology of spontaneous bacterial peritonitis: analysis of 200 cirrhotic patients. Acta Gastroenterol Belg. 2019;82:261–6.
Filik L, Unal S. Clinical and laboratory features of spontaneous bacterial peritonitis. East Afr Med J. 2004;81:474–9.
Béjar-Serrano S, del Pozo P, Fernández-de la Varga M, Benlloch S. Multidrug-resistant bacterial infections in patients with liver cirrhosis in a tertiary referral hospital. Gastroenterología Y Hepatología (English Edition). 2019;42:228–38.
Tsung PC, Ryu SH, Cha IH, Cho HW, Kim JN, Kim YS, et al. Predictive factors that influence the survival rates in liver cirrhosis patients with spontaneous bacterial peritonitis. Clin Mol Hepatol. 2013;19:131–9.
Aziz A, Ashraf S, Talpur MTH, Aamer N, Solangi SA, Shabir KU, et al. Spontaneous bacterial peritonitis in asymptomatic cirrhotic patients with ascites in a tertiary care hospital: a cross-sectional study. Pakistan Armed Forces Med J. 2020;70:1408–12.
Fiore M, Maraolo AE, Gentile I, Borgia G, Leone S, Sansone P, et al. Current concepts and future strategies in the antimicrobial therapy of emerging Gram-positive spontaneous bacterial peritonitis. World J Hepatol. 2017;9:1166–75.
Marciano S, Díaz JM, Dirchwolf M, Gadano A. Spontaneous bacterial peritonitis in patients with cirrhosis: incidence, outcomes, and treatment strategies. Hepatic Medicine: Evid Res. 2019;11:13–22.
Huang C-H, Lee C-H, Chang C. Spontaneous bacterial peritonitis in decompensated liver Cirrhosis—A. Literature Rev Livers. 2022;2:214–32.
Ghobrial C, Mogahed EA, El-Karaksy H. Routine analysis of ascitic fluid for evidence of infection in children with chronic liver disease: is it mandatory? PLoS ONE. 2018;13:e0203808.
Metwally K, Fouad T, Assem M, Abdelsameea E, Yousery M. Predictors of spontaneous bacterial peritonitis in patients with cirrhotic ascites. J Clin Translational Hepatol. 2018;6:372–6.
Wehmeyer MH, Krohm S, Kastein F, Lohse AW, Lüth S. Prediction of spontaneous bacterial peritonitis in cirrhotic ascites by a simple scoring system. Scand J Gastroenterol. 2014;49:595–603.
Kraja B, Sina M, Mone I, Pupuleku F, Babameto A, Prifti S, et al. Predictive value of the Model of End-Stage Liver Disease in Cirrhotic patients with and without spontaneous bacterial peritonitis. Gastroenterol Res Pract. 2012;2012:e539059.
Seyedi SA, Nabipoorashrafi SA, Hernandez J, Nguyen A, Lucke-Wold B, Nourigheimasi S, et al. Neutrophil to lymphocyte ratio and spontaneous bacterial peritonitis among cirrhotic patients: a systematic review and Meta-analysis. Can J Gastroenterol Hepatol. 2022;2022:e8604060.
Alvarez-Silva C, Schierwagen R, Pohlmann A, Magdaleno F, Uschner FE, Ryan P et al. Compartmentalization of Immune Response and Microbial translocation in decompensated cirrhosis. Front Immunol. 2019;10.
Piotrowski D, Sączewska-Piotrowska A, Jaroszewicz J, Boroń-Kaczmarska A. Lymphocyte-To-Monocyte ratio as the best simple predictor of bacterial infection in patients with liver cirrhosis. Int J Environ Res Public Health. 2020;17:1727.
Download references
Acknowledgements
We express our gratitude to the study participants who took part in the study, contributing to the data collection process. Additionally, our sincere thanks extend to the medical staff of St. Paul’s Hospital Millennium Medical College and Yekatit 12 Hospital Medical College for their invaluable support and active involvement in the clinical investigations of the study participants. Special appreciation is also extended to the staff of the microbiology department at St. Paul’s Hospital Millennium Medical College for their collaborative efforts and cooperation in the analysis of the collected samples.
There is no direct funding for this research.
Author information
Authors and affiliations.
Department of Medical Laboratory Science, College of Health Science, Addis Ababa University, Addis Ababa, Ethiopia
Abubeker Shemsu Helil, Shambel Araya Haile, Daniel Melese Desalegn & Kassu Desta
Department of Gastroenterology and Hepatology, College of Health Science, Addis Ababa University, Addis Ababa, Ethiopia
Yohannis Birhanu
Department of Gastroenterology and Hepatology, School of Medicine, St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia
Hailemichael Desalegn
Regional laboratory capacity building, Ethiopian Public Health Institute, Addis Ababa, Ethiopia
Awad Mohammed, Daniel Dejene Wondimagegnehu & Gonfa Ayana
Department of Microbiology, Immunology and Parasitology, School of Medicine, St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia
Rozina Ambachew Geremew, Zenebe Gebreyohannes & Anteneh Mehari Tizazu
Department of Molecular and Translational Science, Monash University, Melbourne, Australia
Shambel Araya Haile
You can also search for this author in PubMed Google Scholar
Contributions
A.S.H, S.A.H, and K.D conceived the study. A.S.H, S.A.H, Y.B, A.M.T and K.D analyzed the data and drafted the manuscript.A.S.H, S.A.H, Y.B, H.D, D.M.D, R.A.G, Z.G, A.M, D.D.W, G.A, A.M.T, and K.D reviewed and edited the manuscript.All authors read the manuscript and approved the submission.
Corresponding author
Correspondence to Abubeker Shemsu Helil .
Ethics declarations
Consent for publication.
Not applicable.
Competing interests
The authors declare no competing interests.
Human ethics and consent to participate
Ethical clearances were granted by the Research review committee of the Department of Medical Laboratory Science, CHS, AAU, the Institutional review board of St. Paul Specialized Hospital Millennium Medical College, and the Research review committee of Yekatit 12 Hospital Medical College. Consent form was issued to each participant in the study, and every participant gave a written informed consent to participate in the study. Confidentiality was maintained during labeling, and names and other unique identifiers were avoided from the questionnaire and the sample. The participants were informed that he/she could withdraw from the study at any time during the study progress.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Helil, A.S., Haile, S.A., Birhanu, Y. et al. Bacterial profile, drug resistance pattern, clinical and laboratory predictors of ascites infection in cirrhosis patients. BMC Infect Dis 24 , 528 (2024). https://doi.org/10.1186/s12879-024-09418-6
Download citation
Received : 03 January 2024
Accepted : 20 May 2024
Published : 26 May 2024
DOI : https://doi.org/10.1186/s12879-024-09418-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Ascites infection
- Bacterila infection
- Liver disease
- Drug resistance
BMC Infectious Diseases
ISSN: 1471-2334
- General enquiries: [email protected]

Introduction
Research design and methods, conclusions, article information, metabolic syndrome traits increase the risk of major adverse liver outcomes in type 2 diabetes.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Ying Shang , Emilie Toresson Grip , Angelo Modica , Helena Skröder , Oskar Ström , Fady Ntanios , Soffia Gudbjörnsdottir , Hannes Hagström; Metabolic Syndrome Traits Increase the Risk of Major Adverse Liver Outcomes in Type 2 Diabetes. Diabetes Care 20 May 2024; 47 (6): 978–985. https://doi.org/10.2337/dc23-1937
Download citation file:
- Ris (Zotero)
- Reference Manager
Type 2 diabetes (T2D) increases the risk for major adverse liver outcomes (MALOs), including cirrhosis and its complications. Patients with T2D frequently have other traits of the metabolic syndrome (MetS). It remains uncertain whether there is a synergistic effect of accumulating MetS traits on future MALO risk.
Patients with T2D without a history of liver disease were identified from national registers in Sweden from 1998 to 2021. MetS traits included hypertension, low HDL level, hypertriglyceridemia, obesity, and albuminuria, in addition to T2D. MALO events were identified based on administrative coding from national registers until 31 October 2022. Data were analyzed using Cox regression models.
In total, 230,992 patients were identified (median age 64 years; 58% male), of whom 3,215 (1.39%) developed MALOs over a median follow-up of 9.9 years. Compared with patients with one MetS trait (only T2D) at baseline, those with more than one MetS trait had a higher rate of MALOs (adjusted hazard ratio [aHR] 2.33, 95% CI 1.53–3.54). The rate of MALOs increased progressively with increasing numbers of MetS traits at baseline (aHR 1.28 per added trait, 95% CI 1.23–1.33). During follow-up, patients who acquired additional MetS traits had a progressively higher rate of MALOs. The MetS trait with the largest association with incident MALOs was hypertension (aHR 2.06, 95% CI 1.57–2.71).
Having or acquiring additional traits of MetS increase the rate of progression to MALOs in patients with T2D. These results could be used to inform screening initiatives for liver disease.
Graphical Abstract

The metabolic syndrome (MetS) is a cluster of metabolic abnormalities that may affect the liver. Metabolic dysfunction–associated steatotic liver disease (MASLD) (recently renamed from nonalcoholic fatty liver disease) is considered the hepatic phenotype of the MetS, affecting 38% of the global adult population ( 1 , 2 ) and >55% of patients with type 2 diabetes (T2D) ( 3 ). Patients with T2D have more than twice as high a risk of developing cirrhosis or liver cancer compared with the general population ( 4 ). Recognizing this high risk for cirrhosis among patients with T2D, several international guidelines now actively recommend screening for MASLD-associated liver fibrosis in patients with T2D. Nevertheless, the implementation of such guidelines in clinical practice is modest at best ( 5 ). Identifying subgroups of patients with T2D who have a particularly high risk might help narrow down the target group intended for directed casefinding initiatives and improve the effectiveness of screening efforts.
A more severe metabolic state is associated with a higher rate of progression to cirrhosis, as evidenced by a study involving patients with a diagnosis of MASLD within a U.S. Veterans Affairs cohort ( 6 ). This study revealed a gradual increase in cirrhosis and liver cancer risk with the presence of additional metabolic traits, with T2D exhibiting the strongest association. However, for patients with T2D who already are at a high risk, it remains unclear whether a similar pattern is present. Additionally, it is unclear if acquiring additional metabolic traits beyond a T2D diagnosis further amplifies this risk and which specific traits hold the strongest association with liver disease. Such information is important as most patients with MASLD will never develop liver-related outcomes ( 7 ). Hence, a risk-based approach to screening or treatment has been advocated for ( 8 , 9 ). That is, instead of solely aiming to diagnose MASLD or fibrosis, one considers the risk a patient has for future liver-related outcomes and tailors appropriate actions accordingly.
Here, we used high-quality Swedish national registers to investigate the association between the number and subtypes of MetS traits and the risk of developing MALOs in Swedish patients with T2D. Our hypothesis was that increasing numbers of MetS traits at or after a diagnosis of T2D would be associated with an increase in the risk of incident liver cirrhosis or complications thereof.
Study Population
We used the Swedish National Diabetes Register (NDR) to identify patients with T2D. In NDR, T2D is defined using the following epidemiological definition: recorded T2D in clinical records, treatment with diet with or without oral antihyperglycemic agents, and treatment with oral antihyperglycemic agents with or without insulin ( 10 ). The NDR was initiated in 1996 and contains data for >90% of all patients aged >18 years with T2D in Sweden. This means that for patients diagnosed with T2D after 1996, their first registration in NDR is typically the time of their first diagnosis. The data are collected by trained nurses and physicians and include information on laboratory tests and medication use obtained from primary care and hospital outpatient clinics.
We included all patients recorded in the NDR between 1 January 1998 and 31 October 2021. The first entry date was defined as baseline. Patients were linked to several national registers using their personal identity number, which is assigned to all Swedish citizens ( 11 ). The National Patient Register (NPR) contains national coverage on hospital discharges since 1987 and contains data on outpatient visits in specialized care since 2001. It has a validity between 85 and 95%, depending on the diagnosis ( 12 ). Specifically, for hepatocellular carcinoma (HCC) and diagnoses related to cirrhosis, the validity rate ranges from 84 to 96% ( 13 ).
A total of 764,944 patients aged >18 years at the time of registration in NDR were eligible for inclusion (see flowchart in Supplementary Fig. 1 ). For the analysis on MetS traits to be comparable to each other, patients in the study sample needed to have information on the values for all MetS traits in this study. Therefore, 520,605 patients who had missing information on one or more of the MetS traits were excluded. We further excluded patients with preexisting MALOs and liver diseases other than MASLD based on ICD-10 codes and surgical procedure data from NPR. We also excluded patients with preexisting alcohol-related disease to minimize the impact of alcohol misuse. Since the use of ICD-10 codes began in 1997 in Sweden, we applied a look-back period of at least 1 year to identify preexisting diseases, with start of identification and follow-up from 1998. To reduce the potential impact of medications that may induce liver steatosis or fibrosis, we excluded patients who had ever used methotrexate or amiodarone prior to the index date from the Prescribed Drug Register. The Prescribed Drug Register was initiated in July 2005 and includes all drug dispensations at any Swedish pharmacy. Patients who emigrated before baseline were also excluded. Diagnostic, surgical, and Anatomical Therapeutic Chemical codes for all diagnoses and medications used in the study are listed in Supplementary Tables 1 – 3 .
MetS and Other Variables
We used World Health Organization (WHO) 1998 criteria to define the traits of the MetS ( 14 ), a definition that has been endorsed by the NDR for identifying the MetS among patients with T2D ( 15 ). The WHO definition may be better suited for populations with T2D because unlike other available definitions of the MetS, the WHO definition specifically mandates the presence of T2D as a diagnostic requirement ( 14 ), which is central to the pathophysiology of the MetS. In addition to T2D, the traits of MetS in this definition include hypertension, obesity, hypertriglyceridemia, a low level of HDL, and albuminuria. These were defined based on laboratory test data from the NDR, diagnoses from the NPR, or dispensed medications from the Prescribed Drug Register. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg, a recorded diagnosis of hypertension, or any dispensed antihypertensive medication. Obesity was determined as BMI ≥30 kg/m 2 . A high level of triglycerides was identified as plasma triglyceride levels ≥1.7 mmol/L. A low level of HDL was defined as <0.9 mmol/L for men and <1.0 mmol/L for women. Finally, albuminuria was determined as a urinary albumin excretion rate ≥20 μg/min or an albumin-to-creatinine ratio ≥30 mg/mmol. We also calculated the number of MetS traits for each patient, which ranged from one to six.
The following information from the NDR recorded at each clinical visit was also included: time since diabetes diagnosis (years), glycated hemoglobin A 1c (HbA 1c ) (mmol/mol), weight (kg), height (m), total cholesterol (mmol/L), LDL (mmol/L), estimated glomerular filtration rate (eGFR) (mL/min/1.73 m 2 ), active smoking, and physical activity (walking or equivalent at least 30 min/day). Furthermore, the NDR contains information on glucose-lowering medications (defined as any oral medication or insulin), use of glucagon-like peptide 1 receptor agonists (GLP-1RAs), and lipid-lowering drugs but not on specific agents or doses. Besides this, clinical history of cardiovascular disease (CVD) (including myocardial infarct, hemorrhagic or ischemic stroke, and heart failure), chronic kidney disease (CKD), and other diseases included in the Charlson comorbidity index were obtained through linkage to the NPR ( 16 ). Hyperlipidemia was determined by recorded diagnosis in the NPR or any lipid-lowering drugs in the NDR or Prescribed Drug Register. Data on other medications, such as sodium–glucose cotransporter 2 (SGLT-2) inhibitors, were obtained from the Prescribed Drug Register.
We linked the NDR data set to the NPR, the Swedish Cancer Register (SCR), and the Causes of Death Register (CDR) to identify liver disease outcomes between 1 January 1998 and 31 October 2022. Malignancies are documented in the SCR, which contains ∼96% of all diagnosed cancers in Sweden ( 17 ). The CDR contains data on causes of death for all Swedish citizens. It is mandatory for Swedish physicians to report the cause of death and any diagnosis that might have contributed to death as recorded the CDR ( 18 ). We used both primary and contributing diagnoses to identify outcomes in the NPR and CDR.
MALOs, as a composite outcome, were defined as having any of the diagnoses defined by ICD-10 codes or surgical procedures in registers. These diagnoses include liver cirrhosis, decompensated cirrhosis, and associated complications (defined as coding for chronic or unspecified hepatic failure, esophageal varices with or without bleeding, portal hypertension, hepatorenal syndromes, ascites, or liver transplant), HCC, or death from any of these (definitions listed in Supplementary Table 1 ). In general, these diagnoses result in contact with health care or in death, leading to a high capture rate of the outcome in the relevant registries. Since the positive predictive value for hepatic ascites is generally low, we excluded outcomes with ascites because of other diseases, such as heart failure or extrahepatic cancer, defined as present at or before the ascites diagnosis.
Statistical Analyses
Baseline characteristics of the study population are expressed as median and interquartile range (IQR) or frequency and percentage, as appropriate. The follow-up time started at the baseline date, and patients were followed until the first event of MALOs or were censored at development of any other liver disease than MASLD, emigration, death, or the end of the study period (31 October 2022), whichever occurred first. Incidence rates (per 1,000 person-years) were calculated as the number of events divided by total person-time at risk, displayed overall by the number of metabolic traits and by each individual trait in the MetS as measured at baseline. A Cox proportional hazards model was used to estimate hazard ratios (HRs) and 95% CIs of the association between the risk of MALOs and prevalent MetS by numbers and by individual traits, where follow-up time was used as the timescale. We reported results from two models: model 1 was a univariate model with only each respective trait in the MetS as the dependent variable, and model 2 was adjusted for all MetS traits, age at baseline, sex, duration of T2D, HbA 1c , CKD, CVD, eGFR, LDL, smoking, hyperlipidemia, and glucose-lowering treatments, all recorded at baseline.
As the competing risk of non–liver-related mortality, e.g., cardiovascular death, is high in patients with T2D, we calculated cause-specific hazard rates of MALOs from a Cox regression model, considering non–liver mortality as a competing event. We did not use the Fine and Gray model since the subdistributional hazard derived from this does not have a straightforward interpretation and only gives the direction and not the strength of the association, which was our primary research question ( 19 , 20 ).
Statistical interaction between the main exposures (number and individual metabolic traits) and median age at baseline (age ≥65 or <65 years) and sex was tested separately. The associations between different combinations of baseline MetS traits and the risk of MALOs were estimated using HRs while adjusting for the same set of covariates at baseline. As patients with T2D may develop additional traits of the MetS during follow-up, we further examined the association between MetS and MALOs using time-varying Cox regression, considering both prevalent and incident metabolic traits. In brief, the time-varying Cox regression analyzed time to event with consideration of time-varying exposures by using all available data on MetS traits measured at multiple visits. We fitted two separate models, considering either the number or the different combinations of metabolic traits developed during follow-up as exposures, and reported corresponding HRs after adjusting for covariates at baseline. The cumulative incidence of MALOs by numbers of metabolic traits at baseline were calculated based on the Aalen-Johansen estimator, accounting for the competing risk of non–liver-related death ( 20 ).
A two-sided P < 0.05 was considered statistically significant, except in the case of interaction analysis, where P < 0.10 indicated the presence of a significant multiplicative interaction. All statistical analyses were performed using Stata MP 17.0 software (StataCorp).
Ethical Considerations
Ethics approval was granted from the ethical review board in Sweden (registration no. 2021-04422).
Data and Resource Availability
Requests of sharing deidentified data from this article will be considered on a case-by-case basis. A detailed proposal for how the data will be used is required, and a data access agreement must be signed upon request.
We identified 230,996 patents with T2D between 1998 and 2021. The median age of patients was 63.6 (IQR 56.0–72.0) years, and 58.2% were men. Among all patients, 19.6% had albuminuria, 92.4% had hypertension, 49.4% had hypertriglyceridemia, 16.2% had low HDL levels, and 46.5% had obesity. The median BMI was 29.6 (IQR 26.5–33.4) kg/m 2 , and the median Charlson comorbidity index was 1 (IQR 1–1). There were 22.2% patients with preexisting CVD and 1.2% with CKD. Baseline characteristics are presented in Table 1 .
Baseline characteristics of study population ( N = 230,996)
Number of MetS Traits and Rate of MALOs
During a median follow-up of 9.9 years (corresponding to 2,235,356 person-years), 3,215 (1.39%; incidence rate 1.44/1,000 person-years) patients developed MALOs ( Table 2 ). Within these outcomes, 1,344 cases of cirrhosis, 2,000 cases of decompensated cirrhosis and its complications, and 839 cases of HCC were identified. Patients with additional metabolic traits had a higher incidence of MALOs (1.46/1,000 person-years for two or more MetS traits vs. 0.56/1,000 person-years for those with only T2D). Furthermore, patients with a higher total number of metabolic traits at baseline had a higher incidence rate of MALOs, and the rate progressively increased as the number of metabolic traits increased, also after adjusting for other covariates (adjusted HR [aHR] 1.28, 95% CI 1.23–1.33, P trend < 0.001) (Table 2A ). Compared with patients with only T2D at baseline, having more than one MetS trait was associated with a 2.3-fold increased rate of MALOs (95% CI 1.53–3.54); for those with all MetS traits at baseline, the aHR was 4.09 (95% CI 2.50–6.68).
Incidence rate and HRs of MALOs associated with numbers and individual traits of the MetS present at baseline in patients with T2D
A : The model was adjusted for numbers of MetS traits (continuous), age, sex, smoking status, diabetes duration, HbA 1c level, CKD, CVD, eGFR, LDL level, hyperlipidemia, any diabetes treatment use, insulin, GLP-1RAs, and SGLT-2 inhibitors. B : The model was adjusted for albuminuria, hypertension, hypertriglyceridemia, low HDL levels, obesity, age, sex, smoking status, diabetes duration, HbA 1c level, CKD, CVD, eGFR, LDL level, hyperlipidemia, any diabetes treatment use, insulin, GLP-1RAs, and SGLT-2 inhibitors.
Individual MetS Traits and Rates of MALOs
Patients with individual MetS traits had a higher incidence rate of MALOs compared with those who did not display such traits (e.g., 1.50/1,000 person-years for patients with hypertension vs. 0.48/1,000 person-years for those without hypertension) (Table 2B ). The strongest association with incident MALOs was found for patients who had hypertension (aHR 2.06, 95% CI 1.57–2.71), followed by obesity (aHR 1.38, 95% CI 1.28–1.50), low HDL levels (aHR 1.37, 95% CI 1.23–1.51), albuminuria (aHR 1.23, 95% CI 1.13–1.35), and hypertriglyceridemia (aHR 1.11, 95% CI 1.02–1.20) (Table 2B ). We did not detect any statistical interaction between the number or individual traits of MetS and age or sex on the rate of MALOs ( P interaction > 0.1 for all). Among the other covariates, CKD (aHR 2.29, 95% CI 1.61–3.25), smoking (aHR 1.36, 95% CI 1.23–1.52), CVD (aHR 1.17, 95% CI 1.06–1.29), and eGFR (aHR 1.01, 95% CI 1.00–1.01) were associated with a higher rate of MALOs, while LDL (aHR 0.81, 95% CI 0.78–0.85) and hyperlipidemia (aHR 0.69, 95% CI 0.64–0.75) were related to a lower rate of MALOs in the fully adjusted models ( Supplementary Table 4 ).
Combinations of MetS Traits and Rates of MALOs
The associations of combinations of baseline MetS traits with MALOs are shown in Fig. 1 . For the different combinations, hypertension consistently had the strongest association with incident MALOs. For instance, patients with hypertension had a higher rate of MALOs (aHR 1.58, 95% CI 1.03–2.42) compared with those with only T2D. For patients with two additional MetS traits, those with comorbid hypertension and obesity had the highest rate of MALOs (aHR 2.77, 95% CI 1.80–4.26), followed by those with hypertension and low HDL (aHR 2.44, 95% CI 1.45–4.09). We consistently observed higher event rates in any combination of hypertension and other MetS traits with regard to all three and four combinations.

HRs and 95% CIs of baseline combinations of metabolic traits with MALOs in patients with T2D. The reference group comprises patients with T2D without any metabolic traits. The numbers of baseline metabolic traits are displayed as a shade of colored squares. NA, not applicable; TGR, triglycerides.
Cumulative incidences of MALOs by the total number of MetS traits over time are shown in Fig. 2 . Overall, patients with additional MetS traits had a higher probability of MALOs than those with only T2D. The highest probability was observed in patients with all traits of the MetS. For instance, the 10-year cumulative incidence of MALOs was 0.49, 0.87, 1.24, 1.23, 1.57, and 1.64% for patients with only T2D, two, three, four, five, and six MetS traits, respectively ( Supplementary Table 5 ). Regarding individual metabolic traits, there was a clear distinction in the probability of MALOs between patients with hypertension and those without. Similarly, there was a difference in the likelihood of outcome development among patients with obesity and without, and among patients with a low HDL level and without ( Supplementary Fig. 2 ). When considering incident traits of the MetS occurring after baseline using time-varying models, we observed slightly higher HRs with regard to both number and different combinations of MetS traits, providing additional validity to our findings ( Supplementary Fig. 3 ).

Cumulative incidence of MALOs by number of MetS traits at baseline.
Sensitivity Analysis
As the association might be driven by the development of HCC, we conducted a sensitivity analysis distinguishing between patients who developed HCC and those who did not. The results indicated that both the individual and the total number of metabolic traits are associated with HCC and non-HCC events; therefore, our estimates are robust ( Supplementary Table 6 ). We also found that each metabolic trait is associated with a higher risk of decompensated cirrhosis and complications and that hypertension, obesity, and low HDL level consistently demonstrate a higher risk across the spectrum of cirrhosis ( Supplementary Table 7 ).
In this population-based cohort study of >230,000 patients with T2D, we found that an increasing number of metabolic traits is associated with an increased risk of MALOs in patients with T2D. Each additional metabolic trait increased the risk for liver-related outcomes in a stepwise manner. We expand on previous knowledge by also showing that acquiring additional traits of the MetS after T2D diagnosis further increases the risk of liver-related outcomes, suggesting a strong association between poor metabolic health and liver disease risk.
Additionally, we investigated which individual parameters included in the MetS, as well as other traits of poor metabolic health that had differing associations with incident liver disease. The strongest association was seen for hypertension and CKD, whereas hyperlipidemia was negatively associated with incident liver disease. This may possibly be explained by a protective effect of statin treatment (which was included to define presence of hyperlipidemia), which has been suggested to have hepatoprotective effects and is associated with a reduced incidence of primarily HCC in patients with T2D ( 21 ). The other plausible explanation could be that patients with mutations in lipid-modifying genes, such as PNPLA3 , may have lower blood lipid levels but a higher risk for development of cirrhosis and HCC ( 22 , 23 ). An alternate explanation might involve hepatic synthetic failure during the early stages of cirrhosis, leading to a decrease in serum lipoprotein levels.
In the absence of primary care diagnoses, similarly to hyperlipidemia, hypertension was also defined by use of antihypertensive treatments in addition to having a recorded diagnosis in specialist care or an elevated blood pressure. As such, the risk increase associated with hypertension in this study may also warrant additional studies that can disentangle the effects associated with hypertension and the associated medical treatment.
In summary, our findings suggest that individualized risk prediction for incident MALOs is possible in patients with T2D. Future studies are needed to examine whether established noninvasive tests, such as the fibrosis 4 score or vibration-controlled transient elastography, can further increase prediction specifically in patients with T2D.
Comparison With Previous Studies
Our findings are in accordance with those of previous studies in this field in that each additional metabolic trait increased the risk of MALOs, regardless of alcohol intake ( 24–26 ). Indeed, the complex relationship between alcohol consumption and MetS complicates its individual impact of the MetS on liver outcomes. We investigated this by excluding all patients who had alcohol-related disease and found that even in the absence of known alcohol overconsumption with health care contacts, metabolic factors are independently related to liver-related outcomes.
The traits of the MetS seemingly have additive effects on the progression to cirrhosis, with hypertension demonstrating the strongest association among the individual traits. Indeed, any combination of hypertension with other MetS traits was associated with a higher risk of MALOs compared with only T2D, while such risk was not observed in the absence of hypertension. The presence of hypertension may indicate the occurrence of MASLD, as suggested by a meta-analysis that showed a bidirectional association between hypertension and MASLD independent of other cardiometabolic risk factors ( 27 ). As >90% of patients in this cohort had hypertension, we examined which combination of MetS traits, in addition to hypertension, conveyed the highest risk of MALOs in our cohort. We found that, in addition to hypertension, patients with obesity had the highest risk of MALOs, followed by those with low HDL, among all the two possible combinations of MetS traits. The risk increased substantially when patients had hypertension, low HDL, and comorbid albuminuria or obesity. This supports previous data from a large U.S. Veterans Affairs cohort of patients with a diagnosis of MASLD, where those with diabetes and coexisting hypertension and obesity or dyslipidemia had the highest risk for cirrhosis or HCC ( 6 ). That study also found a stepwise increase in risk for cirrhosis and HCC with each additional metabolic trait. Our findings extend this by including a less selected population of patients, all of whom have T2D. This is important since current guidelines promote screening for MASLD in all patients with T2D ( 28 , 29 ), aiming to identify patients on the path to cirrhosis. Our findings are important since they are more generalizable to the larger T2D population compared with previous data. A systematic review and meta-analysis found that in >22.8 million individuals with T2D, the overall risk of cirrhosis was 2.25-fold higher compared with people without diabetes ( 26 ). We extend this finding by showing that in patients with T2D, there are distinct risk profiles, possibly allowing for personalization of clinical management and follow-up.
Previous studies of the association between T2D and cirrhosis ( 30–33 ) often used mortality from cirrhosis as the outcome rather than incidence of nonfatal cirrhosis ( 31 , 33 ). Other studies reported an association between diabetes and more severe forms of liver disease, such as occurrence of hepatic failure ( 28 , 32 ). However, these studies did not differentiate between type 1 diabetes and T2D and were unable to study risk factors for development of liver-related outcomes.
Implications
Several important implications can be made based on these data. Although this study cannot show causality, our findings suggest the importance of adequately treating not only hyperglycemia in patients with T2D to reduce diabetes-related complications but also other comorbidities to reduce the risk for liver-related outcomes. Treating and preventing hypertension and obesity are particularly crucial to prevent the development of cirrhosis in this population. In addition to its impact on liver-related outcomes, a more severe metabolic profile in patients with T2D also increases the risk for cardiovascular events and overall mortality ( 34 ). By extension, this suggests that upcoming treatments for MASLD might be holistically more effective if they also improve the full metabolic profile of the patient. Data from randomized controlled trials are needed to corroborate this. In the absence of approved therapies to treat hepatic fibrosis due to MASLD, screening for liver fibrosis in all patients with T2D is currently controversial. The findings from this study may aid in identifying subgroups at high risk of developing future liver disease, which would be particularly efficient for targeted screening initiatives. To determine the cost-effectiveness of different interventions, such as screening for MASLD or advanced fibrosis, health economic evaluation is necessary. Our results suggest that patients with T2D have different metabolic risk factors, with a differing risk of progression to MALOs. Recognizing such distinct risk groups may be important in future clinical guidelines to tailor appropriate interventions and enhance patient outcomes.
Strengths and Limitations
The major strengths of this study are the use of high-quality registers with high coverage, minimal loss to follow-up, and an unprecedented sample size. Hence, estimates are more precise, accurate, and generalizable to the target population than that of previous studies. While large studies are often limited to register-based coding of metabolic traits, we used direct laboratory tests, in combination with medication use and register coding, to define metabolic traits. We further assessed the associations with repeated exposures to investigate whether newly developed metabolic traits also confer a higher risk of outcomes. We used hard outcomes that are validated and unlikely to be misclassified and that are important to patients ( 13 ). Several studies have shown that waist circumference may be a better predictor of central obesity than BMI and may also predict progression to MALO better than BMI; unfortunately, such data are not available in the used data sources ( 35 , 36 ). Additionally, to combine different MetS traits, we used categorized predictors. However, continuous versions of each MetS trait may provide an even more granular understanding. For instance, glycemic control is a better predictor for this purpose than diabetes status as a binary parameter ( 37 ). Combining continuous versions of MetS traits should be a focus for future studies. Another limitation is the lack of direct data on the presence and severity of MASLD, such as radiology or liver-related blood biochemistry, which are not available in the NDR. To address this limitation and further explore the severity of each MetS component, we plan to conduct future linkages to data sources that provide such detailed information. Furthermore, we excluded 486,377 patients because of missing data on at least one MetS trait. These patients tended to be older and more likely to have MetS, CVD, CKD, and severe diabetes as indicated by insulin use compared with those included in the study. Therefore, the estimated associations would have been stronger if we had included all eligible patients in the analysis.
In conclusion, in this large study of patients with T2D in Sweden, we found that an increasing number of metabolic comorbidities is associated with a higher rate of progression to MALOs, most likely attributable to MASLD. Hypertension and CKD were the parameters with the strongest association, whereas no positive association was seen for hyperlipidemia. This finding helps in narrowing down the specific group intended for directed casefinding among patients with T2D, potentially enhancing screening efficiency.
Acknowledgments. The authors thank the staff of the National Diabetes Register and all the study participants.
Funding. Y.S. was supported by Mag-Tarm Fonden, Swedish Gastroenterology Society, Karolinska Institutet research funding, and Region Stockholm. H.H. was supported by grants from Stockholm City County, The Swedish Cancer Society, and The Swedish Research Council. This study received financial support from Pfizer.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. Y.S., E.T.G., A.M., H.S., O.S., F.N., S.G., and H.H. contributed to the data interpretation. Y.S., E.T.G., A.M., and H.H. contributed to the study concept and design. Y.S., E.T.G., H.S., and H.H. contributed to the statistical analysis. Y.S. and H.H. drafted the manuscript. E.T.G., O.S., and H.H. contributed to the data acquisition. All authors contributed to the critical revision of the manuscript. Y.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Handling Editors. The journal editors responsible for overseeing the review of the manuscript were Elizabeth Selvin and Amalia Gastaldelli.
This article contains supplementary material online at https://doi.org/10.2337/figshare.25280482 .
Email alerts
- Online ISSN 1935-5548
- Print ISSN 0149-5992
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account

IMAGES
VIDEO
COMMENTS
Diffuse liver disease includes etiologies such as the various viral and immune hepatitides, storage diseases, hepatic steatosis, fibrosis, and cirrhosis. 38 Ultrasonography findings are often non-specific in the case of diffuse liver disease, and biopsy is usually required to differentiate between the various etiologies. 38 In cases of acute ...
Case Report • 2014 • vol.3 •13-16 CASE STUDY OF PATIENT WITH LIVER CIRRHOSIS Muhammad Rashad, ZubairAhmad, Hassan Ali , shanulqadir , Muhammad umair Faculty of Pharmacy, University of Sargodha. [email protected] Abstract Liver cirrhosis is a disease in which normal tissue of liver replaced with scar tissue, liver cirrhosis is ...
Accepted 16 September 2021. Published 29 November 2021. ABSTRACT. Liver is the second largest organ in human body, more than 5,000 separate b odily functions. .including helping b lood to c lot ...
Recently published guidelines by European Association for Studies of Liver (EASL) 3 and European Society for Clinical Nutrition and Metabolism (ESPEN) 13 provide comprehensive overviews of nutrition in cirrhosis. Herein, we provide a case-based practical guide of the causes of malnutrition, assessments tools, daily requirements and dietary ...
Educational Objective. •Discuss case review of patient with complications of cirrhosis. •Profile. -55 yr old female being evaluated in clinic for new onset ascites and lower extremity edema -Hospitalized 2 weeks ago for UGI bleed: EGD with grade 1 esophageal varices and a 1cm clean-based antral ulcer -Pt was on naproxen 220 mg TID for ...
An 18-year-old woman presented with acute liver failure. The total bilirubin was 19.7 mg per deciliter. Three days earlier, she had received a diagnosis of bronchitis and was treated with azithromy...
The first edition of the clinical practice guidelines for liver cirrhosis was published in 2010, and the second edition was published in 2015 by the Japanese Society of Gastroenterology (JSGE). The revised third edition was recently published in 2020. This version has become a joint guideline by the JSGE and the Japan Society of Hepatology (JSH).
Abstract. We present a case of a 36-year-old female patient who presented with subacute liver disease with a history of alcohol abuse. On basic liver function tests (LFT), she had aspartate transaminase / alanine transaminase > 2.2 and alkaline phosphatase / total bilirubin < 4. This pattern in acute liver failure patients signifies Wilson's ...
This case study examines the physiological nature of alcohol-induced cirrhosis and its pathogenesis, external and internal clinical presentations, and treatment options. ... it can be difficult for ultrasound to penetrate the liver tissue. For example, the lack of penetration when performing Doppler interrogation studies can cause vessels to ...
300+ Nursing Cheatsheets. Start Free Trial. "Would suggest to all nursing students . . . Guaranteed to ease the stress!". ~Jordan. Cirrhosis Case Study (45 min) is mentioned in these lessons. Nursing case study for patient with cirrhosis with unfolding questions and answers. Written by ICU nurses.
This case study examines the physiological nature of alcohol-induced cirrhosis and its pathogenesis, external and internal clinical presentations, and treatment options. Treatments for alcohol-induced cirrhosis include liver transplant for permanent correction as well as varied options to manage symptoms. This case study analyzes alcoholic ...
No. 11. CASE STUDY IN CIRRHOSIS OF THE LIVER 1039. Albumin is an abnormal constituent but it was of the urine, sincere wish of both the and was most likely caused by medical renal conges- and nursing staff to try to. tion, which in turn was caused make by him the as liver comfortable and as con-.
UNFOLDING Reasoning Case Study: STUDENT Cirrhosis History of Present Problem: ... Liver cirrhosis with hepatic encephalopathy and an AKI Admit today - recent admission 6 months ago Hepatic encephalopathy due to advanced liver cirrhosis Hep C +, ETOH use (x25 years), IVDU, liver cirrhosis ...
Cirrhosis is an important cause of morbidity and mortality in people with chronic liver disease worldwide. In 2019, cirrhosis was associated with 2.4% of global deaths. Owing to the rising ...
Abstract. A 25-year-old female nurse was referred to our diabetes outpatient clinic with poorly controlled type 2 diabetes, obesity and elevated liver function tests (LFTs). Following a liver biopsy she was diagnosed with non-alcoholic steatohepatitis (NASH) and liver fibrosis. Treatment with subcutaneous injections of the glucagon-like peptide-1 receptor (GLP-1R) agonist liraglutide was ...
This alcoholic liver case study presents a patient with liver cirrhosis. A 43-year-old man was brought into the hospital with a complaints of loss of appetite, abdominal distention, and arrhythmia. He also experienced itchy skin and blood in the stool. The patient's family rushed him into the hospital, and he was in a half-conscious state.
Some of the common causes of liver cirrhosis include: Alcoholism: Repeated use of alcohol leads to the replacement of healthy liver tissues and leads to scar formation (Hanai et al., 2015).Further, with the progression of disease healthy tissue gets replaced with scar tissues due to which the function of the liver gets compromised.
This chapter discusses the case of a 48-year-old man, Richard, who was diagnosed with decompensated liver disease due to previously undiagnosed liver cirrhosis secondary to alcohol. He is not completely abstinent but has significantly cut down his alcohol intake from 48 units to 12 units per week. Due to his initial diagnosis of alcohol related ...
An alcoholic patient who continues to drink: case presentation. Stuart McPherson, specialist registrar1 and Colin John Rees, consultant gastroenterologist1. Mr Bond is a 42 year old man with alcoholic cirrhosis who was admitted to our unit with haematemesis. He had had three previous admissions with alcohol related problems and had twice bled ...
The document presents a case study of a 35-year-old male auto rickshaw driver diagnosed with cirrhosis of the liver. It provides details on the patient's history, physical examination findings, diagnostic tests, and discusses the etiology, pathophysiology, clinical manifestations, and medical management of cirrhosis of the liver. The patient has a history of alcohol use and malnutrition which ...
Age of patients ranged between 22-106 years with mean age of 52.33 year. The patients predominantly presented with ascites (49.4%), gastrointestinal bleeding (27%), peripheral edema (24.7%), and encephalopathy (21.3%). The in-hospital case fatality rate was 11.2% and the patients presented with decreased urinary output, peripheral edema and ence...
In this chapter Case studies levels 1-3 explore the management of a patient with alcoholic liver disease. The patient has alcoholic liver cirrhosis and first presents with alcohol withdrawal (Case study level 1), then the patient's risk of bleeding and treatment for the maintenance of alcohol abstinence are considered (Case study level 2). The patient then goes on to develop encephalopathy ...
current issue. current issue; browse recently published; browse full issue index; learning/cme
PLANNING Observe for. DAILY PROGRESS NOTEOF. MAJOR NURSING INTERVENTION . Complain of. Medication Diet . Provide the. Liver ultrasound impression: PATHOPHYSIOLOGY OF CIRRHOSIS. A case study on cirrhosis of liver - Download as a PDF or view online for free.
Here, we report the case of a patient presenting with cirrhosis of the liver due to autoimmune hepatitis along with systemic sclerosis. Case presentation A 51-year-old non-obese female with no history of alcoholism was admitted with complaints of gradually progressive abdominal distension and swelling over both lower limbs for the last month.
Ascites is a pathological collection of free fluid in the peritoneal cavity, which is a common complication in patients with cirrhosis, an advanced liver disease. Bacterial infection increases the mortality rate of hospitalized patients with cirrhosis, irrespective of the severity of the liver disease. Around 60% of patients with compensated cirrhosis developed ascites within 10 years during ...
Congenital hemophilia A is an inherited genetic disorder caused by a decrease in the amount or activity of clotting factor VIII, which globally predisposes to bleeding manifestations. 7 Clinical practice guidelines for the management of UGB (Japanese, European, and North American 1,7-9 among others) do not include patients with hemophilia, and information on the management of this entity in ...
A more severe metabolic state is associated with a higher rate of progression to cirrhosis, as evidenced by a study involving patients with a diagnosis of MASLD within a U.S. Veterans Affairs cohort ().This study revealed a gradual increase in cirrhosis and liver cancer risk with the presence of additional metabolic traits, with T2D exhibiting the strongest association.