- Utility Menu

Cognitive Neuropsychology Laboratory
- Peer-Reviewed Articles
- EndNote XML
Cognitive neuroscience research on conceptual knowledge often is discussed with respect to “embodiment” or “grounding.” We tried to disentangle at least three distinct claims made using these terms. One of these, the view that concepts are entirely reducible to sensory-motor representations, is untenable and diminishing in the literature. A second is the view that concepts and sensory-motor representations “interact,” and a third view addresses the question of how concepts are neurally organized—the neural partitions among concepts of different kinds, and where these partitions are localized in cortex. We argue that towards the second and third issues, much fruitful research can be pursued, but that no position on them is specifically related to “grounding.” Furthermore, to move forward on them, it is important to precisely distinguish different kinds of representations—conceptual vs. sensory-motor—from each other theoretically and empirically. Neuroimaging evidence often lacks such specificity. We take an approach that distinguishes conceptual from sensory-motor representations by virtue of two properties: broad generality and tolerance to the absence of sensory-motor associations. We review three of our recent experiments that employ these criteria in order to localize neural representations of several specific kinds of nonsensory attributes: functions, intentions, and belief traits. Building on past work, we find that neuroimaging evidence can be used fruitfully to distinguish interesting hypotheses about neural organization. On the other hand, most such evidence does not speak to any clear notion of “grounding” or “embodiment,” because these terms do not make clear, specific, empirical predictions. We argue that cognitive neuroscience will proceed most fruitfully by relinquishing these terms.
This research studies the neural systems underlying two integration processes that take place during natural discourse comprehension: consistency evaluation and passive comprehension. Evaluation was operationalized with a consistency judgment task and passive comprehension with a passive listening task. Using fMRI, the experiment examined the integration of incoming sentences with more recent, local context and with more distal, global context in these two tasks. The stimuli were stories in which we manipulated the consistency of the endings with the local context and the relevance of the global context for the integration of the endings. A whole-brain analysis revealed several differences between the two tasks. Two networks previously associated with semantic processing and attention orienting showed more activation during the judgment than the passive listening task. A network previously associated with episodic memory retrieval and construction of mental scenes showed greater activity when global context was relevant, but only during the judgment task. This suggests that evaluation, more than passive listening, triggers the reinstantiation of global context and the construction of a rich mental model for the story. Finally, a network previously linked to fluent updating of a knowledge base showed greater activity for locally consistent endings than inconsistent ones, but only during passive listening, suggesting a mode of comprehension that relies on a local scope approach to language processing. Taken together, these results show that consistency evaluation and passive comprehension weigh differently on distal and local information and are implemented, in part, by different brain networks.
The perception of apparent body movement sometimes follows biologically plausible paths rather than paths along the shortest distance as in the case for inanimate objects. For numerous authors, this demonstrates that the somatosensory and motor representations of the observer's own body support and constrain the perception of others’ body movements. In this paper, we report evidence that calls for a re-examination of this account. We presented an apparent upper limb movement perception task to typically developed participants and five individuals born without upper limbs who were, therefore, totally deprived of somatosensory or motor representations of those limbs. Like the typically developed participants, they showed the typical bias toward long and biomechanically plausible path. This finding suggests that the computations underlying the biomechanical bias in apparent body movement perception is intrinsic to the visual system.
Segmentation of the world into meaningful units has typically been described as object individuation, emphasizing the spatially disconnected quality that comes as a result of objecthood. This segmentation can occur rapidly, even in parallel for multiple objects. It remains unclear whether objecthood is a necessary requirement for parallel individuation, or whether target features in distinct locations, such as object parts, may also be individuated in parallel. In a series of six experiments, we used a rapid enumeration task to test whether subitizing, a phenomenon believed to result from parallel individuation, occurs over object parts. We found that subitizing and individuation occur over connected object parts as well as physically separate objects of varied shapes and sizes. We also observed subitizing when target items are indents, features intrinsic to the shape of the object, and when cues for occlusion were removed. The results of these studies suggest that parallel individuation is not bound to objecthood, and can occur over object parts existing in separate locations.
The nature of domain-specific organization in higher-order visual cortex (ventral occipital temporal cortex, VOTC) has been investigated both in the case of visual experience deprivation and of modality of stimulation in sighted individuals. Object domain interacts in an intriguing and revelatory way with visual experience and modality of stimulation: selectivity for artifacts and scene domains is largely immune to visual deprivation and is multi-modal, whereas selectivity for animate items in lateral posterior fusiform gyrus is present only with visual stimulation. This domain-by-modality interaction is not readily accommodated by existing theories of VOTC representation. We conjecture that these effects reflect a distinction between the visual features that characterize different object domains and their interaction with different types of downstream computational systems.
Every day, we interact with people synchronously, immediately understand what they are doing, and easily infer their mental state and the likely outcome of their actions from their kinematics. According to various motor simulation theories of perception, such efficient perceptual processing of others ’ actions cannot be achieved by visual analysis of the movements alone but requires a process of motor simulation — an unconscious, covert imitation of the observed movements. According to this hypothesis, individ- uals incapable of simulating observed movements in their motor system should have difficulty perceiving and interpreting ob- served actions. Contrary to this prediction, we found across eight sensitive experiments that individuals born with absent or se- verely shortened upper limbs (upper limb dysplasia), despite some variability, could perceive, anticipate, predict, comprehend, and mem- orize upper limb actions, which they cannot simulate, as efficiently as typically developed participants. We also found that, like the typically developed participants, the dysplasic participants systematically per- ceived the position of moving upper limbs slightly ahead of their real position but only when the anticipated position was not biomechan- ically awkward. Such anticipatory bias and its modulation by implicit knowledge of the body biomechanical constraints were previously considered as indexes of the crucial role of motor simulation in action perception. Our findings undermine this assumption and the theories that place the locus of action perception and comprehension in the motor system and invite a shift in the focus of future research to the question of how the visuo-perceptual system represents and pro- cesses observed body movements and actions.
Recognizing the identity of a face is computationally challenging, because it requires distinguishing between similar images depicting different people, while recognizing even very different images depicting a same person. Previous human fMRI studies investigated representations of face identity in the presence of changes in viewpoint and in expression. Despite the importance of holistic processing for face recognition, an investigation of representations of face identity across different face parts is missing. To fill this gap, we investigated representations of face identity and their invariance across different face halves. Information about face identity with invariance across changes in the face half was individuated in the right anterior temporal lobe, indicating this region as the most plausible candidate brain area for the representation of face identity. In a complementary analysis, information distinguishing between different face halves was found to decline along the posterior to anterior axis in the ventral stream.
What are the processes involved in determining that there are exactly n objects in the visual field? The core level of representation for this process is based on a mechanism that iteratively individuates each of the set of relevant objects for exact enumeration. In support of this proposal, we review recent electrophysiological findings on enumeration-at-a-glance and consider three temporally distinct responses of the EEG signal that are modulated by object numerosity, and which have been associated respectively with perceptual modulation, attention selection, and working memory. We argue that the neural response associated with attention selection shows the hallmarks of an object individuation mechanism, including the property of simultaneous individuation of a limited number of objects thought to underlie the behavioral subitizing effect. The findings support the view that the core component of exact enumeration is an attention-based individuation mechanism that binds specific features to locations and provides a stable representation of a limited set of relevant objects. The resulting representation is made available for further cognitive operations for exact enumeration.
The placement and development of the visual word form area (VWFA) have commonly been assumed to depend, in part, on its connections with language regions. In this study, we specifically examined the effects of auditory speech experience deprivation in shaping the VWFA by investigating its location distribution, activation strength, and functional connectivity pattern in congenitally deaf participants. We found that the location and activation strength of the VWFA in congenitally deaf participants were highly comparable with those of hearing controls. Furthermore, while the congenitally deaf group showed reduced resting-state functional connectivity between the VWFA and the auditory speech area in the left anterior superior temporal gyrus, its intrinsic functional connectivity pattern between the VWFA and a fronto-parietal network was similar to that of hearing controls. Taken together, these results suggest that auditory speech experience has consequences for aspects of the word form-speech sound correspondence network, but that such experience does not significantly modulate the VWFA's placement or response strength. This is consistent with the view that the role of the VWFA might be to provide a representation that is suitable for mapping visual word forms onto language-specific gestures without the need to construct an aural representation.
Regions in human lateral and ventral occipitotemporal cortices (OTC) respond selectively to pictures of the human body and its parts. What are the organizational principles underlying body part responses in these regions? Here we used representational similarity analysis (RSA) of fMRI data to test multiple possible organizational principles: shape similarity, physical proximity, cortical homunculus proximity, and semantic similarity. Participants viewed pictures of whole persons, chairs, and eight body parts (hands, arms, legs, feet, chests, waists, upper faces, and lower faces). The similarity of multivoxel activity patterns for all body part pairs was established in whole person-selective OTC regions. The resulting neural similarity matrices were then compared with similarity matrices capturing the hypothesized organizational principles. Results showed that the semantic similarity model best captured the neural similarity of body parts in lateral and ventral OTC, which followed an organization in three clusters: (1) body parts used as action effectors (hands, feet, arms, and legs), (2) noneffector body parts (chests and waists), and (3) face parts (upper and lower faces). Whole-brain RSA revealed, in addition to OTC, regions in parietal and frontal cortex in which neural similarity was related to semantic similarity. In contrast, neural similarity in occipital cortex was best predicted by shape similarity models. We suggest that the semantic organization of body parts in high-level visual cortex relates to the different functions associated with the three body part clusters, reflecting the unique processing and connectivity demands associated with the different types of information (e.g., action, social) different body parts (e.g., limbs, faces) convey.
The ability to recognize, create, and use complex tools is a milestone in human evolution. Widely distributed brain regions in parietal, frontal, and temporal cortices have been implicated in using and understanding tools, but the roles of their anatomical connections in supporting tool use and tool conceptual behaviors are unclear. Using deterministic fiber tracking in healthy participants, we first examined how 14 cortical regions that are consistently activated by tool processing are connected by white matter (WM) tracts. The relationship between the integrity of each of the 33 obtained tracts and tool processing deficits across 86 brain-damaged patients was investigated. WM tract integrity was measured with both lesion percentage (structural imaging) and mean fractional anisotropy (FA) values (diffusion imaging). Behavioral abilities were assessed by a tool use task, a range of conceptual tasks, and control tasks. We found that three left hemisphere tracts connecting frontoparietal and intrafrontal areas overlapping with left superior longitudinal fasciculus are crucial for tool use such that larger lesion and lower mean FA values on these tracts were associated with more severe tool use deficits. These tracts and five additional left hemisphere tracts connecting frontal and temporal/parietal regions, mainly overlapping with left superior longitudinal fasciculus, inferior frontooccipital fasciculus, uncinate fasciculus, and anterior thalamic radiation, are crucial for tool concept processing. Largely consistent results were also obtained using voxel-based symptom mapping analyses. Our results revealed the WM structural networks that support the use and conceptual understanding of tools, providing evidence for the anatomical skeleton of the tool knowledge network.
Knowledge of function is critical for selecting objects to meet action goals, even when the affordances of those objects are not mechanical—for instance, both a painting and a vase can decorate a room. To identify neural representations of such abstract function concepts, we asked participants in an fMRI scanner to view a variety of objects and evaluate their utility to each of four goals (two Decoration goals: dress up for a night out and decorate a house, and two Protection goals: protect your body from the cold and keep objects dry in a flooded basement). These task conditions differed in the kind of functional evaluation participants had to perform over objects, but did not vary in the objects themselves. We performed a searchlight multivariate pattern analysis to identify cortical representations in which neural patterns were more similar for the pairs of similar-goal than dissimilar-goal task conditions (Decorate vs. Protect). We report such effects in anterior inferior parietal lobe (aIPL) close to regions typically reported for processing tool-related actions, and thought to be important for representing how they are manipulated. However, the current study design fully controlled for manipulation similarity, which predicted orthogonal relationships among the conditions. We conclude that the aIPL likely has nearby, but distinct, representations of both manipulation and function knowledge, and thereby may have a broader role in understanding how objects can be used, representing not just physical affordances but also abstract functional criteria such as esthetic value or purpose categories such as decorate . This pattern of localization has implications for how semantic knowledge is organized in the brain.
Classical animal visual deprivation studies and human neuroimaging studies have shown that visual experience plays a critical role in shaping the functionality and connectivity of the visual cortex. Interestingly, recent studies have additionally reported circumscribed regions in the visual cortex in which functional selectivity was remarkably similar in individuals with and without visual experience. Here, by directly comparing resting-state and task-based fMRI data in congenitally blind and sighted human subjects, we obtained large-scale continuous maps of the degree to which connectional and functional “fingerprints” of ventral visual cortex depend on visual experience. We found a close agreement between connectional and functional maps, pointing to a strong interdependence of connectivity and function. Visual experience (or the absence thereof) had a pronounced effect on the resting-state connectivity and functional response profile of occipital cortex and the posterior lateral fusiform gyrus. By contrast, connectional and functional fingerprints in the anterior medial and posterior lateral parts of the ventral visual cortex were statistically indistinguishable between blind and sighted individuals. These results provide a large-scale mapping of the influence of visual experience on the development of both functional and connectivity properties of visual cortex, which serves as a basis for the formulation of new hypotheses regarding the functionality and plasticity of specific subregions.
Is visual input during critical periods of development crucial for the emergence of the fundamental topographical mapping of the visual cortex? And would this structure be retained throughout life-long blindness or would it fade as a result of plastic, use-based reorganization? We used functional connectivity magnetic resonance imaging based on intrinsic blood oxygen level-dependent fluctuations to investigate whether significant traces of topographical mapping of the visual scene in the form of retinotopic organization, could be found in congenitally blind adults. A group of 11 fully and congenitally blind subjects and 18 sighted controls were studied. The blind demonstrated an intact functional connectivity network structural organization of the three main retinotopic mapping axes: eccentricity (centre-periphery), laterality (left-right), and elevation (upper-lower) throughout the retino- topic cortex extending to high-level ventral and dorsal streams, including characteristic eccentricity biases in face- and house- selective areas. Functional connectivity-based topographic organization in the visual cortex was indistinguishable from the normally sighted retinotopic functional connectivity structure as indicated by clustering analysis, and was found even in participants who did not have a typical retinal development in utero (microphthalmics). While the internal structural organization of the visual cortex was strikingly similar, the blind exhibited profound differences in functional connectivity to other (non-visual) brain regions as compared to the sighted, which were specific to portions of V1. Central V1 was more connected to language areas but peripheral V1 to spatial attention and control networks. These findings suggest that current accounts of critical periods and experience- dependent development should be revisited even for primary sensory areas, in that the connectivity basis for visual cortex large- scale topographical organization can develop without any visual experience and be retained through life-long experience-dependent plasticity. Furthermore, retinotopic divisions of labour, such as that between the visual cortex regions normally representing the fovea and periphery, also form the basis for topographically-unique plastic changes in the blind.
In congenitally blind individuals, many regions of the brain that are typically heavily involved in visual processing are recruited for a variety of nonvisual sensory and cognitive tasks ( Rauschecker 1995 ; Pascual-Leone et al. 2005 ). This phenomenon — cross-modal plasticity — has been widely documented, but the principles that de- termine where and how cross-modal changes occur remain poorly understood ( Bavelier and Neville 2002 ). Here, we evaluate the hypothesis that cross-modal plasticity respects the type of compu- tations performed by a region, even as it changes the modality of the inputs over which they are carried out ( Pascual-Leone and Hamilton 2001 ). We compared the fMRI signal in sighted and con- genitally blind participants during proprioceptively guided reaching. We show that parietooccipital reach-related regions retain their functional role — encoding of the spatial position of the reach target — even as the dominant modality in this region changes from visual to nonvisual inputs. This suggests that the computational role of a region, independently of the processing modality, codetermines its potential cross-modal recruitment. Our fi ndings demonstrate that preservation of functional properties can serve as a guiding prin- ciple for cross-modal plasticity even in visuomotor cortical regions, i.e. beyond the early visual cortex and other traditional visual areas.
Processing within the dorsal visual stream subserves object-directed action, whereas visual object recognition is mediated by the ventral visual stream. Recent findings suggest that the computations performed by the dorsal stream can nevertheless influence object recognition. Little is known, however, about the type of dorsal stream information that is available to assist in object recognition. Here, we present a series of experiments that explored different psychophysical manipulations known to bias the processing of a stimulus toward the dorsal visual stream in order to isolate its contribution to object recognition. We show that elongated-shaped stimuli, regardless of their semantic category and familiarity, when processed by the dorsal stream, elicit visuomotor grasp-related information that affects how we categorize manipulable objects. Elongated stimuli may reduce ambiguity during grasp preparation by providing a coarse cue to hand shaping and orientation that is sufficient to support action planning. We propose that this dorsal-stream-based analysis of elongation along a principal axis is the basis for how the dorsal visual object processing stream can affect categorization of manipulable objects.
Conspecifics are potential mates, and can be the most dangerous threats. With conspecifics we engage in complex social interactions. Therefore, it is important to rapidly detect the presence of conspecifics in a scene. Images of humans attract attention, and do so already in 9-months-old infants, showing that the distinction between conspecifics and other animals emerges early in development. However, despite a wealth of evidence on the behavioral differences between the processing of conspecifics and other animals, the neural mechanisms that underlie the recognition of conspecifics remain unknown. In this experiment, we used recursive feature elimination to individuate brain regions that show selective effects for the faces of conspecifics, individuating reliable conspecific effects in the right ventrolateral prefrontal cortex (vlPFC). Consistent with the importance of conspecifics recognition for reorienting attention and for social cognition, this region shows functional connectivity with the temporo-parietal junction (TPJ), implicated in reorienting attention and in the attribution of mental states to others. Our results suggest that the right vlPFC plays an important role for the recognition of conspecifics and may function as a gateway for the attribution of mental states to an object.
Every day we encounter dozens of people, and in order to interact with them appropriately we need to recognize their identity. The face is a crucial source of information to recognize a person’s identity. However, recognizing the identity of a face is challenging because it requires distinguishing between very similar images (e.g., the front views of two different faces) while categorizing very different images (e.g., a front view and a profile) as the same person. Neuroimaging has the whole-brain coverage needed to investigate where representations of face identity are encoded, but it is limited in terms of spatial and temporal resolution. In this article, we review recent neuroimaging research that attempted to investigate the representation of face identity, the challenges it faces, and the proposed solutions, to conclude that given the current state of the evidence the right anterior temporal lobe is the most promising candidate region for the representation of face identity.
Publication Type
- Book Chapters
Recent Publications
- Large-scale organizations of the hand action observation network in individuals born without hands
- Plasticity based on compensatory effector use in the association but not primary sensorimotor cortex of people born without hands
- View-invariant representation of hand postures in the human lateral occipitotemporal cortex
- The neural representation of human versus nonhuman bipeds and quadrupeds
- Multimodal representations of person identity individuated with fMRI
- Neural Representations of Belief Concepts: A Representational Similarity Approach to Social Semantics
Copyright Notice
Electronic copies of publications provided on this website are for individual, non-commercial use only. Copyright belongs to those designated within each publication. Files provided herein are not to be disseminated or reposted without permission of the appropriate entities. For any articles not available on this website, please email Prof. Alfonso Caramazza, [email protected] for further discussion.

Behavioral Neuroscience
- Read this journal
- Read free articles
- Journal snapshot
- Advertising information
Journal scope statement
The primary mission of Behavioral Neuroscience ® is to publish original research articles as well as reviews in the broad field of the neural bases of behavior.
We seek empirical papers reporting novel results that provide insight into the mechanisms by which nervous systems produce and are affected by behavior. Experimental subjects may include human and non-human animals and may address any phase of the lifespan, from early development to senescence.
Studies employing brain-imaging techniques in normal and pathological human populations are encouraged, as are studies using non-traditional species (including invertebrates) and employing comparative analyses. Studies using computational approaches to understand behavior and cognition are particularly encouraged.
In addition to behavior, it is expected that some aspect of nervous system function will be manipulated or observed, ranging across molecular, cellular, neuroanatomical, neuroendocrinological, neuropharmacological, and neurophysiological levels of analysis. Behavioral studies are welcome so long as their implications for our understanding of the nervous system are clearly described in the paper.
Behavioral Neuroscience primarily publishes original research articles reporting novel findings. However, the journal also publishes registered reports, negative findings, and replications of previously reported findings. Preregistration of replication studies is strongly recommended, but not required.
We offer the option to publish under either a subscription model at no charge or under an open access model for a modest fee .
Each of these article types may be full-length research papers or brief communications. Brief communications are limited to 3,250 words of text and two figures and/or tables. When appropriate, commentaries on research papers are invited by the editors.
We welcome reviews on any theoretical, empirical, or historical topic related to the role of the nervous system in the production of behavior.
Inquiries about potential review topics can be addressed to the editor.
Relevant information for each of these article types can be found under the Manuscript Submission tab.
Topic areas covered by the journal include:
- learning and memory, attention, decision making
- perception, spatial cognition, sensorimotor processing and integration
- human and non-human animal cognition and emotion
- molecular, cellular, and circuit level analyses of behavior and cognition
- motivation, reward, homeostasis and biorhythms
- animal models of psychopathology, addiction, and neurodegenerative disorders
- developmental and lifespan analyses
Disclaimer: APA and the editors of Behavioral Neuroscience ® assume no responsibility for statements and opinions advanced by the authors of its articles.
Equity, diversity, and inclusion
Behavioral Neuroscience supports equity, diversity, and inclusion (EDI) in its practices. More information on these initiatives is available under EDI Efforts .
Open science
The APA Journals Program is committed to publishing transparent, rigorous research; improving reproducibility in science; and aiding research discovery. Open science practices vary per editor discretion. View the initiatives implemented by this journal .
Author and editor spotlights
Explore journal highlights : free article summaries, editor interviews and editorials, journal awards, mentorship opportunities, and more.
Prior to submission, please carefully read and follow the submission guidelines detailed below. Manuscripts that do not conform to the submission guidelines may be returned without review.
Behavioral Neuroscience ® is a bimonthly, peer-reviewed journal that publishes research articles in the broad field of the neural bases of behavior. A detailed description of the editorial coverage policy appears on the inside of the front cover of each issue.
Behavioral Neuroscience publishes direct replications. Submissions should include “A Replication of XX Study” in the subtitle of the manuscript as well as in the abstract.
Behavioral Neuroscience is a member of the Neuroscience Peer Review Consortium .
To submit to the editorial office of Geoffrey Schoenbaum , please submit manuscripts electronically through the Manuscript Submission Portal in Microsoft Word or Open Office format.
Prepare manuscripts according to the Publication Manual of the American Psychological Association using the 7 th edition. Manuscripts may be copyedited for bias-free language (see Chapter 5 of the Publication Manual ). APA Style and Grammar Guidelines for the 7 th edition are available.
Submit Manuscript
General correspondence may be directed to the editorial office .
The manuscript file for new submissions or revisions should include the text, tables, and figures; should be in Word (.doc), Rich Text Format (.rtf) or PDF formats; and should not exceed 20MB.
In addition to addresses and phone numbers, please supply email addresses for potential use by the editorial office and later by the production office.
Behavioral Neuroscience is now using a software system to screen submitted content for similarity with other published content. The system compares the initial version of each submitted manuscript against a database of 40+ million scholarly documents, as well as content appearing on the open web. This allows APA to check submissions for potential overlap with material previously published in scholarly journals (e.g., lifted or republished material).
Registered reports
In addition to full-length research papers reporting novel findings, the journal publishes registered reports, negative findings, replications, commentaries and reviews. Preregistration of replication studies is strongly recommended, but not required.
Registered reports require a two-step review process.
The first step is the submission of the registration manuscript. This is a partial manuscript that includes hypotheses, rationale for the study, experimental design, and methods. The partial manuscript will be reviewed for significance and methodological approach.
If the partial manuscript is accepted this amounts to provisional acceptance of the full report regardless of the outcome of the study. The full manuscript will receive rapid editorial review, for adherence to the preregistered design, and expedited production for publication in the journal.
All articles can be published as full-length articles or as brief communications. Brief communications must not exceed 3,250 words of text, with no more than two figures and/or tables.
Submission letter
Include the following in your submission letter:
- A statement designating the type of article being submitted: report of novel findings, registered report (report registration or manuscript reporting completed study), negative finding, replication, commentary, or review.
- For brief communications, a statement that the article is 3,250 words of text and two figures and/or tables, or less.
- A statement of compliance with APA ethical standards in the treatment of your sample, human or animal, or a description of the details of the treatment.
- A statement that the manuscript or data have not been published previously and that they are not under consideration for publication elsewhere.
- A statement to reflect that all listed authors have contributed significantly to the manuscript and consent to their names on the manuscript.
- A statement to disclose any possible conflict of interest in the conduct and reporting of research (e.g., financial interests in a test or procedure, funding by pharmaceutical companies for drug research).
- The current date.
- If your manuscript is a follow-up to another manuscript previously published in Behavioral Neuroscience , please include the manuscript number and title of the previous manuscript in your submission letter, if you would like us to try to invite the reviewers of the previous manuscript.
Authors are encouraged to suggest five reviewers who are especially qualified to review their work and would not have a conflict of interest serving as a referee. The editors of Behavioral Neuroscience suggest that you include one or more of our consulting editors from the editorial board in your list of suggested reviewers .
Also please consider the gender and racial/ethnic diversity of the reviewers you propose, including members of underrepresented groups.
Review policy
Masked reviews are optional, and authors who wish masked reviews must specifically request them when submitting their manuscripts.
Each copy of a manuscript to be mask-reviewed should include a separate title page with authors' names and affiliations, and these should not appear anywhere else on the manuscript. Footnotes that identify the authors should be typed on a separate page.
Additionally, authors should make every effort to see that a manuscript intended for masked review itself contains no clues to their identities, including grant numbers, names of institutions providing IRB approval, self-citations, and links to online repositories for data, materials, code, or preregistrations (e.g., Create a View-only Link for a Project ).
If your manuscript was mask reviewed, please ensure that the final version for production includes a byline and full author note for typesetting.
Journal Article Reporting Standards
Authors should review the APA Style Journal Article Reporting Standards (JARS) for quantitative, qualitative, and mixed methods. The standards offer ways to improve transparency in reporting to ensure that readers have the information necessary to evaluate the quality of the research and to facilitate collaboration and replication.
- Recommend the division of hypotheses, analyses, and conclusions into primary, secondary, and exploratory groupings to allow for a full understanding of quantitative analyses presented in a manuscript and to enhance reproducibility;
- Offer modules for authors reporting on replications, clinical trials, longitudinal studies, and observational studies, as well as the analytic methods of structural equation modeling and Bayesian analysis;
- Include guidelines on reporting of study preregistration (including making protocols public); participant characteristics (including demographic characteristics); inclusion and exclusion criteria; psychometric characteristics of outcome measures and other variables; and planned data diagnostics and analytic strategy.
The guidelines focus on transparency in methods reporting, recommending descriptions of how the researcher’s own perspective affected the study, as well as the contexts in which the research and analysis took place.
Transparency and openness
APA endorses the Transparency and Openness Promotion (TOP) Guidelines by a community working group in conjunction with the Center for Open Science ( Nosek et al. 2015 ). Empirical research, including meta-analyses, submitted to Behavioral Neuroscience must meet the “disclosure” level for all eight aspects of research planning and reporting. Authors should include a subsection in the method section titled “Transparency and openness.” This subsection should detail the efforts the authors have made to comply with the TOP guidelines. For example:
- We report how we determined our sample size, all data exclusions (if any), all manipulations, and all measures in the study, and the study follows JARS (Appelbaum, et al., 2018). All data, analysis code, and research materials are available at [stable link to repository]. Data were analyzed using R, version 4.0.0 (R Core Team, 2020) and the package ggplot , version 3.2.1 (Wickham, 2016). This study’s design and its analysis were not pre-registered.
Data, materials, and code
Authors must state whether data and study materials are posted to a trusted repository and, if so, how to access them. Recommended repositories include APA’s repository on the Open Science Framework (OSF), or authors can access a full list of other recommended repositories . Trusted repositories adhere to policies that make data discoverable, accessible, usable, and preserved for the long term. Trusted repositories also assign unique and persistent identifiers.
In a subsection titled "Transparency and Openness" at the end of the method section, specify whether and where the data and material will be available or include a statement noting that they are not available. For submissions with quantitative or simulation analytic methods, state whether the study analysis code is posted to a trusted repository, and, if so, how to access it.
For example:
- All data have been made publicly available at the [repository name] and can be accessed at [persistent URL or DOI].
- Materials and analysis code for this study are available by emailing the corresponding author.
- Materials and analysis code for this study are not available.
- The code behind this analysis/simulation has been made publicly available at the [repository name] and can be accessed at [persistent URL or DOI].
Preregistration of studies and analysis plans
Preregistration of studies and specific hypotheses can be a useful tool for making strong theoretical claims. Likewise, preregistration of analysis plans can be useful for distinguishing confirmatory and exploratory analyses. Investigators may preregister prior to conducting the research via a publicly accessible registry system (e.g., OSF , ClinicalTrials.gov, or other trial registries in the WHO Registry Network).
There are many available templates; for example, APA, the British Psychological Society, and the German Psychological Society partnered with the Leibniz Institute for Psychology and Center for Open Science to create Preregistration Standards for Quantitative Research in Psychology (Bosnjak et al., 2022).
Articles must state whether or not any work was preregistered and, if so, where to access the preregistration. If any aspect of the study is preregistered, include the registry link in the method section.
- This study’s design was preregistered prospectively, before data were collected; see [STABLE LINK OR DOI].
- This study’s design and hypotheses were preregistered after data had been collected but before analyses were undertaken; see [STABLE LINK OR DOI].
- This study’s analysis plan was preregistered; see [STABLE LINK OR DOI].
- This study was not preregistered.
Author contributions statements using CRediT
The APA Publication Manual (7th ed.) stipulates that "authorship encompasses…not only persons who do the writing but also those who have made substantial scientific contributions to a study." In the spirit of transparency and openness, Behavioral Neuroscience has adopted the Contributor Roles Taxonomy (CRediT) to describe each author's individual contributions to the work. CRediT offers authors the opportunity to share an accurate and detailed description of their diverse contributions to a manuscript.
Submitting authors will be asked to identify the contributions of all authors at initial submission according to this taxonomy. If the manuscript is accepted for publication, the CRediT designations will be published as an author contributions statement in the author note of the final article. All authors should have reviewed and agreed to their individual contribution(s) before submission.
CRediT includes 14 contributor roles, as described below:
- Conceptualization : Ideas; formulation or evolution of overarching research goals and aims.
- Data curation : Management activities to annotate (produce metadata), scrub data and maintain research data (including software code, where it is necessary for interpreting the data itself) for initial use and later re-use.
- Formal analysis : Application of statistical, mathematical, computational, or other formal techniques to analyze or synthesize study data.
- Funding acquisition : Acquisition of the financial support for the project leading to this publication.
- Investigation : Conducting a research and investigation process, specifically performing the experiments, or data/evidence collection.
- Methodology : Development or design of methodology; creation of models.
- Project administration : Management and coordination responsibility for the research activity planning and execution.
- Resources : Provision of study materials, reagents, materials, patients, laboratory samples, animals, instrumentation, computing resources, or other analysis tools.
- Software : Programming, software development; designing computer programs; implementation of the computer code and supporting algorithms; testing of existing code components.
- Supervision : Oversight and leadership responsibility for the research activity planning and execution, including mentorship external to the core team.
- Validation : Verification, whether as a part of the activity or separate, of the overall replication/reproducibility of results/experiments and other research outputs.
- Visualization : Preparation, creation and/or presentation of the published work, specifically visualization/data presentation.
- Writing — original draft : Preparation, creation and/or presentation of the published work, specifically writing the initial draft (including substantive translation).
- Writing — review & editing : Preparation, creation and/or presentation of the published work by those from the original research group, specifically critical review, commentary or revision — including pre- or post-publication stages.
Authors can claim credit for more than one contributor role, and the same role can be attributed to more than one author.
All manuscripts must include a title page, typed on a separate page within the manuscript file, which includes the title of the manuscript, all author names and institutional affiliations, any funding received from one of the listed organizations in the author questionnaire, any disclosure of interests, and contact information for at least the corresponding author. For masked manuscripts, see review policy above.
Abbreviations and metrics
Nonstandard abbreviations should be introduced by placing the abbreviation in parentheses after the first occurrence of the term being abbreviated in both the abstract and the text. The metric system should be followed for all volumes, lengths, weights, and so on. Temperatures should be expressed in degrees Celsius (centigrade). Units should conform to the International System of Units (SI; see the Publication Manual ).
Revised manuscripts are processed electronically and should also be uploaded through the Manuscript Submission Portal. Manuscripts need not be accompanied by a copy of the original version. Revisions not returned within 2 months of the last action date will be treated as a new submission.
All proofs must be corrected and returned within 48 hours of receipt. Any extensive nonessential changes and extensive changes due to author error may incur charges. With the proofs will be a form providing the author with the opportunity to order reprints.
Direct inquiries to the APA Journals Office can be emailed ; sent to 800-374-2721; or faxed to 202-336-5549.
Manuscript preparation
Prepare manuscripts according to the Publication Manual of the American Psychological Association (7 th edition). Manuscripts may be copyedited for bias-free language (see Chapter 5 of the 7 th edition).
Review APA's Journal Manuscript Preparation Guidelines before submitting your article.
Double-space all copy. Other formatting instructions, as well as instructions on preparing tables, figures, references, metrics, and abstracts, appear in the Manual . Additional guidance on APA Style is available on the APA Style website .
Below are additional instructions regarding the preparation of display equations, computer code, and tables.
Display equations
We strongly encourage you to use MathType (third-party software) or Equation Editor 3.0 (built into pre-2007 versions of Word) to construct your equations, rather than the equation support that is built into Word 2007 and Word 2010. Equations composed with the built-in Word 2007/Word 2010 equation support are converted to low-resolution graphics when they enter the production process and must be rekeyed by the typesetter, which may introduce errors.
To construct your equations with MathType or Equation Editor 3.0:
- Go to the Text section of the Insert tab and select Object.
- Select MathType or Equation Editor 3.0 in the drop-down menu.
If you have an equation that has already been produced using Microsoft Word 2007 or 2010 and you have access to the full version of MathType 6.5 or later, you can convert this equation to MathType by clicking on MathType Insert Equation. Copy the equation from Microsoft Word and paste it into the MathType box. Verify that your equation is correct, click File, and then click Update. Your equation has now been inserted into your Word file as a MathType Equation.
Use Equation Editor 3.0 or MathType only for equations or for formulas that cannot be produced as Word text using the Times or Symbol font.
Computer code
Because altering computer code in any way (e.g., indents, line spacing, line breaks, page breaks) during the typesetting process could alter its meaning, we treat computer code differently from the rest of your article in our production process. To that end, we request separate files for computer code.
In online supplemental material
We request that runnable source code be included as supplemental material to the article. For more information, visit Supplementing Your Article With Online Material .
In the text of the article
If you would like to include code in the text of your published manuscript, please submit a separate file with your code exactly as you want it to appear, using Courier New font with a type size of 8 points. We will make an image of each segment of code in your article that exceeds 40 characters in length. (Shorter snippets of code that appear in text will be typeset in Courier New and run in with the rest of the text.) If an appendix contains a mix of code and explanatory text, please submit a file that contains the entire appendix, with the code keyed in 8-point Courier New.
Use Word's Insert Table function when you create tables. Using spaces or tabs in your table will create problems when the table is typeset and may result in errors.
Academic writing and English language editing services
Authors who feel that their manuscript may benefit from additional academic writing or language editing support prior to submission are encouraged to seek out such services at their host institutions, engage with colleagues and subject matter experts, and/or consider several vendors that offer discounts to APA authors .
Please note that APA does not endorse or take responsibility for the service providers listed. It is strictly a referral service.
Use of such service is not mandatory for publication in an APA journal. Use of one or more of these services does not guarantee selection for peer review, manuscript acceptance, or preference for publication in any APA journal.
Submitting supplemental materials
APA can place supplemental materials online, available via the published article in the APA PsycArticles ® database. Please see Supplementing Your Article With Online Material for more details.
Abstract and keywords
All manuscripts must include an abstract containing a maximum of 250 words typed on a separate page. After the abstract, please supply up to five keywords or brief phrases.
List references in alphabetical order. Each listed reference should be cited in text, and each text citation should be listed in the references section.
Examples of basic reference formats:
Journal article
McCauley, S. M., & Christiansen, M. H. (2019). Language learning as language use: A cross-linguistic model of child language development. Psychological Review , 126 (1), 1–51. https://doi.org/10.1037/rev0000126
Authored book
Brown, L. S. (2018). Feminist therapy (2nd ed.). American Psychological Association. https://doi.org/10.1037/0000092-000
Chapter in an edited book
Balsam, K. F., Martell, C. R., Jones. K. P., & Safren, S. A. (2019). Affirmative cognitive behavior therapy with sexual and gender minority people. In G. Y. Iwamasa & P. A. Hays (Eds.), Culturally responsive cognitive behavior therapy: Practice and supervision (2nd ed., pp. 287–314). American Psychological Association. https://doi.org/10.1037/0000119-012
Data set citation
Alegria, M., Jackson, J. S., Kessler, R. C., & Takeuchi, D. (2016). Collaborative Psychiatric Epidemiology Surveys (CPES), 2001–2003 [Data set]. Inter-university Consortium for Political and Social Research. https://doi.org/10.3886/ICPSR20240.v8
Software/Code citation
Viechtbauer, W. (2010). Conducting meta-analyses in R with the metafor package. Journal of Statistical Software , 36(3), 1–48. https://www.jstatsoft.org/v36/i03/
Wickham, H. et al., (2019). Welcome to the tidyverse. Journal of Open Source Software, 4 (43), 1686, https://doi.org/10.21105/joss.01686
All data, program code, and other methods must be cited in the text and listed in the references section.
Preferred formats for graphics files are TIFF and JPG, and preferred format for vector-based files is EPS. Graphics downloaded or saved from web pages are not acceptable for publication. Multipanel figures (i.e., figures with parts labeled a, b, c, d, etc.) should be assembled into one file. When possible, please place symbol legends below the figure instead of to the side.
- All color line art and halftones: 300 DPI
- Black and white line tone and gray halftone images: 600 DPI
Line weights
- Color (RGB, CMYK) images: 2 pixels
- Grayscale images: 4 pixels
- Stroke weight: 0.5 points
APA offers authors the option to publish their figures online in color without the costs associated with print publication of color figures.
The same caption will appear on both the online (color) and print (black and white) versions. To ensure that the figure can be understood in both formats, authors should add alternative wording (e.g., “the red (dark gray) bars represent”) as needed.
For authors who prefer their figures to be published in color both in print and online, original color figures can be printed in color at the editor's and publisher's discretion provided the author agrees to pay:
- $900 for one figure
- An additional $600 for the second figure
- An additional $450 for each subsequent figure
Permissions
Authors of accepted papers must obtain and provide to the editor on final acceptance all necessary permissions to reproduce in print and electronic form any copyrighted work, including test materials (or portions thereof), photographs, and other graphic images (including those used as stimuli in experiments).
On advice of counsel, APA may decline to publish any image whose copyright status is unknown.
- Download Permissions Alert Form (PDF, 13KB)
Publication policies
For full details on publication policies, including use of Artificial Intelligence tools, please see APA Publishing Policies .
APA policy prohibits an author from submitting the same manuscript for concurrent consideration by two or more publications.
See also APA Journals ® Internet Posting Guidelines .
APA requires authors to reveal any possible conflict of interest in the conduct and reporting of research (e.g., financial interests in a test or procedure, funding by pharmaceutical companies for drug research).
- Download Full Disclosure of Interests Form (PDF, 41KB)
In light of changing patterns of scientific knowledge dissemination, APA requires authors to provide information on prior dissemination of the data and narrative interpretations of the data/research appearing in the manuscript (e.g., if some or all were presented at a conference or meeting, posted on a listserv, shared on a website, including academic social networks like ResearchGate, etc.). This information (2–4 sentences) must be provided as part of the author note.
Ethical Principles
It is a violation of APA Ethical Principles to publish "as original data, data that have been previously published" (Standard 8.13).
In addition, APA Ethical Principles specify that "after research results are published, psychologists do not withhold the data on which their conclusions are based from other competent professionals who seek to verify the substantive claims through reanalysis and who intend to use such data only for that purpose, provided that the confidentiality of the participants can be protected and unless legal rights concerning proprietary data preclude their release" (Standard 8.14).
APA expects authors to adhere to these standards. Specifically, APA expects authors to have their data available throughout the editorial review process and for at least 5 years after the date of publication.
Authors are required to state in writing that they have complied with APA ethical standards in the treatment of their sample, human or animal, or to describe the details of treatment.
- Download Certification of Compliance With APA Ethical Principles Form (PDF, 26KB)
The APA Ethics Office provides the full Ethical Principles of Psychologists and Code of Conduct electronically on its website in HTML, PDF, and Word format. You may also request a copy by emailing or calling the APA Ethics Office (202-336-5930). You may also read "Ethical Principles," December 1992, American Psychologist , Vol. 47, pp. 1597–1611.
Other information
See APA’s Publishing Policies page for more information on publication policies, including information on author contributorship and responsibilities of authors, author name changes after publication, the use of generative artificial intelligence, funder information and conflict-of-interest disclosures, duplicate publication, data publication and reuse, and preprints.
Visit the Journals Publishing Resource Center for more resources for writing, reviewing, and editing articles for publishing in APA journals.
Geoffrey Schoenbaum, MD, PhD National Institute on Drug Abuse, National Institutes of Health, United States
Associate editors
Mihaela Iordanova, PhD Concordia University, Canada
Alicia Izquierdo, PhD UCLA, United States
Elisabeth Murray, PhD National Institute of Mental Health, United States
Yael Niv, PhD Princeton University, United States
Mark Walton, PhD University of Oxford, United Kingdom
Catharine Winstanley, PhD University of British Columbia, Canada
Consulting editors
Ted G. Abel, PhD University of Iowa, United States
Cristina M. Alberini, PhD New York University, Unites States
Jeffrey R. Alberts, PhD Indiana University, Unites States
Timothy A. Allen, PhD Florida International University, United States
Jocelyne Bachevalier, PhD Yerkes National Primate Research Center, Emory University, United States
Bernard W. Balleine, PhD University of New South Wales, Australia
Moshe Bar, PhD Bar-Ilan University, Israel
Carol A. Barnes, PhD University of Arizona, United States
Kevin G. Bath, PhD Columbia University, United States
Mark S. Blumberg, PhD University of Iowa, United States
Jennifer M. Bossert, PhD NIDA/NIH/IRP, United States
Mark E. Bouton, PhD University of Vermont, United States
Sara N. Burke, PhD University of Florida, United States
Michael D. Burton, PhD Neuroimmunology and Behavior Laboratory, University of Texas at Dallas, United States
Denise J. Cai, PhD Neuroscience Department, Icahn School of Medicine at Mount Sinai, United States
Xinying Cai, PhD Neural and Cognitive Sciences, NYU Shanghai, China
Regina M. Carelli, PhD University of North Carolina at Chapel Hill, United States
Andrea A. Chiba, PhD University of California - San Diego, United States
Uraina S. Clark, PhD Icahn School of Medicine at Mount Sinai, United States
Lique M. Coolen, PhD University of Mississippi Medical Center, United States
Roshan Cools, PhD Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Department of Psychiatry, the Netherlands
Alice Cronin-Golomb, PhD Boston University, United States
Nathaniel D. Daw, PhD Princeton University, United States
John F. Disterhoft, PhD Northwestern University, United States
Juan M. Dominguez, PhD University of Texas at Austin, United States
S. Tiffany Donaldson, PhD Honors College and Department of Psychology, Developmental and Brain Sciences, University of Massachusetts Boston, United States
Sarah DuBrow, PhD University of Oregon, Department of Psychology and Institute of Neuroscience, United States
Amelia J. Eisch, PhD University of Pennsylvania Perelman School of Medicine, United States
Michael S. Fanselow, PhD University of California – Los Angeles, United States
Marcelo Febo, PhD University of Florida, United States
Stan B. Floresco, PhD University of British Columbia, Canada
John H. Freeman, PhD University of Iowa, United States
Karyn M. Frick, PhD University of Wisconsin-Milwaukee, United States
Stephen C. Gammie, PhD University of Wisconsin, United States
Erica R. Glasper, PhD The Ohio State Wexler Medical Center, United States
Paul E. Gold, PhD Syracuse University, United States
Katalin M. Gothard, MD, PhD University of Arizona, United States
Thomas J. Gould, PhD Pennsylvania State University, United States
James W. Grau, PhD Texas A&M University, United States
Amy L. Griffin, PhD University of Delaware, United States
Patricia Sue Grigson, PhD Pennsylvania State University, College of Medicine, United States
Stephanie M. Groman, PhD University of Minnesota, United States
Jung-Soo Han, PhD Konkuk University, Republic of Korea
Catherine A. Hartley, PhD Department of Psychology and Center for Neural Science, New York University, United States
Michael E. Hasselmo, PhD Boston University, United States
Fred J. Helmstetter, PhD University of Wisconsin-Milwaukee, United States
J. David Jentsch, PhD Binghamton University, United States
Ni Ji, PhD Chinese Institute for Brain Research, Beijing
Theresa A. Jones, PhD University of Texas at Austin, United States
Janice M. Juraska, PhD University of Illinois—Champaign/Urbana, United States
Thorsten Kahnt, PhD NIDA-IRP, United States
Donald B. Katz, PhD Brandeis University, United States
Martin Kavaliers, PhD University of Western Ontario, Canada
Leslie M. Kay, PhD University of Chicago, United States
Brock Kirwan, PhD Brigham Young University, United States
Bryan E. Kolb, PhD University of Lethbridge, Canada
Kevin S. LaBar, PhD Duke University, United States
Matthew Lattal, PhD Oregon Health & Science University, United States
Mark Laubach, PhD American University, United States
Michael A. Leon, PhD University of California—Irvine, United States
Christiane Linster, PhD Cornell University, United States
Joseph S. Lonstein, PhD Michigan State University, United States
Carmen S. Maldonado-Vlaar, PhD University of Puerto Rico, United States
Ludise Malkova, PhD Georgetown University Medical Center, United States
Nathan J. Marchant, PhD VU University Medical Center, The Netherlands
Michael A. McDannald, PhD Boston College, United States
Jill A. McGaughy, PhD University of New Hampshire, United States
Gavin P. McNally, PhD University of New South Wales, Australia
Sheri J. Y. Mizumori, PhD University of Washington, United States
Lisa M. Monteggia, PhD Vanderbilt University, United States
Jonathan D. Morrow, MD, PhD University of Michigan, United States
Mark B. Moss, PhD Boston University School of Medicine, United States
T. Celeste Napier, PhD Rush University Medical Center, United States
Nandakumar S. Narayanan, MD, PhD University of Iowa, United States
Randy J. Nelson, PhD West Virginia University, United States
Sean B. Ostlund, PhD University of California, Irvine, United States
Jelena Radulovic, MD, PhD Northwestern University, United States
Michael E. Ragozzino, PhD University of Illinois at Chicago, United States
Steve Ramirez, PhD Boston University Department of Psychological and Brain Sciences, Department of Biomedical Engineering, Center for Systems Neuroscience, Neurophotonics Center, United States
Catharine H. Rankin, PhD University of British Columbia, Canada
Peter R. Rapp, PhD National Institute on Aging, United States
Stephen Reilly, PhD University of Illinois at Chicago, United States
Rick Richardson, PhD University of New South Wales, Australia
Trevor W. Robbins, PhD University of Cambridge, United Kingdom
Angela C. Roberts, PhD Department of Physiology, Development and Neuroscience, University of Cambridge, United Kingdom
Mike J. F. Robinson, PhD Concordia University, Canada
Peter H. Rudebeck, DPhil Icahn School of Medicine at Mount Sinai, United States
Federico Sanabria, PhD Arizona State University, United States
Martin F. Sarter, PhD University of Michigan, United States
Bernard G. Schreurs, PhD West Virginia University, United States
Barry Setlow, PhD University of Florida, United States
Matthew L. Shapiro, PhD Albany Medical School, United States
David M. Smith, PhD Cornell University, United States
Sade Spencer, PhD University of Minnesota, United States
Mark E. Stanton, PhD University of Delaware, United States
Chantal E. Stern, DPhil Boston University, United States
Neal R. Swerdlow, MD, PhD University of California - San Diego, United States
Susan E. Swithers, PhD Purdue University, United States
Michael A. Taffe, PhD University of California, San Diego, United States
Jeffrey S. Taube, PhD Dartmouth College, United States
Victoria L. Templer, PhD Providence College, United States
Kate M. Wassum, PhD UCLA, United States
Mascha van ’t Wout, PhD Brown University, United States
Tara L. White, PhD Brown University, United States
Ingo Willuhn, PhD Netherlands Institute for Neuroscience, Royal Netherlands Academy of Arts and Sciences & Department of Psychiatry, Amsterdam University Medical Centers, University of Amsterdam, the Netherlands
Robert C. Wilson, PhD University of Arizona, United States
Tianming Yang, PhD Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, China
Michael A. Yassa, PhD University of California, Irvine, United States
Jingfeng Zhou, PhD Chinese Institute for Brain Research, Beijing, China
Abstracting and indexing services providing coverage of Behavioral Neuroscience ®
- Abstracts in Anthropology
- Academic OneFile
- Academic Search Alumni Edition
- Academic Search Complete
- Academic Search Elite
- Academic Search Index
- Academic Search Premier
- Academic Search Research & Development
- AgBiotech News and Information
- Animal Behavior Abstracts
- Animal Breeding Abstracts
- Animal Production Database
- Animal Science Database
- Aquatic Science & Fisheries Abstracts (ASFA)
- Biological & Agricultural Index Plus
- Biological Abstracts
- Biological Sciences
- BIOSIS Previews
- Biotechnology and BioEngineering Abstracts
- CAB Abstracts
- Cabell's Directory of Publishing Opportunities in Psychology
- Chemical Abstracts
- Chemoreception Abstracts
- Current Abstracts
- Current Contents: Life Sciences
- EBSCO MegaFILE
- Embase (Excerpta Medica)
- Engineering Research Database
- Expanded Academic ASAP
- General OneFile
- Global Health
- InfoTrac Custom
- International Index of Music Periodicals (IIMPA)
- Journal Citations Report: Social Sciences Edition
- Nematological Abstracts
- Neuroscience Citation Index
- Neurosciences Abstracts
- NSA Collection
- Nutrition Abstracts and Reviews
- OmniFile Full Text Mega
- Poultry Abstracts
- Professional ProQuest Central
- ProQuest Discovery
- ProQuest Platinum Periodicals
- ProQuest Psychology Journals
- ProQuest Research Library
- ProQuest Social Science Journals
- Psychology Collection
- Review of Aromatic and Medicinal Plants
- Science Citation Index Expanded
- Social Sciences Abstracts
- Social Sciences Citation Index
- Social Sciences Full Text
- Soils and Fertilizers Abstracts
- Sugar Industry Abstracts
- Technology Research Database
- TOC Premier
- Veterinary Science Database
Special issue of the APA journal Behavioral Neuroscience, Vol. 133, No. 3, June 2019. The issue highlights advances in neuroethology based on research presented at the 13th International Congress of Neuroethology and associated satellite symposia in Brisbane, Australia, on July 15–20, 2018.
Special issue of the APA journal Behavioral Neuroscience, Vol. 132, No. 5, October 2018. The articles highlight progress made in understanding the anatomy and function of the retrosplenial cortex in both animals and humans.
Special issue of the APA journal Behavioral Neuroscience, Vol. 130, No. 3, June 2016. The articles advance knowledge about the relation of sleep to cognition, memory, emotional reactivity, and mood, with several of the articles emphasizing the relation between sleep and human clinical conditions.
Special issue of the APA journal Behavioral Neuroscience, Vol. 128, No. 3, June 2014. The papers range from the molecular biology of clock genes to the behavior of free-living animals, and cover a wide variety of species ranging from insects, to rodents, to humans.
Transparency and Openness Promotion
APA endorses the Transparency and Openness Promotion (TOP) Guidelines by a community working group in conjunction with the Center for Open Science ( Nosek et al. 2015 ). The TOP Guidelines cover eight fundamental aspects of research planning and reporting that can be followed by journals and authors at three levels of compliance.
- Level 1: Disclosure—The article must disclose whether or not the materials are posted to a trusted repository.
- Level 2: Requirement—The article must share materials via a trusted repository when legally and ethically permitted (or disclose the legal and/or ethical restriction when not permitted).
- Level 3: Verification—A third party must verify that the standard is met.
Empirical research, including meta-analyses, submitted to Behavioral Neuroscience must, at a minimum, meet Level 1 (Disclosure) for all eight aspects of research planning and reporting. Authors should include a subsection in their methods description titled “Transparency and openness.” This subsection should detail the efforts the authors have made to comply with the Transparency and Openness Promotion (TOP) guidelines.
The list below summarizes the minimal TOP requirements of the journal. Please refer to the Center for Open Science TOP guidelines for details, and contact the editor (Geoffrey Schoenbaum, MD, PhD) with any further questions. APA recommends sharing data, materials, and code via trusted repositories (e.g., APA’s repository on the Open Science Framework (OSF)). Trusted repositories adhere to policies that make data discoverable, accessible, usable, and preserved for the long term. Trusted repositories also assign unique and persistent identifiers.
We encourage investigators to preregister their studies and to share protocols and analysis plans prior to conducting their research. There are many available preregistration forms (e.g., the APA Preregistration for Quantitative Research in Psychology template, ClininalTrials.gov , or other preregistration templates available via OSF ). Completed preregistration forms should be posted on a publicly accessible registry system (e.g., OSF , ClinicalTrials.gov, or other trial registries in the WHO Registry Network).
A list of participating journals is also available from APA.
The following list presents the eight fundamental aspects of research planning and reporting, the TOP level required by Behavioral Neuroscience , and a brief description of the journal's policy.
- Citation: Level 1, Disclosure—All data, program code, and other methods developed by others should be cited in the text and listed in the references section.
- Data Transparency: Level 1, Disclosure—Article states whether the raw and/or processed data on which study conclusions are based are posted to a trusted repository and, if so, how to access them.
- Analytic Methods (Code) Transparency: Level 1, Disclosure—Article states whether computer code or syntax needed to reproduce analyses in an article is available and, if so, where to access it.
- Research Materials Transparency: Level 1, Disclosure—Article states whether materials described in the method section are available and, if so, where to access them.
- Design and Analysis Transparency (Reporting Standards): Level 1, Disclosure—The journal encourages the use of APA Style Journal Article Reporting Standards (JARS-Quant, JARS-Qual, and/or MARS).
- Study Preregistration: Level 1, Disclosure—Article states whether the study design and (if applicable) hypotheses of any of the work reported was preregistered and, if so, how to access it. Authors may submit a masked copy via stable link or supplemental material or may provide a link after acceptance.
- Analysis Plan Preregistration: Level 1, Disclosure—Article states whether any of the work reported preregistered an analysis plan and, if so, how to access it. Authors may submit a masked copy via stable link or supplemental material or may provide a link after acceptance.
- Replication: Level 3, Verification—The journal publishes replications and Registered Reports.
Other open science initiatives
- Open Science badges: Not offered
- Public significance statements: Not offered
- Author contribution statements using CRediT: Required
- Registered Reports: Published
- Replications: Published
Explore open science at APA .
Journal equity, diversity, and inclusion statement
As the editorial team of Behavioral Neuroscience , we are strongly committed to equity, diversity, and inclusion (EDI) in the operation of the journal. We hope to show this commitment in how we operate at all levels, and we are open to suggestions for improving this aspect of our management. The following are several concrete commitments we have made to address EDI issues at Behavioral Neuroscience :
- We are committed to recruiting a diverse team to handle manuscripts, in our board of consulting editors and in the reviewers we choose. Our intention is that these groups represent our field both scientifically as well as in terms of gender, race, ethnicity, orientation, disability status, geographical location, and career stage.
- We encourage our authors to consider diversity in suggesting reviewers and to include in their manuscripts an inclusive list of references that recognizes the scholarship of women, people of color, and those from different locations and institutions.
- We work to solicit papers that reflect the diversity in our field. To that end, we offer the option of registered reports and the authors’ choice of a double-blind peer review (where both the author and reviewer identities are masked) to help address concerns of bias in the reviewing process. To the extent possible, decisions to triage papers without review are made based on an initial reading of the abstract and paper, prior to consideration of any materials that include the authors and their affiliations.
Inclusive study designs
- Registered Reports
Definitions and further details on inclusive study designs are available on the Journals EDI homepage .
Inclusive reporting standards
- Bias-free language and community-driven language guidelines (required)
- Author contribution roles using CRediT (required)
- Data sharing and data availability statements (required)
More information on this journal’s reporting standards is listed under the submission guidelines tab .
Other EDI offerings
Orcid reviewer recognition.
Open Research and Contributor ID (ORCID) Reviewer Recognition provides a visible and verifiable way for journals to publicly credit reviewers without compromising the confidentiality of the peer-review process. This journal has implemented the ORCID Reviewer Recognition feature in Editorial Manager, meaning that reviewers can be recognized for their contributions to the peer-review process.
Masked peer review
This journal offers masked peer review (where both the authors’ and reviewers’ identities are not known to the other). Research has shown that masked peer review can help reduce implicit bias against traditionally female names or early-career scientists with smaller publication records (Budden et al., 2008; Darling, 2015).
Behavioral Neuroscience is a member of the Neuroscience Peer Review Consortium
Announcements
- New editor appointed
- Special sections
Editor Spotlight
- Read an interview with Editor Geoffrey Schoenbaum
From APA Journals Article Spotlight ®
- Executive function and emotion processing in attention-deficit / hyperactivity and bipolar disorders
- Cortical activity during REM sleep associated with improved decision-making
Journal Alert
Sign up to receive email alerts on the latest content published.
Welcome! Thank you for subscribing.
Subscriptions and access
- Pricing and individual access
- APA PsycArticles database
Calls for Papers
Access options
- APA publishing resources
- Educators and students
- Editor resource center
APA Publishing Insider
APA Publishing Insider is a free monthly newsletter with tips on APA Style, open science initiatives, active calls for papers, research summaries, and more.
Social media
Contact Journals
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Method matters: an empirical study of impact in cognitive neuroscience
Affiliation.
- 1 University of Pennsylvania, USA. [email protected]
- PMID: 15969904
- DOI: 10.1162/0898929054021139
A major thrust of cognitive neuroscience is the elucidation of structure-function relationships in the human brain. Over the last several years, functional neuroimaging has risen in prominence relative to the lesion studies that formed the historical core of work in this field. These two methods have different strengths and weaknesses. Among these is a crucial difference in the nature of evidence each can provide. Lesion studies can provide evidence for necessity claims, whereas functional neuroimaging studies do not. We hypothesized that lesion studies will continue to have greater scientific impact even as the relative proportion of such studies in the cognitive neuroscience literature declines. Using methods drawn from systematic literature review, we identified a set of original cognitive neuroscience articles that employed either functional imaging or lesion techniques, published at one of two time points in the 1990s, and assessed the effect of the method used on each article's impact across the decade. Functional neuroimaging studies were cited three times more often than lesion studies throughout the time span we examined. This effect was in large part due to differences in the influence of the journals publishing the two methods; functional neuroimaging studies appeared disproportionately more often in higher impact journals. There were also differences in the degree to which articles using one method cited articles using the other method. Functional neuroimaging articles were less likely to include such cross-method citations.
PubMed Disclaimer
- A madness to the methods in cognitive neuroscience? Chatterjee A. Chatterjee A. J Cogn Neurosci. 2005 Jun;17(6):847-9. doi: 10.1162/0898929054021085. J Cogn Neurosci. 2005. PMID: 15969903 No abstract available.
Publication types
- Search in MeSH
Related information
- Cited in Books
Grants and funding
- F32 AG22806/AG/NIA NIH HHS/United States
- R01 DC04817/DC/NIDCD NIH HHS/United States
- R01 MH60414/MH/NIMH NIH HHS/United States
- R21 NS045074/NS/NINDS NIH HHS/United States
- T32 NS07413/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full text sources.
- Silverchair Information Systems

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
What is neurophilosophy: Do we need a non-reductive form?
- Open access
- Published: 17 October 2020
- Volume 199 , pages 2701–2725, ( 2021 )
Cite this article
You have full access to this open access article

- Philipp Klar ORCID: orcid.org/0000-0003-1702-8029 1 , 2
11k Accesses
4 Citations
2 Altmetric
Explore all metrics
Neurophilosophy is a controversial scientific discipline lacking a broadly accepted definition and especially a well-elaborated methodology. Views about what neurophilosophy entails and how it can combine neuroscience with philosophy, as in their branches (e.g. metaphysics, epistemology, ethics) and methodologies, diverge widely. This article, first of all, presents a brief insight into the naturalization of philosophy regarding neurophilosophy and three resulting distinguishable forms of how neuroscience and philosophy may or may not be connected in part 1, namely reductive neurophilosophy, the parallelism between neuroscience and philosophy which keeps both disciplines rather strictly separated and lastly, non-reductive neurophilosophy which aims for a bidirectional connection of both disciplines. Part 2 presents a paradigmatic example of how these three forms of neuroscience and philosophy approach the problem of self, mainly concerning its ontological status (existence and reality). This allows me to compare all three neurophilosophical approaches with each other and to highlight the benefits of a non-reductive form of neurophilosophy. I conclude that especially non-reductive neurophilosophy can give full justice to the complementary position of neurophilosophy right at the intersection between neuroscience, philosophy, and psychology.
Similar content being viewed by others

- Neurophilosophy

Postscript: Neurophilosophy, Darwinian Naturalism, and Subjectivity

Neuropragmatism and the Reconstruction of Scientific and Humanistic Worldviews
Avoid common mistakes on your manuscript.
1 Introduction and an overview of the distinct forms of neurophilosophy
Neurophilosophy is a scientific discipline connecting neuroscience and philosophy and that intends to research former genuine philosophical topics, such as the ancient and major topics of consciousness, the self, and free will. These philosophical topics faced the enormous development of imaging-methods (neuroimaging) in the last past 35–40 years, hence resulting in an increasing interest of neuroscience in them which allows different kinds of interaction between both disciplines today. To chronologically introduce the development of each form of neurophilosophy, a threefold differentiation between reductive neurophilosophy, parallelism between neuroscience and philosophy, and non-reductive neurophilosophy will be defined in the perspective of the following main principles concerning the possible connection of neuroscience with philosophy:
Naturalization of philosophy;
Branches of philosophy and linkage to empirical sciences;
Philosophical and empirical methodology; and
Stance towards the brain and mind or consciousness.
Since naturalization of philosophy is a prerequisite for the connection of neuroscience with philosophy and therefore neurophilosophy, it shall be highlighted in the following part 1.1, while the threefold differentiation between reductive neurophilosophy (part 1.2), parallelism between neuroscience and philosophy (part 1.3), and non-reductive neurophilosophy (part 1.4) follow subsequently.
1.1 Naturalization of philosophy as a prerequisite for neurophilosophy
Naturalization of philosophy stands as a prerequisite to enable the connection of empirical science, namely neuroscience, with philosophy. In a first instance, the differentiation between empirical science and philosophy in a classical sense is necessary so that consequently it becomes more comprehensible how the strict classical dissociation of both disciplines is in principle dissolvable via the naturalization of philosophy.
Philosophy, in a classical sense, qualifies as an a priori analytic science that mainly operates on the rational-argumentative basis of linguistic concepts which are primarily focused on logical conditions within imaginable possible worlds. The main branches of philosophy among others are metaphysics, epistemology, ethics, and phenomenology. Linguistic concepts are used to explain philosophical topics, problems, and the therewith connected approach. On the other hand, empirical sciences are classified as a posteriori and synthetic; that is, they are based on the observational-experimental methodology and investigation in third-person-perspective. Footnote 1 This scientific methodology of empirical sciences focuses primarily on processes and mechanisms that underly phenomena within the natural and real world. In other words, it focuses more on the how in the sense of functionality instead of on the what in the sense of ontology (existence and reality) as usually pursued in philosophy. The observational-experimental investigation may then provide certain possible inferences to the underlying processes and mechanisms of phenomena. Altogether in a classical perspective, empirical sciences and philosophy differ completely from each other as diametral confronted extremes regarding their branches and methodologies. As long as this categorical distinction is maintained, neurophilosophy does not become an option.
However, in the middle of the twentieth century, American philosopher Willard van Orman Quine (1908–2000) elaborated a possible naturalization of philosophy and stated that the principal distinction between empirical sciences and philosophy is not reasonable (Quine 1951 , 1969 ). Quine ( 1951 , 1969 ) further argued that philosophical linguistic concepts and the rational-argumentative methodology within logical conditions and reasoning can be seen as an abstraction of scientific results and its observational-experimental methodology. This allows a mutual quantitative continuum between empirical sciences and philosophy to open up, and in which genuine empirical sciences and genuine philosophy are represented solely by their respective endpoint on that continuum (Fig. 1 ). Based on this reasoning, Quine ( 1951 , 1969 ) argued on the following three fundamental levels:
There is a continuum between analytic and synthetic sentences;
There is a continuum between a priori and a posteriori reasoned knowledge; and
Consequently, a mutual continuum exists between empirical sciences and philosophy.
Following the introduction of the naturalization of philosophy, the categorical distinction between analytic and synthetic sentences as previously defined by Kant ( 1781 /1996), was replaced. Footnote 2 Rather than a categorial distinction between analytic and synthetic sentences, Quine ( 1951 , 1969 ) preferred a quantitative continuum between both, which he explained in detail within his writings.
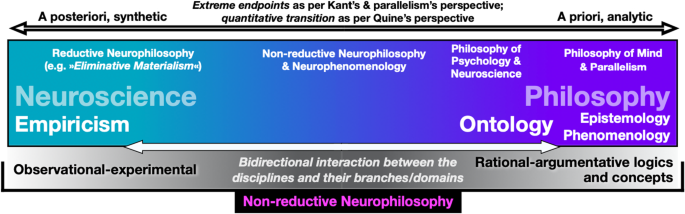
Empirical sciences and naturalized philosophy are located on the same mutual continuum . The branches/domains and methodologies (observational-experimental vs. rational-argumentative) of both disciplines are thus faced with a possible union. Naturalized philosophy consequently allows for an interdisciplinary and systematical bidirectional interaction between neuroscience and philosophy, as represented by non-reductive neurophilosophy (part 1.4), to become possible
Naturalization of philosophy can be achieved in two different major ways that touch the implementation of neurophilosophy: (1) replacement naturalism and (2) cooperative naturalism . In replacement naturalism, the empirical observational-experimental methodology strongly dominates or even overrules the conceptual-logical methodology of classic philosophy. This gives rise to reductive neurophilosophy (part 1.2), which is common in Anglo-American countries today, as represented by the Churchlands ( 1981 , 1985 , 1989 , 2013b ) among others. Therefore, philosophy, including its branches and methodology, is ultimately reduced to the empirical realm. Cooperative naturalism, on the other hand, avoids this reduction by allowing a bidirectional interaction between the branches and methodologies of empirical sciences and philosophy, which results in a non-reductive neurophilosophy, and thus a truthful interdisciplinary interaction. Hence cooperative naturalism is a necessary prerequisite for non-reductive neurophilosophy (part 1.4), which may allow for a more comprehensive perspective on the concrete phenomena of investigation. The naturalization of philosophy, including the main principles concerning the possible connections between neuroscience and philosophy, is summarized in Table 1 . These main principles will be discussed subsequently within parts 1.2–1.4 concerning the threefold differentiation of possible connections between neuroscience and philosophy.
1.2 Reductive neurophilosophy
The term neurophilosophy was explicitly shaped for the very first time in the year 1986 by Canadian philosopher Patricia S. Churchland (1943–) in her eponymous book» Neurophilosophy « (Churchland 1989 ). Together with her husband Paul Churchland (1942–), who is also a philosopher, she developed a strong reductive neurophilosophy (» Eliminative materialism «) which states that philosophy, including its branches consisting of metaphysics, ontology, epistemology, ethics, phenomenology, etc., is ultimately reduced to the observational-experimental methodology and empirical research of neuroscience, and that philosophy, as well as folk psychology, will be reduced more and more into neuroscience as the latter advances in its scientific research (Bickle 2006 ; Churchland 1981 , 2013a ). Reductive neurophilosophy is especially common in the Anglo-American countries based on their understanding of neurophilosophy as a discipline today (Bickle 2003 , 2009 , 2019 ).
The abstract principles and practical methodological approach of the Churchlands ( 1989 , 2002a , b ) are heavily shaped by the application of the observational-experimental methodology within the empirical level of neuroscience to former genuine philosophical topics, and consequently reflect the replacement naturalism form of the naturalization of philosophy. This approach ultimately concludes that this form of neurophilosophy can, therefore, be considered as reductive, as similarly to neuroscience, it also takes a brain-reductive stance : the person and her or his (self-)conscious phenomenal first-person-perspective’s experience with its aspects like point-of-view, intentionality, sense-of-self, sense-of-agency, that is especially phenomenology itself, are reduced to the neuronal activity of the brain. The mind or consciousness does not correspond to the neuronal level of the brain, but instead, it is considered to be reducible to it. Moreover, according to Churchland’s ( 2002a ) metaphysics of eliminative materialism, the mind (e.g. consciousness) does not have an ontological status (existence and reality). Instead, consciousness, the self, and mental features are scientifically eliminated and are replaced by a complete focus on the neuronal level of empirical neuroscience in favor of an isolated observation of only the brain. This brain-reductive stance is also well reflected in the denial of the existence and reality of the self as stated by the German philosopher Thomas Metzinger ( 2004 ) in his book» Being No One «, just as Patricia Churchland ( 2013b ) considers the self illusionary and to be nothing but the brain.
Therefore, reductive neurophilosophy applies a unidirectional inference from mere empirical data and findings to philosophical concepts which concludes that the empirical level of neuroscience strongly overrules philosophical branches and their respective concepts, since concepts are unidirectionally created and adapted to empirical data and interpretations. This unidirectional inference shall be demonstrated with an example: since classic philosophical concepts of the self, such as a substance in form of a mental entity (Descartes 1641 /1993), cannot be found as a physical entity within the brain, philosophers like Metzinger ( 2004 ) and Churchland ( 2013b ) consequently consider the self to be non-existent. In other terms, the self has no ontological status. According to the two philosophers, the self does not exist because inside the mere empirical realm of neuroscience , the philosophical concept of a self as a substance is not directly in itself findable, and hence its ontological status needs to be eliminated. Footnote 3 Instead of adapting or creating entirely new concepts of the self in accordance and matching correspondence with empirical data, reductive neurophilosophy primarily infers from sole empirical data and facts to the existence and reality of philosophical concepts. The foundation of concepts thus exclusively relies on their empirically neuroscientific plausibility within natural conditions, while conceptual plausibility concerning logical conditions is either neglected or even ignored. Finally, reductive neurophilosophy also dismisses philosophically generated concepts as input for scientific investigations and research; by contrast, it creates concepts unidirectionally as mere outputs from empirical data and facts, so that philosophical concepts are left as entire empirical induced outputs .
Additionally, today’s philosophy of neuroscience can be subsumed under the umbrella of reductive neurophilosophy as it discusses principles and methodological aspects of plain neuroscience (Bechtel et al. 2001 ), similarly as the philosophy of psychology critically discusses the methodology of psychology (Bermúdez 2005 ). The reductive form of neurophilosophy as discussed above is also subsumed under the broader umbrella of the philosophy of neuroscience by American philosopher John Bickle ( 2019 ). According to Bickle ( 2019 ), in contrast to the philosophy of neuroscience, neurophilosophy has “fallen” from its initial vision and aims of revolutionizing philosophy by explicitly introducing neuroscientific research and its implications to philosophy as a discipline. However, famous approaches that avoid a rather complete reduction of philosophical concepts, branches, and its methodology, such as neurophenomenology or non-reductive neurophilosophy (part 1.4), are neither considered nor even mentioned within Bickle’s criticism. The formerly elucidated reductive notion of neurophilosophy is now so prominent in the Anglo-American countries, that other forms of neurophilosophy which avoid today’s reductionism seem to be non-existent among the corresponding academical philosophy of science’s circles.
Furthermore, neurophilosophy has to be separated from the philosophy of mind, which especially asks about the existence and reality of the mind and the latter’s relationship to the matter. The topics of philosophy of mind are rather basically of an analytic-metaphysical nature, i.e., they involve mainly the mind–body problem and the consequences which result in different positions regarding the latter (Brüntrup 2018 ; Kutschera 2006 , 2009 ; Newen 2013 ). After introducing the widely known reductive neurophilosophy, a strict parallelism between neuroscience and philosophy, which denies any form of bidirectional interaction between both, hence denying the possibility of neurophilosophy at all, will be presented.
1.3 Parallelism between neuroscience and philosophy
Besides the possible forms of neurophilosophy, there is also the conviction that strict parallelism between the empirical realm of neuroscience and philosophy is required (Bennett and Hacker 2003 ). Maxwell Bennett (1939–), an Australian neuroscientist, and Peter Hacker (1939–), an English philosopher (Philosophy of Language, Philosophy of Mind, and an expert for the philosophy of Wittgenstein), published the book» Philosophical Foundations of Neuroscience « together in 2003. The book covers comprehensive analysis and criticism of cognitive neuroscience, with particular reference to how cognitive neuroscientists accidentally mislead themselves. First of all, Bennett and Hacker ( 2003 ) argue that a wrong and confusing usage of terms and concepts concerning empirical investigation is very common especially in cognitive neuroscience. Furthermore, there are conceptually confused interpretations of findings since a conceptual-theoretical confused input will lead to even more confusing investigations and results (Bennett and Hacker 2003 ). For example, Bennett and Hacker ( 2003 ) state that this was the case for the first generation of modern neuroscientists in the twentieth century, when they either explicitly argued in favor of ontological substance dualism between the brain and mind, like neurophysiologist Charles S. Sherrington (1857–1952) and neurosurgeon Wilder Penfield (1891–1976), or they demonstrated implicitly an unintentionally induced substance dualism by conceptual confusion, as for example by neuroscientist Edgar D. Adrian (1889–1977).
According to Bennett and Hacker ( 2003 ), a far more subtle yet erroneous neo-Cartesianism lives on in cognitive neuroscience today. In other terms, (self-)consciousness, free will, and mental features or psychological attributes like attention, memory, knowledge or sense-of-agency, are considered as exclusive brain functions in form of distinct entities or processes, which in turn shall be reducible to the brain’s neuronal activity in the neuroscientist’s perspective. Bennett and Hacker ( 2003 ) instead argue that the mind in general and the distinct mental features in particular, are nothing but capabilities and behavioral executions of the organism as a whole and not of the brain. The attribution of mental features and psychological attributes to the brain would represent the especially pointed out mereological fallacy , which is a part-whole confusion. The reification of the above capabilities, i.e., as mind or mental features, is simply wrong and instead, the latter has to be seen merely as a linguistic expression of these capabilities .
According to Bennett and Hacker ( 2003 ), it is especially and fundamentally important to thereby distinguish between scientific empirical and conceptual questions . Concerning neuroscience and philosophy, this means that neuroscience is constrained to research the brain in a strict empirical manner throughout empirical scientific questions, while philosophy and its respective branches focus on genuine conceptual questions concerning the mind, e.g. (self-)consciousness, mental features or psychological attributes. Consequently, Bennett and Hacker ( 2003 ) refrain from merging a bidirectional interaction between neuroscience and philosophy since neuroscience must concentrate solely on the empirical realm and its observational-experimental methodology, while philosophy should focus exclusively on the definition of concepts, terms, and categories including their elaboration in distance to the empirical realm. Hence, any form of neurophilosophy is simply not an option. On the contrary, Bennett and Hacker ( 2003 ) argue in favor of a classical branch and methodological monism regarding each discipline. Furthermore, philosophy is not able to generate real new knowledge as it is the case in empirical sciences, but instead, philosophy is principally and most widely a linguistic-logic based analytic science which allows for precise verification and reflection concerning human knowledge; e.g. what knowledge was obtained through empirical sciences, after confused concepts, terms and categories were revealed and revised (Bennett and Hacker 2003 ; Hacker 2010 ).
However, philosophy is nevertheless allowed to at least suggest exactly defined concepts and terms, which are not to be confused with possible topics of investigation for the empirical research in neuroscience, as well as to provide interpretations and verifications concerning the question if neuroscientists interpret their findings and empirical data wrong, particularly when applying these empirical findings to philosophical concepts. An example is linguistic confusions and conceptual fallacies, like the already mentioned mereological fallacy that was widely pointed out to be very present in today’s neuroscience, precisely when psychological predicates are attributed to the brain, instead of to the organism as a whole. This is the limit that philosophy can offer to neuroscience (Bennett and Hacker 2003 ). On the contrary, real and genuine philosophical problems and hence therewith connected topics, e.g. in connection to neuroscience, do not exist according to Bennett and Hacker ( 2003 ) but are especially induced by linguistic confusions. According to Hacker ( 2010 ), this is also particularly the case for consciousness studies.
This strict separation of neuroscience and philosophy is also partially present in today’s philosophy of mind whenever the focus of the investigation is extensively laid on genuine philosophical branches like metaphysics, while empirical data and findings are not significantly included. This is especially the case when philosophical investigations focus on concepts concerning conceivable possible worlds and their inherent logical plausibility (instead of their empirical plausibility of natural conditions regarding the real and natural world). This is well reflected by the common presupposition of a metaphysical mind, which consequently leads to the question of how the mind is related to the matter, hence maintaining the mind–body problem. Instead of challenging the question if a metaphysical mind exists at all, philosophy of mind typically takes the mind for granted and then starts its investigation upon it. In summary, it is now possible that philosophy of mind is partially subsumed under the umbrella of parallelism between neuroscience and philosophy, since the philosophy of mind can be considered more on the side of genuine philosophy in comparison with neurophilosophy when considering a quantitative continuum (Fig. 1 ) in Quine’s perspective ( 1951 , 1969 ), between empirical sciences and philosophy.
1.4 Non-reductive neurophilosophy
While Churchland ( 1989 ) introduced the term neurophilosophy with a reductive-eliminative imprint, she was not the first person in the history of philosophy to practice neurophilosophy. Non-reductive neurophilosophy originates further back in the nineteenth century. In the year 1818, 29 years old Arthur Schopenhauer (1788–1860) finished his main work» Die Welt als Wille und Vorstellung « (The World as Will and Representation) which was published in 1819, and in which he took the vantage point of Kant’s philosophy by interpreting his a priori categories and forms of intuition as brain functions; i.e., not a mental entity like the mind shall be responsible for the subjective-phenomenal experience of the first-person-perspective, but the brain (Schopenhauer 1819 /2011). Using the above, Schopenhauer introduced the brain explicit into the philosophical investigation which led to a brain-based approach instead of a mind-based approach (as it is still common in genuine philosophy today) to neurophilosophy. Hence, he can be considered to be the very first neurophilosopher ever (Northoff 2018a ; Göhmann 2018 ). It took over a hundred years more, particularly until the middle of the twentieth century, before non-reductive neurophilosophy was realized implicitly once again. The French phenomenological philosopher Maurice Merleau-Ponty (1908–1961) can also be considered as an early neurophilosopher who likewise introduced the brain to philosophy whilst connecting the brain and the body (accounting for embodiment) to perception and phenomenology. Based on their respective arguments and approaches, it can be implicitly considered that both Schopenhauer and Merleau-Ponty were against a reductive-eliminative approach as put forward by the Churchlands (Merleau-Ponty 1945 /2013; Schopenhauer 1819 /2011). Subsequently, in the second half of the twentieth century, more precisely in the year 1977, Australian neuroscientist John C. Eccles (1903–1997) and Austrian-British philosopher Karl R. Popper (1902–1994) came up with a different approach in their famous book» The Self and Its Brain « (Popper and Eccles 1985 ). Popper and Eccles ( 1985 ) argued in favor of ontological substance dualism between the mind and brain, more specifically trialism, whose explanation is beyond the aim of this article. Footnote 4
As a first modern approach to combine neuroscience with philosophy in bidirectional interaction using the heavily increasing development of neuroscience, Chilean neuroscientist and philosopher Francisco Varela (1946–2001) founded» Neurophenomenology « in the 1990s, which can be considered as non-reductive neurophilosophy. Neurophenomenology presents a methodological strategy that takes a vantage point from phenomenology, that is the conscious experience of the first-person-perspective, to especially research consciousness and its connection to the neuronal level of the third-person-perspective of the observational-experimental empirical research of neuroscience (Khachouf et al. 2013 ; Lutz and Thompson 2003 ; Varela 1996 ). Neurophenomenology by Varela ( 1996 ) seriously considers subjective-phenomenal experience, for example, shaped by the aspects of intentionality, self, point-of-view, and sense-of-self/agency, as non-illusionary and real so that phenomenology is not considered to be simply reducible to neuronal activity in the brain. Varela ( 1996 ) especially contemplated embodiment regarding consciousness. In embodiment, the brain’s sensorimotor functions in direct connection to the body, and its linkage to the environment is viewed as a major constituting factor for consciousness. Furthermore, at the end of the 1990s, more precisely in the year 1998, the investigation into the topic of free will by German physician and philosopher Henrik Walter (1962–) in his book» Neurophilosophy of Free Will « (Walter 1998 /2009) can be emphasized as a truthful neurophilosophical approach towards an original philosophical topic.
Another modern and more advanced approach for a bidirectional interaction between neuroscience and philosophy, consequently leading to non-reductive neurophilosophy which neither aims for a reductionist engulf of philosophy to neuroscience nor aims for an absolute distinction without interaction between both disciplines, stems from the German physician (psychiatrist), neuroscientist and philosopher Georg Northoff (1963–). Northoff ( 2012 ) points out that the brain is undisciplined , i.e. the borders between the distinctive scientific disciplines of philosophy, neuroscience, psychology, psychiatry, etc. are ultimately artifacts of the human mind that shall be overcome by interdisciplinary scientific research.
Considering the four main principles relevant to the connection of neuroscience with philosophy as listed in part 1 and Table 1 , non-reductive neurophilosophy, first of all, represents the cooperative naturalism type of the naturalization of philosophy; i.e., philosophical branches and their respective concepts are not reduced to the empirical realm of neuroscience. Northoff’s ( 2004 , 2014a , b , c , 2018b , 2019a ) approach applies a bidirectional connection of both sciences. Philosophical concepts require empirical evidence; i.e., concepts need to be established on the empirical level within natural conditions. Their empirical plausibility, as Northoff ( 2004 , 2014a ) terms it, is primarily weighted over their mere logical plausibility within the borders of genuine philosophy and its logical conditions concerning conceivable possible words. In conclusion, a domain and methodological pluralism (Northoff 2014a ) becomes possible and hence introduces truthful neurophilosophical investigations.
Referring to the bidirectional interaction between neuroscience and philosophy, concept-fact iterativity (Northoff 2014a ) stands out as the main principle of non-reductive neurophilosophy which shall now be presented and further elaborated. As described above in part 1.2, reductive neurophilosophy unidirectionally infers concepts solely from empirical data and evidence as mere output while non-reductive neurophilosophy starts the investigation of a certain topic with its prior philosophical concept. First of all, philosophical concepts offer an input for empirical research which may be followed by empirically plausible modification of a priori established philosophical concepts, while on the other side there is also the possibility to put the results of empirically conceptualized and operationalized concepts as well as their resulting neurophilosophical investigation back as output into philosophy to evaluate their conceptual plausibility in a second step. Proceeding from this empirical-theoretical interaction, a modified neurophilosophical investigation may follow so that concepts and their bidirectional connected empirical facts and modification according to the latter pass through the research-loop of concept-fact iterativity, thus allowing for a converging interdisciplinary approach to reality, as the empirically modified concepts are in return reviewed for their logical plausibility as well.
Northoff ( 2014a ) mentions that in a genuine philosophical perspective the principle of concept-fact iterativity may reflect a category error. According to classical philosophy, empirical facts cannot be connected with logical argumentation, and respectively, natural and logical conditions require a strict separation. However, neurophilosophy primarily strives for empirical plausibility of concepts, which is then connected with logical plausibility. According to Northoff ( 2014a ), while empirical plausibility is valued to be fundamentally important, logical plausibility is not neglected. However, at the same time, it is not possible to infer unidirectionally from mere empirical data and facts to ontological postulations. Such unidirectional inferences would correspond to an empirical-ontological fallacy as Kant ( 1781 /1996) pointed out concerning British physician and philosopher John Locke ( 1690 /1996). Instead, a matching-process between the empirical and philosophical realm, particularly between empirical facts and corresponding ontological assumptions, is required. Concept-fact iterativity thus reflects a principle of branch pluralism between empirical sciences and philosophy: the branches of metaphysics, epistemology, ethics, etc. are systematically connected with empirical facts, instead of merely investigating into either only empirical or logical plausibility.
Concerning the brain, non-reductive neurophilosophy takes a brain-based stance: even though consciousness, the self, and mental features are based on the brain, the latter is only a necessity but is not a sufficient condition for these. Taking the temporo-spatial theory of consciousness (TTC) (Northoff 2013a , 2014b , c , 2016a , b , 2018b ; Northoff and Huang 2017 ) as an example, consciousness, the self, and mental features are based on a relational structure between the brain, body, and environment conceptualized as empirical-ontological » World-brain relation « which entails embodiment and embeddedness. Without going into the details of the empirical and philosophical aspects of this theory, it ought to be mentioned that consciousness and the self are here considered to have an ontological status. They are existent and real but they correspond neither to a physical entity nor to a mental entity as it is the case in common property-based ontologies. Instead, consciousness and the self, including mental features, are fundamentally based on empirical-ontological relations between the brain, body, and world, hence forming a balanced structure, which is then for example altered in neuropsychiatric disorders such as depression, schizophrenia or mania. These neuropsychiatric disorders are situated on the more extreme ends of a hybrid relational continuum, than being located in the more healthy and functioning centered areas (Northoff 2014c , 2016a , b , 2018b ; Northoff and Tumati 2019 ).
2 Forms of neuroscience and philosophy: distinct approaches to the topic of the self as a paradigmatic presentation
After briefly introducing three forms of how or how not to connect neuroscience and philosophy in part 1, it is now possible to paradigmatically conceptualize and operationalize their respective approaches to the original and formerly genuine philosophical topic of the self regarding their presented main principles (Table 1 ). These divergent methodologies consequently entail different notions and arising concepts of the self to both the empirical and/or the philosophical realm.
Philosophy discusses the topic of the self, e.g. regarding its existence and reality, for centuries. It is this very ontological status of the self, more precisely the question if the self is real at all, which represents the hereby chosen example of how forms of neuroscience and philosophy practically differ in their approaches to genuine philosophical concepts when facing empirical sciences. The sequence of these presentational approaches is equal to part 1: reductive neurophilosophy’s approach to the self sets the beginning (2.1); which is then followed by the parallelism between neuroscience and philosophy (2.2); and finally, non-reductive neurophilosophy concludes the paradigmatically approaches (2.3).
2.1 Reductive neurophilosophy and its approach to the self
Nobody ever was or had a self. All that ever existed were conscious self-models that could not be recognized as models. […] subjective experience of being someone emerges if a conscious information-processing system operates under a transparent self-model (Metzinger 2004 , p.1).
As elaborated in part 1, one main principle of reductive neurophilosophy is the reduction of philosophical concepts, which arise from the rational-argumentative method of analytic reasoning within logical conditions, to the observational-experimental methodology of empirical neuroscience. Already at the onset of the investigation, reductive neurophilosophy is defined by a specific methodological step, particularly by taking a vantage point from within the empirical realm of neuroscience (Churchland 1989 , 2002a ). More precisely, none of the many established philosophical concepts of the self is chosen as heuristical input, instead, the investigation starts with a specific and only empirical phenomenon. For example, abnormalities of (self-)consciousness in the neuropsychiatric disorders of depression and schizophrenia, that is their altered or diminished first-person-perspective’s subjective sense-of-self, may serve as a starting point to investigate into the concept in question. Since a genuine philosophical concept as input is missing, the investigation into the topic of the self fully shifts from the formerly metaphysical, ontological, epistemological and phenomenological realms to only the empirical realm of neuroscience.
lready with this initiating step, the self is transformed into a matter of empirical research that reflects a rather complete replacement naturalism of the relationship between neuroscience and philosophy, more precisely with a strong focus on general biological functions of the brain’s neuronal activity. Any possible and resulting concept of the self is therefore unidirectionally inferred from plain empirical facts and data , which are obtained by the observational-experimental methodology of neuroscience. Beyond that, there is no further sufficient philosophical consideration of the empirically induced concept, neither regarding its conceptual-logical plausibility nor regarding its philosophical implications, e.g. concerning its ontological or epistemological significance (Fig. 2 ).
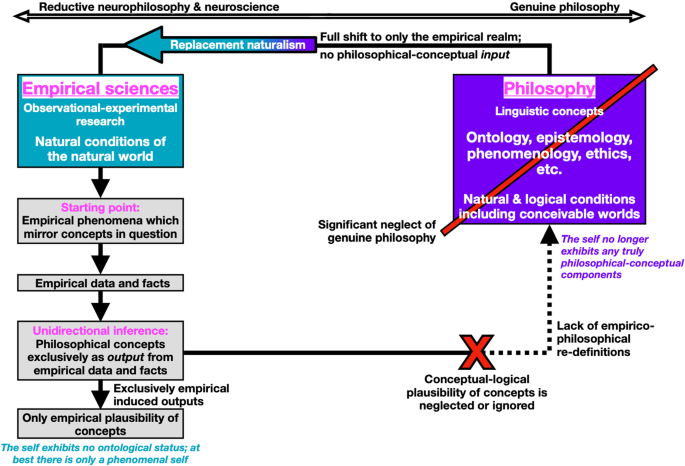
Reductive neurophilosophy shifts its investigation fully into the empirical realm of neuroscience. Firstly, no significant input by philosophy of the concept in question is given for empirical research. Consequently, empirical sciences already represent the starting point of the investigation. Secondly, empirical phenomena that are believed to mirror the philosophical concept in question are investigated by only the observational-experimental methodology of neuroscience. Lastly, philosophical concepts are accordingly and unidirectionally inferred from mere empirical data without further and sufficient philosophical consideration including critical reflection, that is regarding their conceptual-logical plausibility and implications within the branches of ontology, epistemology, phenomenology, etc.
However, scientific research now faces a major “problem” concerning the self: it is not possible to find a distinct self such as an “object”, “core”, substance, or entity within the mere empirical realm of neuroscience. This problem derives from the brain-reductive stance , i.e., reductive neurophilosophy’s complete and isolated focus on only the brain. Within the brain, only its neuronal activity, which is electrical action-potentials and biochemical substances between chemical synapses (neurotransmitters), be it on the molecular, cellular, or the area and network level are detectable. Even though neuroscience offers various empirical concepts and effects such as self-referential reflection (D'Argembeau et al. 2005 ), self-reference effects (SRE) (Klein 2012 ) or self-referential processing (D'Argembeau 2013 ; Liu et al. 2014 ; Knyazev 2013 ) among many others, a self defined as a traditional physical or even a mental entity or property is simply neither detectable nor deductible by using only the empirical methodology when investigating exclusively into the isolated brain and its biological functionality.
Reductive neurophilosophers like Thomas Metzinger ( 1999 , 2004 , 2009 ) correspondingly infer that any former traditional philosophical concept of the self, especially defined as a mental entity, is simply a false inference from the phenomenal experience of the self, i.e., originating from (self-)consciousness as in sense-of-self or sense-of-agency, to the ontological and underlying reality. According to Metzinger’s ( 1999 , 2001 , 2004 , 2009 ) representational theory and naturalization of (self-)consciousness, phenomenal so-called» self-models « developed over phylogenesis and are caused by neuronal activity. Metzinger ( 1999 , 2001 , 2004 , 2009 ) does not deny the immediate phenomenal experience of the self but instead denies any underlying ontological reality or status of the self, more precisely because the only ontological reality is only the brain including its body. The brain causes a self-model that is “transparent” to us–we principally cannot experience the fact that the self is a model within our phenomenological naive realism. Hence, real selves do not exist and this is culminated in eliminating all ontological characterizations of the self and the title of his famous book» Being No One « (Metzinger 2004 ). On the grounds of the above, formerly philosophical concepts of the self are then eliminated in favor of the empirical reality of only the brain (Churchland 2002b , 2013b ; Metzinger 2004 , 2009 ). Consequently, new conceptual definitions of the self (no-self theories) originate solely as output from the mere empirical realm.
However, this reductive approach makes it obvious that concepts still implicitly frame the empirical starting-point into data and facts . Firstly, the self is a philosophical concept which was implicitly given as a conceptual frame; and secondly, only a specific concept of the self was implicitly presupposed and is then rejected on the grounds of the obtained empirical data and facts which are not in accordance to the self as this particular entity. In reductive neurophilosophy’s conclusion, no reality of the self, neither as a mental entity nor as a physical entity or property, truly exists–ontologically conceived, the self is considered to be an illusion.
2.2 Parallelism between neuroscience and philosophy and its approach to the self
It should be evident that the philosophical conception of self-consciousness not only deviates from the common or garden notions but is also a product of philosophical confusions rooted in the notion of apperception transmitted from Locke to Leibniz and from Wolf to Kant (Hacker 2013 , p. 57).
In the perspective of a rather strict parallelism between neuroscience and philosophy, neither replacement nor cooperative naturalism between empirical sciences and philosophy becomes an option in the light of truthful interdisciplinary collaboration. When taking the example of the self into reflection, this clear-cut stance of parallelism is equivalent to the notion that the self, both concerning its ontological status as well as towards its phenomenological aspects, is only a philosophical topic that cannot be investigated by neuroscience in principal. The investigation of the self within the empirical realm of neuroscience would reflect nothing but a category error. Firstly, there would be confusion between scientific empirical and conceptual questions in general. Secondly and more precisely, a confusion of capabilities and behaviors of the organism and person as a whole with empirical data and facts of specific brain functions would occur.
Most fundamentally in the perspective of parallelism, problems which require both empirical (neuro-)sciences and philosophy to be solved do not even exist, more accurately because topics and problems which allegedly span across the disciplines are nothing but errors which are initially induced by conceptual confusions already inherent in the philosophical realm and then transferred to empirical sciences. Bennett and Hacker ( 2003 ) exemplify that philosophical misconceptions also involve the case of (self-)consciousness, especially proceeding from the notion of the self as an entity by Descartes ( 1641 /1993) to the self as a psychological feature which is supposed to be accessible via introspection by Locke ( 1690 /1996), over to the corresponding notion of a phenomenal self in present-days cognitive neuroscience and its relation to specific brain regions and networks (Damasio 1999 , 2000 , 2010 ; Frewen et al. 2020 ; Gazzaniga 2000 , 2005 ; LeDoux 2003 ; Panksepp 1998 , 2003 ; Turk et al. 2003 ; Wolff et al. 2018 ). These postulations which account for any additional self within consciousness and related questions concerning the underlying ontological status of such a self are fallacious and meaningless (Bennett and Hacker 2003 ; Hacker 2007 , 2013 ).
It is a misconception, specifically a mereological fallacy, to ask how the pure physical brain can have a state, i.e., in form of a distinct entity, of (self-)consciousness or how the latter can arise from the brain’s neuronal activity (Bennett and Hacker 2003 ; Hacker 2013 ). This is so precisely because it is the living being as a whole which exists and which is conscious. Consciousness is a capability that is inherent within the living being and that the physical brain lacks on the contrary. (Self-)consciousness is a linguistically expressed capability of the human being (Bennett and Hacker 2003 ; Hacker 2007 , 2013 ). Consequently, the search for neuronal correlates of (self-)consciousness is simply meaningless because based on the grounds of the above, the self is a linguistically induced concept of which no immediate correspondence within the physical brain exists, as a result of the fact that the self does not exist. Conceptual confusions about the self’s existence and reality, including its phenomenological aspects, need to be eliminated right from the onset of research. More precisely, misconceptions need to be detected and eliminated already within the philosophical realm. Otherwise, the neuroscientist will investigate topics and problems whose implicit or explicit presupposition as input is already erroneous. Consequently, any following interpretation of empirically induced outputs will be nothing but a result of misguided research and faulty interpretations of empirical data and facts which are not related to the real concepts or phenomena in question.
Since the enterprise of neurophilosophy combines neuroscience and philosophy, its approach is doomed right from the beginning in the parallelism’s perspective. Therefore, a neurophilosophy of the self cannot have a stand. In the framework of parallelism, philosophy does not create genuine new knowledge concerning the self or any empirical facts, instead, philosophy provides a better understanding of already established knowledge and experience concerning the way humans think about themselves and the world (Bennett and Hacker 2003 ; Hacker 2010 ). For example, a better understanding of (self-)conscious experience, including its grammatical and linguistic expressions, may shed light on how the latter relates to conceptual confusions towards the self, e.g. its existence and reality or the “self’s” phenomenological aspects (which are in fact experiences of a conscious living being and only linguistically mediated expressions of a self).
Most fundamentally, in the perspective of Bennett and Hacker ( 2003 ), real philosophical problems do not exist. Philosophical problems solely occur based on confusions within specifically presupposed conceptual schemes. Hence, the enterprise of neurophilosophy, including its investigation into neuroscience to advance questions, e.g. concerning the self, is not just erroneously, that is it would require correction and could then be properly investigated, but it is completely meaningless right from the onset. No matter how promising and complex these enterprises appear and how special their resulting outcomes seem to be, such misconceptions, e.g. about the self, ultimately become obvious once their erroneous presuppositions are carefully analyzed and revealed. Regarding the parallelism between neuroscience and philosophy, the paradigmatically chosen question if the self is real at all is a good example of such a misdirected enterprise. All that is required is to avoid and dissolve initial misconceptions so that erroneous investigations, both in neuroscience and philosophy, are prevented. This is one possible contribution of philosophy to neuroscience. As a result, neuroscience and philosophy cannot operate in any immediate and merging interaction, instead, they require a strict separation from each other (Fig. 3 ). In conclusion, the ontological characterization of the self is erroneous. The self’s ontological characterization is a misconception that already arose in the traditional philosophical realm and now resurfaces in neuroscience as well as in neurophilosophy (Bennett and Hacker 2003 ; Hacker 2007 , 2013 ).
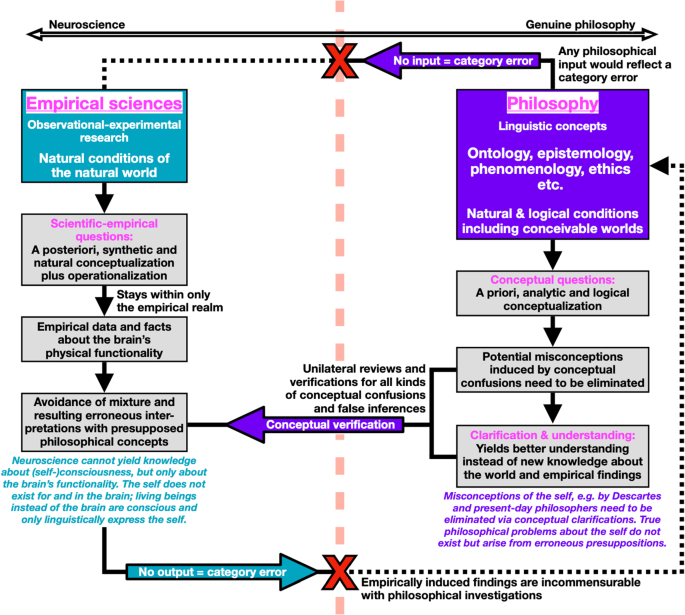
In the perspective of Bennett’s and Hacker’s ( 2003 ) parallelism, neuroscience and philosophy require a principal and most basic separation as individual disciplines. It is not just their methodology which completely differs (observational-experimental vs. conceptual-linguistic), but the categories in which the topics of investigation fall. Neuroscience, as part of the empirical realm, researches the brain’s functionality, which is bio-physiological processes within the brain and body. On the contrary, philosophy’s aim is the creation of precise concepts and clarification of already established knowledge about ourselves and the world. Furthermore, philosophy offers clarification of findings that originate from empirical sciences. Hence, philosophy, unlike science, does not create new knowledge, but better understanding. Neuroscience cannot contribute to philosophical knowledge because true philosophical problems do not exist. However, philosophy can unilaterally correct misconceptions, such as wrong interpretations of empirical data and facts, made by neuroscientists. That is, not the data and facts themselves change, but the corresponding flawed concepts which served as input and/or erroneous interpretations and which represent the output of empirical research
2.3 Non-reductive neurophilosophy and its approach to the self
[…] such concept of self as structure and organization is embodied, e.g., intrinsically linked to the body, and embedded, e.g., intrinsically linked to the environment. Hence, the virtual structure of the self spans across the brain, body, and environment with the brain’s midline structure activity being a neural predisposition for its constitution, while at the same time being dependent upon the respective environmental context (Northoff 2013a , b , p. 11).
A most fundamental principle of non-reductive neurophilosophy is reflected by its pluralism of branches which consequently entails methodological pluralism between neuroscience and philosophy. Firstly, right at the onset of the investigation, non-reductive neurophilosophy’s starting-point is defined by considering philosophical concepts, e.g. of the self, concerning their ontological determination. On one side, this distinguishes non-reductive neurophilosophy from its reductive variant, as the reductive approach conceives the empirical realm, including its data and facts, as a starting point. On the other side, philosophical concepts as input distinguish non-reductive neurophilosophy from the parallelism between neuroscience and philosophy, since the parallelism considers philosophical concepts not as starting-point, but as a realm by itself which is completely separate from neuroscience.
Accordingly, non-reductive neurophilosophy chooses specific and genuine philosophical concepts (e.g. of the self) as input . Consequently, a specific philosophical concept of the self has to be empirically conceptualized and/or operationalized to the extent that its empirical observational-experimental investigation becomes possible. Therefore and within this step of research, a strong focus on empirical data and facts subsequently allows for validation concerning the empirical plausibility of the philosophical concept (e.g. the self as entity or structure) in question. The empirically obtained data and facts can then be taken into a matching-process with the specifically chosen philosophical concept, e.g. of the self.
In this further step of matching-process, the specific concept of the self eventually requires a re-definition, which is per empirical data and facts, hence approving and ensuring its empirical plausibility. However, precisely this step is likewise provided by reductive neurophilosophy, specifically when philosophical concepts are unilaterally adapted to empirical facts as Searle ( 1999 , 2004 ) favors in a weak version of reductive neurophilosophy, or if concepts are completely deduced from the empirical realm of neuroscience without philosophical input as per Churchland’s ( 1989 , 2013b ) strong reductive approach.
On the contrary, non-reductive neurophilosophy, as developed by Northoff ( 2014a ), goes two steps further. (1) The now re-defined empirically plausible and formerly genuine philosophical concept is put back into the philosophical realm where it additionally faces its validation in respect to its logical-conceptual plausibility. Furthermore, implications regarding the distinct branches of philosophy, i.e., the re-defined concept’s implications for the ontological, epistemological, or phenomenological realm, allow for wide-ranging philosophical considerations up to investigations. (2) The finally resulting empirico-philosophical re-defined concept of the self can now be taken as another starting-point for renewed and advanced deepening research. It re-enters a loop of bidirectional empirical-conceptual investigation between neuroscience and philosophy, hence reflecting cooperate naturalism in general and especially what Northoff ( 2014a ) labels as concept-fact iterativity in particular. Such concept-fact iterativity represents truthful neurophilosophical research and resulting in interdisciplinary developed concepts.
From the initial onset to the preliminary end of the investigation, concepts include both neuroscientific research as well as a philosophical reflection both as input and output into both directions . (1) Firstly, there is an initiating philosophical-conceptual input for neuroscience; (2) neuroscience then returns an empirically plausible concept as output; (3) this output serves as input for neurophilosophical re-definition and investigation; and finally (4) the interdisciplinary re-defined neurophilosophical concept is taken as the vantage point for further investigation within new research-loops. This concept-fact iterativity consequently guarantees that neither the self stays remains as a sole philosophical concept lacking empirical data and facts, which match and correspond to a specific re-defined concept of the self, nor that philosophical concepts are completely reduced to neuroscience, as a consequence of only using the plain observational-experimental methodology of neuroscience. Altogether, there is philosophical input of the self to the empirical realm, which consequently ensures an empirico-philosophical output of interdisciplinary re-defined concepts of the self, as well as a constant loop of freshly developed concepts into a further and deepening investigation regarding future research (Fig. 4 ).
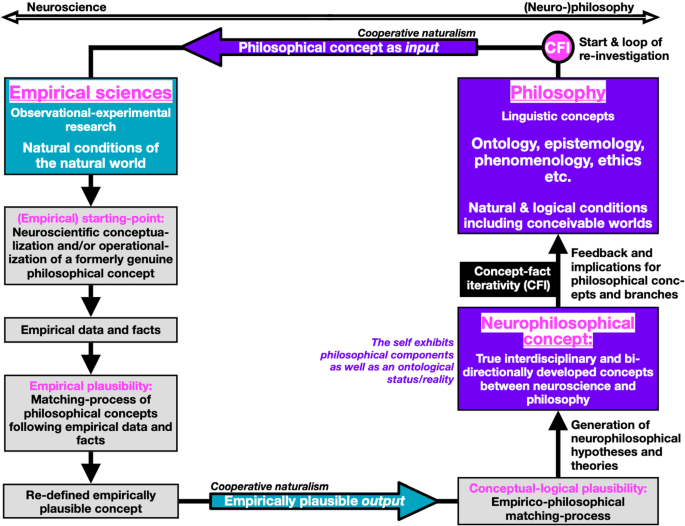
Non-reductive neurophilosophy aims for a bidirectional, and hence a truthful interdisciplinary connection of neuroscience and philosophy. Firstly, genuine philosophical concepts serve as input for subsequent empirical conceptualization and/or operationalization. Secondly, the empirical plausibility of philosophical concepts is verified within a matching-process to empirical data and facts. Consequently, a re-defined empirically plausible concept arises, which is then given as output and is additionally investigated concerning its philosophical plausibility, which is its conceptual-logical one. Thus real neurophilosophical concepts become possible, which are lastly placed back into the philosophical realm and its respective branches such as ontology or epistemology. These completely re-defined interdisciplinary concepts can then serve as a starting point for further investigations. This research loop allows us to increasingly determine concepts concerning both empirical and conceptual plausibility and hence within a broader framework that non-reductive neurophilosophy covers
While it is beyond the scope of this article to present a full-blown elaboration of the non-reductive approach to the self, nevertheless, an overview shall be provided in comparison to its reductive sibling which is nowadays rejected by Bickle ( 2019 ). When empirically investigating the brain’s neuronal functionality, the self as a physical, or even a mental entity, is certainly not traceable. If the investigation would stop at this point, since the presupposed narrow framework of reductive neurophilosophy does only consider the isolated brain, the conclusion would be correct that there is no empirical (neuronal) mirror of the self’s hereby implicitly chosen ontological determination as an entity or property-based concept. So far, reductive neurophilosophers like Metzinger ( 2004 , 2009 ) and Churchland ( 2013b ) are correct insofar that the self’s ontological determination as a physical or as a mental entity seems absent in the brain’s neuronal activity. Nevertheless, unlike reductive approaches, non-reductive neurophilosophy does not rely on the straightforward elimination of the self’s ontological status in favor of unidirectional inferences from plain empirical data and facts of an isolated observed brain to the denial and rejection of philosophical concepts.
Consequently, other concepts of the self need to be firstly provided as philosophical input for the empirical realm of neuroscience, and secondly developed within a bidirectional enterprise, thus resulting in a broader framework consisting of both neuroscientific investigation and philosophical reflection. Instead of the common entity and property-based ontologies, structure- or process-based ontologies may better reflect the empirical reality of the brain’s functionality. Recent neuroscientific findings speak in favor of a neuro-ecological structure of the brain’s empirical reality, which accounts for the concept of» World-brain relation « (Northoff 2018b , 2019a ). Footnote 5 Most basically, the brain’s temporo-spatial structure of its spontaneous activity’s dynamics has to align itself to the wider temporo-spatial context of the world on adaptational grounds. Such constant alignment of the brain to the world virtually spans the temporo-spatial structure across the brain, body, and environment, hence reflecting a neuro-ecological structure (Northoff 2013a , 2018b , 2019a ; Northoff and Huang 2017 ). The brain’s neuro-ecological structure and world-brain relation require relational based ontologies instead of entity-based ontologies. This could amount to structural realism (SR), more precisely moderate ontic structural realism (OSR) (Esfeld and Lam 2008 , 2011 ) which assumes that relations and structures are ontologically more fundamental than relata/elements. OSR is also favored by the non-reductive neurophilosophical approach to the brain and (self-)consciousness (Northoff 2018b ).
Conceiving the brain in this broader framework (compared to the narrow and reductive framework of an isolated brain) may then opens the door for the possibility of the self’s ontological determination. In other terms, these obtained empirical data and facts then serve as output for further philosophical reflection and implications concerning the self within philosophical branches. Following empirical findings and philosophical reflection, both mind based as well as reductive-eliminative concepts of the self is rejected and replaced by a structural determination of the self. Ultimately, such a truthful neurophilosophical concept is chosen as the starting point regarding forthcoming bidirectional empirico-philosophical research. This re-investigation loop particularly reflects the non-reductive principle of concept-fact iterativity (Northoff 2014a ).
In summary, non-reductive neurophilosophy takes a brain-based stance (in opposite to a brain-reductive stance of reductive neurophilosophy) which absolutely includes the brain but also goes beyond it by taking the world in respect to the brain’s functionality as well as mental features into consideration. Accordingly, the feedback-loop system of non-reductive neurophilosophy’s research as described above searches for a “common currency” as the linkage between mental features and the brain’s neuronal activity (Northoff 2019a , b ; Northoff et al. 2019 ). A reduction of both subjective first-person phenomenal experience including the ontological determination of the self to only the brain’s empirical functionality is thus rejected. Footnote 6
Consequently, (self-)consciousness as well as specific mental features are considered to hold an ontological status within the perspective of non-reductive neurophilosophy, constituted by the relational neuro-ecological structure between the brain, body (accounting for embodiment) and the environment (accounting for embeddedness), which is conceptualized and ultimately traced back to the empirical-ontological» World-brain relation « within the Temporo-spatial theory of consciousness (TTC) (Northoff 2014b , c , 2016a , b , 2018b ; Northoff and Huang 2017 ). Such ecological view of the brain contradicts a brain-reductive stance, as the latter claims that consciousness, the self, and mental features are reducible to and especially caused by the brain’s neuronal activity, which is still commonly presupposed in theories about consciousness by empirical neuroscience, as seen in the Integrated Information Theory (IIT) (Tononi 2004 , 2008 ; Tononi and Koch 2008 ; Tononi et al. 2016 ), the Global Neuronal Workspace Theory (GNWT) (Baars 2005 ; Baars and Franklin 2007 ) and reductive neurophilosophy (Churchland 1985 , 1989 , 2002a , b , 2013a , b ). Footnote 7 On the contrary in the perspective of non-reductive neurophilosophy, (self-)consciousness including mental features are neither seen as reducible to the brain’s neuronal activity nor caused by the latter. Instead, an intrinsic correspondence, that is, a neuro-mental transformation by the brain’s temporo-spatial dynamics between neuronal activity and mental features, is suggested. Therefore, the distinction into two distinct entities as well as the reduction from one level to the other is rejected (Northoff et al. 2019 ).
3 Conclusion
Even though Patricia Churchland ( 1989 ) explicitly introduced the term neurophilosophy into the academic discourse of philosophy and its possible connection with neuroscience more than 30 years ago, neither widely accepted abstract principles of neurophilosophy nor the methodologies concerning its practical implementation about neurophilosophical research exist as at date. Therefore, the chosen threefold differentiation in the article between reductive neurophilosophy, non-reductive neurophilosophy, and parallelism as a strict separation between the disciplines of neuroscience and philosophy, presented a brief insight into today’s distinguishable perspectives on the project of neurophilosophy. While parallelism between neuroscience and philosophy denies the possibility of a merging collaboration between their branches and methodologies, even in the light of fascinating results and possibilities that neuroscience developed especially within the last 25 years, non-reductive neurophilosophy reaches out for exactly this bidirectional interaction: a neuro-phenomenological linkage consisting of neuroscientific third-person-perspective data with corresponding first-person-perspective’s experience of (self-)consciousness is one of its aims, leading to a broader understanding of consciousness in general as well as specific mental features in particular, and therefore ultimately of human existence. Correspondingly, the project of non-reductive neurophilosophy goes along without a reductionism of ourselves to the brain’s neuronal activity. As paradigmatically presented, this approach also applies to neurophilosophical inspired research on the topic of the self. While philosophy in the past focused on many contrasting concepts of the self, e.g. as mental substance (Descartes, 1641 /1993), which are most widely rejected today, or as the distinction between the subjective “I” and objective “me” (James 1890a , b ), it is common in contemporary reductive neurophilosophy and neuroscience to reduce or eliminate the phenomenal self in favor of the brain (Churchland 2013b ; Metzinger 2004 , 2009 ). In other terms, the self is only empirically conceptualized, e.g. as a higher-order cognitive function (Churchland 2002b ; Damasio 1999 , 2000 ; Dennett 1991 ), without further and sufficient philosophical consideration, including respective implications. This one-sided notion on the self consequently leads to significant neglect of phenomenological aspects of the self and its present-day frequent ontological denial, similar to the self’s rejection by Hume ( 1739 –1740/2003), so that wide parts of neuroscience, and especially reductive neurophilosophy, reverted into the other one-sided extreme in form of a neuronal reductionism. Non-reductive neurophilosophy, however, takes both neuroscience and philosophy seriously for any field of investigation, e.g. in respect to the self (Northoff 2014b , 2016c , 2018b , 2019a , b ), therefrom reflecting a brain-based stance and cooperative naturalism form of the naturalization of philosophy (rather than a brain-reductive and replacement naturalistic stance). In conclusion, non-reductive neurophilosophy does not stand in competition with neuroscience and philosophy, instead, its approach should be seen as complementary to genuine particular sciences and it especially preserves philosophy alive by actively considering and taking philosophical concepts into the interdisciplinary investigation, that is, both as input and output.
The terms a priori analytic and a posteriori synthetic used here are based on the definitions of Kant’s transcendental philosophy ( 1781 /1996).
According to Kant ( 1781 /1996), an analytic sentence already contains the predicate (P) within the subject (S), and therefore the predicate does not offer any further information: S = P, whereas a synthetic sentence is empirical: it is based on experience. He further elaborated that synthetic sentences a priori are possible and are especially highlighted as a prerequisite fundament concerning a reinterpretation of metaphysics (Kant 1781 /1996). Kant’s examples for synthetic a priori sentences refer to mathematics and physics by Newton among others (Kant 1781 /1996; Kutschera 2006 ).
Whereas other concepts of the self, for example as relational constituted structure between the brain, body, and environment (Northoff 2013b , 2014c , 2016a , 2018b ), or as the mind, i.e., conscious experience itself (Vacariu 2016 ), can be per with neuroscientific data.
It is worthwhile to mention that most of the famous modern neuroscientists of the first generation in the twentieth century, like Charles S. Sherrington (1857–1952) or the neurosurgeon Wilder Penfield (1891–1976), explicitly or implicitly argued in favor of ontological substance dualism (Bennett and Hacker 2003 , 2012 ; Penfield 1975 ).
In perspective of the self, hereof involved are especially the overlapping cortical midline structures (CMS) and the default-mode-network (DMN); in accordance to the empirical phenomena of self-relatedness, both the CMS and DMN are neuroscientifically associated with the self (Northoff 2013a ; Qin and Northoff 2011 ; Scalabrini et al. 2018 ; Qin et al. 2013 ; Qin et al. 2016 ; Wolff et al. 2018 ).
Phenomenal experience of the self, i.e., what phenomenology defines as» ipseity « of the» experiential self, core self or minimal self « (Gallagher 2000 ; Parnas and Henriksen 2019 ; Zahavi 2005 , 2014 , 2019 ), that is immediate and intrinsically melt of a basic sense-of-self within the stream-of-consciousness, is not reduced to the brain’s neuronal activity within the approach of non-reductive neurophilosophy.
Since this causal relationship between neuronal activity and mental features implies that both are sort of distinct entities, it may very well reflect the criticized neo-Cartesianism in current neuroscience by Bennett and Hacker ( 2003 ) as well as by German psychiatrist and philosopher Thomas Fuchs ( 2018 ).
Baars, B. J. (2005). Global workspace theory of consciousness: Toward a cognitive neuroscience of human experience. Progress in Brain Research, 150, 45–53.
Google Scholar
Baars, B. J., & Franklin, S. (2007). An architectural model of conscious and unconscious brain functions: Global Workspace Theory and IDA. Neural Networks, 20 (9), 955–961.
Bechtel, W., Mandik, P., Mundale, J., & Stufflebeam, R. S. (Eds.). (2001). Philosophy and the neurosciences: A reader . Oxford: Wiley.
Bennett, M. R., & Hacker, P. M. S. (2003). Philosophical foundations of neuroscience . Oxford: Blackwell.
Bennett, M. R., & Hacker, P. M. S. (2012). History of cognitive neuroscience . Oxford: Wiley.
Bermúdez, J. L. (2005). Philosophy of psychology. A contemporary introduction . New York: Routledge.
Bickle, J. (2003). Philosophy and neuroscience: A ruthlessly reductive account . Dordrecht: Kluwer Academic Publishers.
Bickle, J. (2006). Reducing mind to molecular pathways: Explicating the reductionism implicit in current cellular. Synthese, 151 (3), 411–434.
Bickle, J. (Ed.). (2009). The Oxford handbook of philosophy and neuroscience . New York: Oxford University Press.
Bickle, J. (2019). Lessons for experimental philosophy from the rise and “fall” of neurophilosophy. Philosophical Psychology, 32 (1), 1–22.
Brüntrup, G. (2018). Philosophie des Geistes. Eine Einführung in das Leib-Seele-Problem . Stuttgart: Kohlhammer.
Churchland, P. M. (1981). Eliminative materialism and the propositional attitudes. The Journal of Philosophy, 78 (2), 67–90.
Churchland, P. M. (1985). Reduction, qualia and the direct introspection of brain states. The Journal of Philosophy, 82 (1), 8–28.
Churchland, P. S. (1989). Neurophilosophy: Toward a unified science of the mind–brain . Cambridge, MA: MIT Press.
Churchland, P. S. (2002a). Brain-wise: Studies in neurophilosophy . Cambridge, MA: MIT Press.
Churchland, P. S. (2002b). Self-representation in nervous systems. Science, 12 (296), 308–310.
Churchland, P. M. (2013a). Matter and consciousness . Cambridge, MA: MIT Press.
Churchland, P. S. (2013b). Touching a nerve. The self as brain . New York: W.W. Norton.
Damasio, A. R. (1999). How the brain creates the mind. Scientific American, 281 (6), 112–117.
Damasio, A. R. (2000). The feeling of what happens. Body and emotion in the making of consciousness . Boston, MA: Mariner books.
Damasio, A. R. (2010). Self comes to mind. Constructing the conscious brain . New York: Pantheon Books.
D’Argembeau, A., Collette, F., Van der Linden, M., Laureys, S., Del Fiore, G., Delgueldre, C., et al. (2005). Self-referential reflective activity and its relationship with rest: A PET study. NeuroImage, 25 (2), 616–624.
D’Argembeau, A. (2013). On the role of the ventromedial prefrontal cortex in self-processing: The valuation hypothesis. Frontiers in Human Neuroscience, 7, 372.
Dennett, D. C. (1991). Consciousness explained . Boston: Little, Brown and Company.
Descartes, R. (1641/1993). Meditations on first philosophy . Indianapolis: Hackett Publishing Company.
Esfeld, M., & Lam, V. (2008). Moderate structural realism about space-time. Synthese, 160 (1), 27–46.
Esfeld, M., & Lam, V. (2011). Ontic structural realism as a metaphysics of objects. In A. Bokulich & P. Bokulich (Eds.), Scientific structuralism (pp. 143–159). Dordrecht: Springer.
Frewen, P., Schroeter, M. L., Riva, G., Cipresso, P., Fairfield, B., Padulo, C., et al. (2020). Neuroimaging the consciousness of self: Review, and conceptual-methodological framework. Neuroscience and Biobehavioral Reviews, 112, 164–212.
Fuchs, T. (2018). Ecology of the brain . New York: Oxford University Press.
Gallagher, S. (2000). Philosophical conceptions of the self: Implications for cognitive science. Trends in Cognitive Sciences, 4 (1), 14–21.
Gazzaniga, M. S. (2000). The mind’s past . Berkeley, CA: University of California Press.
Gazzaniga, M. S. (2005). Forty-five years of split-brain research and still going strong. Nature Reviews Neuroscience, 6 (8), 653–659.
Göhmann, D. (2018). Neurophilosophie. In D. Schubbe & M. Koßler (Eds.), Schopenhauer-Handbuch (pp. 375–378). Stuttgart: J. B. Metzler.
Hacker, P. M. S. (2007). Human nature. The categorial framework . Oxford: Blackwell Publishing.
Hacker, P. M. S. (2010). Hacker’s challenge. The Philosophers’ Magazine, 51 (51), 23–32.
Hacker, P. M. S. (2013). The intellectual powers. A study of human nature . New York: Wiley.
Hume, D. (1739–1740/2003). A treatise of human nature . Mineola, NY: Dover Publications.
James, W. (1890a). The principles of psychology (Vol. 1). New York, NY: Holt.
James, W. (1890b). The principles of psychology (Vol. 2). New York, NY: Holt.
Kant, I. (1781/1996). Critique of pure reason . Indianapolis: Hackett Publishing Company.
Khachouf, O. T., Poletti, S., & Pagnoni, G. (2013). The embodied transcendental: A Kantian perspective on neurophenomenology. Frontiers in Human Neuroscience, 7, 611.
Klein, S. B. (2012). Self, memory, and the self-reference effect: An examination of conceptual and methodological issues. Personality and Social Psychology Review, 16 (3), 283–300.
Knyazev, G. G. (2013). EEG correlates of self-referential processing. Frontiers in Human Neuroscience, 7, 264.
Kutschera, F. (2006). Die Wege des Idealismus . Paderborn: Mentis.
Kutschera, F. (2009). Philosophie des Geistes . Paderborn: Mentis.
LeDoux, J. (2003). Synaptic self. How our brains become who we are . New York: Penguin Books.
Locke, J. (1690/1996). An essay concerning human understanding . Indianapolis: Hackett Publishing Company.
Liu, J., Corbera, S., & Wexler, B. E. (2014). Neural activation abnormalities during self-referential processing in schizophrenia: An fMRI study. Psychiatry Research: Neuroimaging, 222 (3), 165–171.
Lutz, A., & Thompson, E. (2003). Neurophenomenology integrating subjective experience and brain dynamics in the neuroscience of consciousness. Journal of Consciousness Studies, 10 (9–10), 31–52.
Merleau-Ponty, M. (1945/2013). Phenomenology of perception . London: Routledge.
Metzinger, T. (1999). Subjekt und selbstmodell . Paderborn: Mentis.
Metzinger, T. (Ed.). (2001). Bewusstsein . Mentis: Beiträge zur Gegenwartsphilosophie. Paderborn.
Metzinger, T. (2004). Being no one. The self-model theory of subjectivity . Cambridge, MA: MIT Press.
Metzinger, T. (2009). The ego tunnel. The science of the mind and the myth of the self . New York: Basic Books.
Newen, A. (2013). Philosophie des Geistes. Eine Einführung . München: Verlag C. H. Beck.
Northoff, G. (2004). What is neurophilosophy? A methodological account. Journal for General Philosophy of Science, 35 (1), 91–127.
Northoff, G. (2012). Das disziplinlose Gehirn—Was nun, Herr Kant? Auf den Spuren unseres Bewusstseins mit der Neurophilosophie . München: Irsania.
Northoff, G. (2013a). What the brain’s intrinsic activity can tell us about consciousness? A tri-dimensional view. Neuroscience and Biobehavioral Reviews, 37 (4), 726–738.
Northoff, G. (2013b). Brain and self—a neurophilosophical account. Child and Adolescent Psychiatry and Mental Health, 7, 28.
Northoff, G. (2014a). Minding the brain. A guide to philosophy and neuroscience . Basingstoke: Palgrave Macmillan.
Northoff, G. (2014b). Unlocking the brain. Volume 1: Coding . New York: Oxford University Press.
Northoff, G. (2014c). Unlocking the brain. Volume 2: Consciousness . New York: Oxford University Press.
Northoff, G. (2016a). Spatiotemporal psychopathology I: No rest for the brain’s resting state activity in depression? Spatiotemporal psychopathology of depressive symptoms. Journal of Affective Disorders, 190, 854–866.
Northoff, G. (2016b). Spatiotemporal psychopathology II: How does a psychopathology of the brain’s resting state look like? Spatiotemporal approach and the history of psychopathology. Journal of Affective Disorders, 190, 867–879.
Northoff, G. (2016c). Neuro-philosophy and the healthy mind. Learning from the unwell brain . New York: W.W. Norton.
Northoff, G. (2018a). Neurophilosophy and Neuroethics: Template for Neuropsychoanalysis? In H. Boeker, P. Hartwich, & G. Northoff (Eds.), Neuropsychodynamic psychiatry (pp. 599–615). Berlin: Springer.
Northoff, G. (2018b). The spontaneous brain. From the mind–body to the world-brain problem . Cambridge, MA: MIT Press.
Northoff, G. (2019a). Lessons from astronomy and biology for the mind-Copernican revolution in neuroscience. Frontiers in Human Neuroscience, 13, 319.
Northoff, G. (2019b). Phenomenological psychopathology and neuroscience. In G. Stanghellini, M. R. Broome, A. V. Fernandez, P. Fusar-Poli, A. Raballo, & R. Rosfort (Eds.), The Oxford handbook of phenomenological psychopathology (pp. 909–924). New York: Oxford University Press.
Northoff, G., & Huang, Z. (2017). How do the brain’s time and space mediate consciousness and its different dimensions? Temporo-spatial theory of consciousness (TTC). Neuroscience and Biobehavioral Reviews, 80, 630–645.
Northoff, G., & Tumati, S. (2019). Average is good, extremes are bad”—Non-linear inverted U-shaped relationship between neural mechanisms and functionality of mental features. Neuroscience and Biobehavioral Reviews, 104, 11–25.
Northoff, G., Wainio-Theberge, S., & Evers, K. (2019). Is temporo-spatial dynamics the “common currency” of brain and mind? In Quest of “Spatiotemporal Neuroscience”. Physics of Life Reviews ( in press ).
Panksepp, J. (1998). The pre-conscious substrates of consciousness: Affective states and the evolutionary origin of the SELF. Journal of Consciousness Studies, 5 (5–6), 566–582.
Panksepp, J. (2003). The neural nature of the core SELF: Implications for understanding schizophrenia. In T. Kircher & A. David (Eds.), The self in neuroscience and psychiatry (pp. 197–213). Oxford: Oxford University Press.
Parnas, J., & Henriksen, M. G. (2019). Selfhood and its disorders. In G. Stanghellini, M. R. Broome, A. V. Fernandez, P. Fusar-Poli, A. Raballo, & R. Rosfort (Eds.), The Oxford handbook of phenomenological psychopathology (pp. 465–474). New York: Oxford University Press.
Penfield, W. (1975). The mystery of the mind: A critical study of consciousness and the human brain . Princeton: Princeton University Press.
Popper, K. R., & Eccles, J. C. (1985). The self and its brain . Berlin: Springer.
Qin, P., & Northoff, G. (2011). How is our self related to midline regions and the default-mode network? NeuroImage, 57 (3), 1221–1233.
Qin, P., Duncan, N. W., & Northoff, G. (2013). Why and how is the self-related to the brain midline regions? Frontiers in Human Neuroscience, 7, 909.
Qin, P., Grimm, S., Duncan, N. W., Fan, Y., Huang, Z., Lane, T., et al. (2016). Spontaneous activity in default-mode network predicts ascription of self-relatedness to stimuli. Social Cognitive and Affective Neuroscience, 11 (4), 693–702.
Quine, W. V. O. (1951). Two dogmas of empiricism. The Philosophical Review, 60, 20–43.
Quine, W. V. O. (1969). Ontological relativity and other essays . New York: Columbia University Press.
Scalabrini, A., Mucci, C., & Northoff, G. (2018). Is our self related to personality? A neuropsychodynamic model. Frontiers in Human Neuroscience, 12, 346.
Schopenhauer, A. (1819/2011). Die welt als wille und vorstellung . München: Deutscher Taschenbuch Verlag.
Searle, J. R. (1999). The future of philosophy. Philosophical Transactions of the Royal Society of London, 354 (1392), 2069–2080.
Searle, J. R. (2004). Mind. A brief introduction . Oxford/New York: Oxford University Press.
Tononi, G. (2004). An information integration theory of consciousness. BMC Neuroscience, 5, 42.
Tononi, G. (2008). Consciousness as integrated information: A provisional manifesto. Biological Bulletin, 215 (3), 216–242.
Tononi, G., & Koch, C. (2008). The neural correlates of consciousness: An update. Annals of the New York Academy of Sciences, 1124 (1), 239–261.
Tononi, G., Boly, M., Massimini, M., & Koch, C. (2016). Integrated information theory: From consciousness to its physical substrate. Nature Reviews: Neuroscience, 17 (7), 450–461.
Turk, D. J., Heatherton, T. F., Macrae, C. N., Kelley, W. M., & Gazzaniga, M. S. (2003). Out of contact, out of mind: The distributed nature of the self. Annals of the New York Academy of Sciences, 1001, 65–78.
Vacariu, G. (2016). Illusions of human thinking. On concepts of mind, reality, and the universe in psychology, neuroscience, and physics . Wiesbaden: Springer Fachmedien.
Varela, F. J. (1996). Neurophenomenology: A methodological remedy for the hard problem. Journal of Consciousness Studies, 3 (4), 330–349.
Walter, H. (1998/2009). Neurophilosophy of free will. From libertarian illusions to a concept of natural autonomy . Cambridge, MA: MIT Press.
Wolff, A., Di Giovanni, D. A., Gómez-Pilar, J., Nakao, T., Huang, Z., Longtin, A., & Northoff, G. (2018). The temporal signature of self: Temporal measures of resting-state EEG predict self-consciousness. Human Brain Mapping, 40 (3), 789–803.
Zahavi, D. (2005). Subjectivity and selfhood. Investigating the first-person perspective . Cambridge, MA: MIT Press.
Zahavi, D. (2014). Self and other: Exploring subjectivity, empathy, and shame . Oxford: Oxford University Press.
Zahavi, D. (2019). Self. In G. Stanghellini, M. R. Broome, A. V. Fernandez, P. Fusar-Poli, A. Raballo, & R. Rosfort (Eds.), The Oxford handbook of phenomenological psychopathology (pp. 299–305). New York: Oxford University Press.
Download references
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and affiliations.
Cécile and Oskar Vogt Institute of Brain Research, Heinrich Heine University Düsseldorf, Düsseldorf, Germany
Philipp Klar
Mönchengladbach, Germany
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Philipp Klar .
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Klar, P. What is neurophilosophy: Do we need a non-reductive form?. Synthese 199 , 2701–2725 (2021). https://doi.org/10.1007/s11229-020-02907-6
Download citation
Received : 24 April 2020
Accepted : 08 October 2020
Published : 17 October 2020
Issue Date : December 2021
DOI : https://doi.org/10.1007/s11229-020-02907-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Philosophy of mind
- Philosophy of neuroscience
- Consciousness
- Find a journal
- Publish with us
- Track your research
Loading metrics
Open Access
Peer-reviewed
Meta-Research Article
Meta-Research Articles feature data-driven examinations of the methods, reporting, verification, and evaluation of scientific research.
See Journal Information »
Empirical assessment of published effect sizes and power in the recent cognitive neuroscience and psychology literature
Roles Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Department of Psychology, University of Cambridge, Cambridge, United Kingdom
Roles Conceptualization, Writing – review & editing
Affiliation Meta-Research Innovation Center at Stanford (METRICS) and Department of Medicine, Department of Health Research and Policy, and Department of Statistics, Stanford University, Stanford, California, United States of America
- Denes Szucs,
- John P. A. Ioannidis

- Published: March 2, 2017
- https://doi.org/10.1371/journal.pbio.2000797
- See the preprint
- Reader Comments
5 Mar 2021: Szucs D, Ioannidis JPA (2021) Correction: Empirical assessment of published effect sizes and power in the recent cognitive neuroscience and psychology literature. PLOS Biology 19(3): e3001151. https://doi.org/10.1371/journal.pbio.3001151 View correction
We have empirically assessed the distribution of published effect sizes and estimated power by analyzing 26,841 statistical records from 3,801 cognitive neuroscience and psychology papers published recently. The reported median effect size was D = 0.93 (interquartile range: 0.64–1.46) for nominally statistically significant results and D = 0.24 (0.11–0.42) for nonsignificant results. Median power to detect small, medium, and large effects was 0.12, 0.44, and 0.73, reflecting no improvement through the past half-century. This is so because sample sizes have remained small. Assuming similar true effect sizes in both disciplines, power was lower in cognitive neuroscience than in psychology. Journal impact factors negatively correlated with power. Assuming a realistic range of prior probabilities for null hypotheses, false report probability is likely to exceed 50% for the whole literature. In light of our findings, the recently reported low replication success in psychology is realistic, and worse performance may be expected for cognitive neuroscience.
Author summary
Biomedical science, psychology, and many other fields may be suffering from a serious replication crisis. In order to gain insight into some factors behind this crisis, we have analyzed statistical information extracted from thousands of cognitive neuroscience and psychology research papers. We established that the statistical power to discover existing relationships has not improved during the past half century. A consequence of low statistical power is that research studies are likely to report many false positive findings. Using our large dataset, we estimated the probability that a statistically significant finding is false (called false report probability). With some reasonable assumptions about how often researchers come up with correct hypotheses, we conclude that more than 50% of published findings deemed to be statistically significant are likely to be false. We also observed that cognitive neuroscience studies had higher false report probability than psychology studies, due to smaller sample sizes in cognitive neuroscience. In addition, the higher the impact factors of the journals in which the studies were published, the lower was the statistical power. In light of our findings, the recently reported low replication success in psychology is realistic, and worse performance may be expected for cognitive neuroscience.
Citation: Szucs D, Ioannidis JPA (2017) Empirical assessment of published effect sizes and power in the recent cognitive neuroscience and psychology literature. PLoS Biol 15(3): e2000797. https://doi.org/10.1371/journal.pbio.2000797
Academic Editor: Eric-Jan Wagenmakers, University of Amsterdam, Netherlands
Received: August 10, 2016; Accepted: February 6, 2017; Published: March 2, 2017
Copyright: © 2017 Szucs, Ioannidis. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting Information files.
Funding: James S. McDonnell Foundation 21st Century Science Initiative in Understanding Human Cognition (grant number 220020370). Received by DS. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Abbreviations: D, effect size; df, degree of freedom; fMRI, functional magnetic resonance imaging; FRP, False Report Probability; JPR, Journal of Psychiatry Research; NHST, Null Hypothesis Significance Testing; TRP, True Report Probability
Introduction
Low power and selection biases, questionable research practices, and errors favoring the publication of statistically significant results have been proposed as major contributing factors in the reproducibility crisis that is heavily debated in many scientific fields [ 1 – 5 ]. Here, we aimed to get an impression about the latest publication practices in the closely related cognitive neuroscience and (mostly experimental) psychology literature. To this end, we extracted close to 30,000 records of degrees of freedom (df) and t -values from papers published between Jan 2011 to Aug 2014 in 18 journals. Journal impact factors ranged from 2.367 (Acta Psychologica) to 17.15 (Nature Neuroscience). The data allowed us to assess the distribution of published effect sizes (D), to estimate the power of studies, and to estimate the lower limit of false report probability (FRP). The text-mining approach we used enabled us to conduct a larger power survey than classical studies.
Low power is usually only associated with failing to detect existing (true) effects, and therefore, with wasting research funding on studies which a priori have a low chance to achieve their objective. However, low power also has two other serious negative consequences: it results in the exaggeration of measured effect sizes and it also boosts FRP, the probability that statistically significant findings are false [ 5 – 7 ].
First, if we use Null Hypothesis Significance Testing (NHST), then published effect sizes are likely to be, on average, substantially exaggerated when most published studies in a given scientific field have low power [ 6 , 8 ] (see S1A Fig for the mechanism of effect size exaggeration). This is because even if we assume that there is a fixed true effect size, actual effect sizes measured in studies will have some variability due to sampling error. Underpowered studies will be able to classify as statistically significant only the occasional large deviations from real effect sizes. Conversely, most measured effects will remain under the statistical significance threshold even if they reflect true relationships [ 9 – 11 ]. Effect size inflation is greater when studies are even more underpowered. Consequently, while meta-analyses may provide the illusion of precisely estimating real effects, they may, in fact, estimate exaggerated effects detected by underpowered studies while at the same time not considering unpublished negative findings (see, e.g., [ 12 ]).
Secondly, from the Bayesian perspective, the long-run FRP of the NHST framework can be defined as the probability that the null hypothesis (a hypothesis to be “nullified”) is true when we get a statistically significant finding. The long-run True Report Probability (TRP) can be defined as the probability that the alternative hypothesis is true when we get a statistically significant finding [ 13 , 5 ]. Note that the concepts of FRP and TRP do not exist in the NHST framework: NHST only allows for the rejection of the null hypothesis and does not allow for the formal acceptance of the alternative hypothesis. However, here we do not apply NHST but rather, characterize its long-run (“frequentist”) performance from the Bayesian point of view. This approach allows us to talk about true and false null and alternative hypotheses (see more on this in [ 13 , 5 ]).
Computationally, FRP is the number of statistically significant false positive findings divided by the total number of statistically significant findings. TRP is the number of statistically significant true positive findings divided by the total number of statistically significant findings. FRP and TRP can be computed by applying Bayes theorem (see S1 Text , Section 5 for details).
Because published effect sizes are likely to be inflated, it is most informative to determine the power of studies to detect predefined effect sizes. Hence, we first computed power from the observed degrees of freedom using supporting information from manually extracted records to detect effect sizes traditionally considered small (d = 0.2), medium (d = 0.5), and large (d = 0.8) [ 16 – 18 ]. Second, we also computed power to detect the effect sizes computed from t -values published in studies. Given that many of these published effect sizes are likely to be inflated compared to the true ones (as explained above), this enabled us to estimate the lower limit of FRP [ 5 , 13 ].
Materials and methods
We extracted statistical information from cognitive neuroscience and psychology papers published as PDF files. We sampled 18 journals frequently cited in cognitive neuroscience and psychology. Our aim was to collect data on the latest publication practices. To this end, we analyzed 4 y of regular issues for all journals published between Jan 2011 to Aug 2014. The time period was chosen to represent recent publication practices (during the closest possible period before the start of data analysis). Particular journals were chosen so as to select frequently cited journals with a range of impact factors from our disciplines of interest.
We categorized ten journals as focused more on (cognitive) neuroscience (Nature Neuroscience, Neuron, Brain, The Journal of Neuroscience, Cerebral Cortex, NeuroImage, Cortex, Biological Psychology, Neuropsychologia, Neuroscience) and five journals focused more on psychology (Psychological Science, Cognitive Science, Cognition, Acta Psychologica, Journal of Experimental Child Psychology). We also searched three more medically oriented journals which are nevertheless often cited in cognitive neuroscience papers so as to increase the representativeness of our sample (Biological Psychiatry, Journal of Psychiatric Research, Neurobiology of Ageing). Journal impact factors ranged from 2.367 (Acta Psychologica) to 17.15 (Nature Neuroscience). Five-year impact factors were considered as reported in 2014 (see S1 Table ).
When there were fewer than 20 empirical papers in a journal issue, all empirical research reports with any reported t statistics were analyzed. When there were more than 20 papers in an issue, a random sample of 20 papers were analyzed merely because this was the upper limit of papers accessible in one query. This procedure sampled most papers in most issues and journals. All algorithms and computations were coded in Matlab 2015b ( www.mathworks.com ). Initial PDF file text extraction relied on the PdfToolbox Matlab package.
Data extraction
In summary, a computer algorithm searched through each paper for frequently occurring word and symbol combinations for reporting degrees of freedom and effect sizes provided as Cohen’s d . We extracted statistical information about t tests and F tests ( t -values, F -values, degrees of freedom, p -values, and effect sizes). Only t -test data is used in this paper, so here we limit data extraction description to t -tests.
In psychology and cognitive neuroscience, full t -test records are typically reported in the text as, for example, ' t (df) = x.xx; p = y.yy'. D-value reports are often added to these reports as, e.g., ' t (df) = x.xx; p = y.yy; d = z.zz'. Hence, in a first text parsing phase, the algorithm opened each PDF file from each journal and identified each point of text which contained a “ t (” character combination or a “ t ” character. If these characters were identified, then a line of 65 characters were read out from the PDF file starting at the “ t (” character combination or at the “ t ” character. Spaces between letters and symbols were removed from these lines of text. That is, it did not matter how many spaces separated relevant statistical entries. Lines of text were kept for further analysis if they contained the characters “=“, “<”, or “>” and an additional “p =“, “p<”, or “p>” character combination. This parsing phase identified lines potentially containing independent full t -test records. In building this parsing phase, the performance of the algorithm was initially evaluated by reviewing identified lines of text and extracted data from the first 30 papers analyzed for each journal. If specific journals used special characters (as identified by the PdfToolbox package) for typesetting some information (e.g., equation signs), then this was identified and taken into account in the code.
In a second parsing phase, Matlab regular expressions were used to identify full t -test records using the templates noted above (e.g., “ t (df) = x.xx” or “ d = z.zz”). All text searches were done after converting lines to lowercase characters, so upper- or lowercase differences did not matter in searches.
After data extraction, some error checks were done. First, the algorithm detected a few records twice. This may have happened if for any reason an additional “ t ” appeared within the statistical reporting text (e.g., if researchers used the ‘ t ’ character very close to a statistical record, then that record may have been picked up twice). So, records which had identical statistical information to preceding records were removed. Second, records where negative degrees of freedom (two records) and/or negative p -values (one record) were detected were removed. These may have occurred in response to odd character sets or to errors in the text. After cleaning the data, several informal spot-checks were run: hundreds of lines of extracted text were visually compared with the numerical records extracted from the text.
A limitation is that the algorithm only extracted information from the text but not from tables. Further, in order to limit false positive detections (see also later), we restricted our initial search for full p -value records, so some reported nonsignificant results and stand-alone t -values may have been missed (e.g., t < 1; t = 0.23). It is important to note that we only assured that our extraction algorithm works fine for the journals and publication years analyzed here. It has not been validated as a more “universal” extraction algorithm like statcheck [ 19 ], for example, which we did not know about when starting this project. The extraction algorithm is published as supporting material ( S1 Code ).
Formal data validation
In a formal validation procedure, we randomly selected 100 papers with t -value, df , and effect size reports. The selected papers were manually checked for all statistical records. The content of the identified records was then compared to the content of automatically extracted records. This was done to see the accuracy of the computer algorithm and to gather information on the data.
Validation results showed that the automatic extraction algorithm had highly satisfactory performance. The randomly selected papers for validation included 1,478 records of data. The algorithm correctly identified about 95% of t -values and degrees of freedom in these records. The algorithm missed only 76 records (5.14%), usually due to atypical punctuation or line breaks within a statistical record. There were no false alarms; that is, all data extracted really belonged to t -value records. This is plausible because regular expressions had to fulfill several conditions in order to be identified as potential t -test records. For example, it is unlikely that an expression like “ t (df) = x.x” would stand for anything else than a t -value record.
The good performance of the extraction algorithm is also reflected in the similarity between the distributions of automatically and manually extracted degrees of freedom shown in Fig 1 (two-sample Kolgomorov-Smirnov test comparing the distributions: test statistic = 0.04; p > 0.127). This suggests that the degrees of freedom distribution underlying our effect size analysis was extracted accurately.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
Note that the distributions are close to overlapping.
https://doi.org/10.1371/journal.pbio.2000797.g001
Using the validation data, we found that the overwhelming majority of extracted two sample t -test records reported close-to-equal group numbers (median ratio of group numbers = 1). The ratio of the participant numbers in the larger group to the participant numbers in the smaller group was smaller than 1.15 in 77% of records. We also established that with degrees of freedom of ten or less, about 94% of tests were one sample or matched t -tests, whereas about 72% of records with higher degrees of freedom were one-sample or matched t -tests.
Computing effect sizes from t tests
t -test data was used for effect size, power, and FRP analysis as it is straightforward to estimate effect sizes from published t -values. After checks for reporting errors, seven records with degrees of freedom > 10,000 were excluded from analysis as outliers. This left 27,414 potential records. Of these records, 26,841 from 3,801 papers had both degrees of freedom and t -values reported. We used this data for the effect size analysis. 17,207 t -test records (64.1%) were statistically significant ( p ≤ 0.05) and 9,634 (35.9%) t -test records were statistically nonsignificant ( p > 0.05). 2,185 t -test records also reported Cohen's d as a measure of effect size (1,645 records with p ≤ 0.05 [75.3%] and 540 records with p > 0.05 [24.7%]).
As it is not possible to establish the exact participant numbers in groups for our large sample size, making a few reasonable assumptions is inevitable. First, based on our validation data from 1,478 records, we made the assumption that participant numbers in two-sample t -test groups were equal. The number of participants in groups was approximated as the upwards rounded value of half the potential total number of participants in the study, i.e, N subgroup = round upper ((df+2)/2), where df = degree of freedom. This formula even slightly exaggerates participant numbers in groups, so it can be considered generous when computing power. Second, regarding matched t -tests, we assumed that the correlation between repeated measures was 0.5. In such a case, the effect sizes can be approximated in the same way for both one-sample and matched t -tests. These assumptions allowed us to approximate effect sizes associated with all t -tests records in a straightforward way [ 20 – 21 ]. Computational details are provided in S1 Text , Section 2.
The power of t -tests was computed from the noncentral t distribution [ 22 ] assuming the above mixture of one-sample, matched-, and independent-sample t -tests. Computational details are provided in S1 Text , Section 3 . Power was computed for each effect size record. (Note that NHST is amalgamation of Fisher’s significance testing method and the Neyman-Pearson theory. However, the concept of power is only interpreted in the Neyman-Pearson framework. For extended discussion, see [ 23 – 24 ]).
First, we calculated power to detect small, medium, and large effect sizes. Power was computed for each extracted statistical record, taking into account the extracted degrees of freedom, a fixed (small, medium, or large) effect size with a significance level of α = 0.05.
Second, we also calculated power to detect the published effect sizes. Importantly, these published effect sizes are likely to be highly exaggerated. Using these exaggerated effect sizes for power calculations will then overestimate power. Hence, if we calculate FRP based on power calculated from published effect size reports, we are likely to estimate the lower limits of FRP. So, we estimated the lower limits for FRP, using the probably highly inflated effect sizes (computed from published t -values) to calculate power for various H 0 :H 1 odds and bias values and with α = 0.05. (The computation of FRP is laid out in detail in S1 Text , Section 5 .)
Most importantly, factoring in very small but statistically significant effect sizes as false reports into our calculations would only further increase FRP relative to the nill–null hypothesis testing model outlined above. That is, our calculations here really reflect a best-case scenario, the lowest possible levels of FRP when researchers use NHST.
The extracted degrees of freedom distributions are shown in Fig 2A (degrees of freedom reflect the sample sizes of the studies, e.g., for an independent sample t -test, the degrees of freedom are the sample size minus two). The median of degrees of freedom was 20 for statistically significant and 19 for statistically nonsignificant results (mode = 15 for both). During the validation process, we assessed the proportion of one-sample, matched, and two-sample t -tests, which enabled us to use a mixture model to compute published effect sizes and power. The distribution of the effect sizes computed from 26,841 t -value records showed an excellent match to the effect size distribution determined from 2,185 records where effect size (D)-values were reported ( Fig 2B ) . This suggests that the mixture model we used is likely to well approximate the proportions of one-sample, matched, and two-sample t -tests. The computed D-value distribution was more spread out to the right relative to the reported D-value distribution, but both the medians (computed = 0.654; reported = 0.660) and means (computed = 0.938; reported = 0.889) were very similar.
The histograms are zoomed in for better visibility, but all data were used in calculations. (A) Extracted df distribution in all 26,841 records. (The distributions were nearly overlapping for statistically significant and nonsignificant results.) (B) The distribution of D-values in statistically significant ( p ≤ 0.05) and nonsignificant ( p > 0.05) records in the whole data set (“computed”) and in the subset of data with D-value reports (“reported”). (C) The bivariate distribution of D-values and degrees of freedom in the whole data set. The density of statistical records is color coded, as shown by the calibration bar on the right. Curves in the figure are described in a left-to-right order. The leftmost dashed curve shows the expected value of effect sizes if the null hypothesis is true. The dotted curve shows the mean effect size from nonsignificant records in the data (the median was nearly the same). The middle thick continuous red curve denotes the significance threshold with p ≤ α where α = 0.05, in terms of D-values. The dotted-dashed blue curve and the rightmost continuous thin blue curve show the median and mean effect sizes only for statistically significant effect sizes, for various degrees of freedom.
https://doi.org/10.1371/journal.pbio.2000797.g002
Fig 2C shows the bivariate distribution of the 26,841 computed D-values and degrees of freedom and represents the mean and median effect sizes for statistically significant and nonsignificant records and for the whole dataset. Most statistically significant results were reported in the df 10–20 range, and the density of nonsignificant results also increased in this range. The effect size discrepancy between statistically significant and nonsignificant results is clear (medians, 25th and 75th quantiles for statistically significant and nonsignificant D-values, respectively: d = 0.932 [0.637–1.458]; d = 0.237 [0.106–0.421]).
Assuming 1:1 H 0 :H 1 odds [ 5 ], it is apparent that many statistically nonsignificant results are missing from the data; with 1:1 H 0 :H 1 odds, a large density of nonsignificant t -values could be expected on the left of the significance threshold and an even higher density of nonsignificant than significant results can be expected if H 0 :H 1 odds are larger than 1 (see the extracted t -value distribution in S2 Fig and compare the shape of this extracted t -value distribution to the expected shapes shown in S1A and S1B Fig ). Some nonsignificant results are missing because our extraction method could not pick up stand-alone p -values. However, the bias towards having mostly significant records in the data (amounting to three quarters of the records here) is also consistent with strong selective reporting biases. Such biases have been demonstrated in distributions of p -values reported in abstracts and full texts of biomedical papers [ 14 ]. Overall, effect sizes computed from the extracted data are biased towards larger effect sizes. Again, this means that the FRPs we estimate here represent lower limits.
For a certain effect size, power is determined by sample size, which determines degrees of freedom. Subfields showed large differences in degrees of freedom with most records having much lower degrees of freedom in cognitive neuroscience than in psychology and medicine ( Fig 3A ; 25th and 75th centiles for all records for cognitive neuroscience journals: df = 10–28; psychology: df = 17–60; medical journals: df = 15–54). Reported effect sizes also differed markedly by subfields (25th and 75th centiles for all records for cognitive neuroscience journals: d = 0.34–1.22; psychology: d = 0.29–0.96; medical journals: d = 0.23–0.91). The larger reported effect sizes in cognitive neuroscience may well be the consequence of effect size exaggeration due to having smaller sample sizes (as shown above) and consequential low power as the following power analyses suggest.
(A) The cumulative distribution of degrees of freedom in subfields. (i.e., the fraction of records with at least a certain level of degrees of freedom by science subfield.) (B) The cumulative distribution of power in subfields. The top three lines denote power for an effect size of D = 0.5. The bottom three lines denote power for an effect size of D = 0.2. Power is not shown for effect size of D = 0.8.
https://doi.org/10.1371/journal.pbio.2000797.g003
Taking into account the reported degrees of freedom, we computed power (at α = 0.05) for effect sizes, which are traditionally considered small (d = 0.2), medium (d = 0.5), and large (d = 0.8) [ 16 – 18 ]. The cumulative probability of records reaching a certain level of power is shown in Fig 3B (for power calculation details see S1 Text , Section 3 ).
Median and mean power for subfields are shown in Table 1 . Under the assumption that standardized effect sizes are similar in all subfields tested, it is apparent that cognitive neuroscience studies had the lowest level of power. For example, to detect a small true effect (d = 0.2), 90% of cognitive neuroscience records had power < 0.234. This is a much worse chance to detect a true effect than relying on flipping a coin [ 17 ].
The bottom row shows mean power computed from 25 power surveys.
https://doi.org/10.1371/journal.pbio.2000797.t001
A comparison to prominent older surveys of power estimates 25 and >50-y-ago showed that median power to detect medium-sized effects has increased slightly in psychology journals but remained about the same for small and large effects (see Table 1 [ 16 – 18 ]). Power for cognitive neuroscience and for all subfields together was lower than median and mean power reported in 1962, more than half a century ago.
Median degrees of freedom and median effect sizes for each journal are depicted in Fig 4 . It is apparent that cognitive neuroscience journals report the largest effect sizes but at the same time have the smallest degrees of freedom. Consequently, they also have the lowest power levels assuming similar true effect sizes across fields and are most subject to effect size exaggeration. As a further consequence, journal impact factors negatively correlated with median power because, on the average, cognitive neuroscience journals had the largest impact factors in our sample (correlation for small, medium, and large effect sizes, respectively with 95% accelerated and bias corrected bootstrap confidence intervals [10 5 permutations]: r = −0.42 [−0.63; −0.09]; −0.46 [−0.71; −0.09]; −0.45 [−0.77; −0.02]).
Median effect sizes and degrees of freedom in the journals analyzed. Red crosses denote cognitive neuroscience journals. Green circles denote psychology journals. Blue triangles denote medically oriented journals. Journal markers to the left of the significance threshold show medians for statistically nonsignificant records (range of medians: d = 0.15–0.29). Journal markers to the right of the significance threshold show medians for statistically significant records (range of medians: d = 0.57–1.17). Journal abbreviations: Neuroscience: Nature Neuroscience (NN), Neuron, Brain (B), The Journal of Neuroscience (JN), Cerebral Cortex (Ccrx), NeuroImage (Ng), Cortex (Cx), Biological Psychology (BP), Neuropsychologia (NPy), Neuroscience (NSci). Psychology: Psychological Science (Psi), Cognitive Science (CS), Cognition (Cogn), Acta Psychologica (Acta), Journal of Experimental Child Psychology (JC). Medically oriented journals: Biological Psychiatry (BPS), Journal of Psychiatric Research (JPR), Neurobiology of Ageing (Nage).
https://doi.org/10.1371/journal.pbio.2000797.g004
The somewhat higher power in the journals we classified as more medically oriented was driven by the Journal of Psychiatry Research (JPR in Fig 4 ; median power to detect small, medium and large effects: 0.23, 0.74, 0.86), which includes more behavioral studies than the other two journals we classified as “medical.” These other two journals, more focused on neuroimaging, still performed better than cognitive neuroscience journals and at about the same level as psychology journals (median power to detect small, medium, and large effects: 0.14, 0.53, 0.78).
FRP depends on power (which depends on sample size and effect size), the prestudy odds of true H 0 to H 1 data, and on reporting bias [ 5 ]. In this context, we use the term “bias” in a general abstract sense as a model parameter as defined by Ioannidis [ 5 ]. That is, bias stands for any kind of implicit or explicit technique, manipulation, or error which can result in the outcome that a certain proportion of results which would otherwise be reported as statistically nonsignificant will be reported as statistically significant (see details and mathematical definition in S1 Text , Section 5 ). For example, if the bias parameter equals 0.1, that means that 10% of results which would be reported as statistically nonsignificant in the absence of bias will be now reported as statistically significant. Such bias can easily appear due to data dredging techniques even if formal NHST parameters are maintained [ 5 ]. For example, if the main (prespecified) analysis does not yield a formally significant result, investigators may remove or add cases [ 25 ], change the model specification [ 26 ] and/or data preprocessing parameters in neuroimaging [ 27 ], change the statistical analytical method, report on a different outcome, or report a statistically nonsignificant result as significant (e.g., reporting p = 0.058 as p < 0.05; [ 28 ]. Altogether, there are many ways that nonsignificant results may become significant. Frank publication bias (suppression/nonpublication of nonsignificant results), and the rarer fraud with fabrication of nonexistent data or distorting data, yielding significant results will all lead to an excess of reported statistically significant results [ 29 ].
The continuous lines in Fig 5 estimate lower limits for FRP, using the probably inflated effect sizes computed from published t -values, for various prestudy H 0 :H 1 odds and bias values and for α = 0.05. H 0 :H 1 odds are difficult to determine empirically. First, the nil–null hypothesis is never exactly true. From this perspective, it could be argued that even a very small deviation from the null hypothesis, i.e., a very small effect size, could be considered not only statistically but also practically “significant.” However, very small effect sizes are practically meaningless (see the Materials and Methods section on further elaboration on this). So, when considering H 0 :H 1 odds, it makes more sense to think about these in the context of effect sizes which could be considered practically meaningful/useful to know about. From such a perspective, it would be unrealistic to assume that most tested hypotheses are really correct (i.e., that they are associated with reasonable effect sizes; [ 5 ]); and a recent reanalysis of the Open Science Collaboration replication project [ 1 ] also suggests that H 0 :H 1 odds are likely to be as high as 13:1 (93% true H 0 situations), at least in psychology [ 30 ].
(A) FRP for a wide range (0.1–1,000) of H 0 :H 1 odds on a 10-based logarithmic scale (horizontal axis). (B) FRP for the 1–30 range. The dotted vertical lines denote the same H 0 :H 1 odds in both panels for easier comparison (H 0 :H 1 odds of 1, 2, 3, 4, 5, 10, 15, 20, 25, and 30). Note that the vertical axis begins at 0.1 in Panel B for better visibility. FRP is provided for a range of bias values. For example, bias = 0.1 means that 10% of results that would be reported as statistically nonsignificant in the absence of bias will be now reported as statistically significant. See further elaboration on bias in the text.
https://doi.org/10.1371/journal.pbio.2000797.g005
Fig 5A represents the lower limits of FRP computed from our data for a wide range of H 0 :H 1 odds on a 10-based logarithmic scale. Observe that FRP is under 10% only if H 0 :H 1 odds are smaller than one. In such a case, researchers would mostly come up with correct alternative hypotheses. This is perhaps possible in conservative, very incremental research. On the contrary, in the range of explorative research where H 0 :H 1 odds are larger than 100, FRP is above 90%.
Fig 5B shows FRP zooming into the 1–30 H 0 :H 1 odds range for better visibility. This range of H 0 :H 1 odds would still represent a relatively high proportion of correct alternative hypotheses but would keep the ratio of true null hypotheses slightly or moderately higher than the ratio of true alternative hypotheses. Hence, this range of H 0 :H 1 odds represents a kind of compromise between those who would assume that most null hypotheses are false and those who would assume that most null hypotheses are correct. In the best case of having H 0 :H 1 odds = 1:1 = 1 and zero bias, FRP is 13.5%. A 10% bias pushes this to 23%. Staying in the optimistic zone when every second to every sixth of hypotheses work out (1 ≤ H 0 :H 1 odds ≤ 5) and with relatively modest 10%–30% experimenter bias, FRP is 23%–71% (median = 51%). That is, between one- to three-quarters of statistically significant results will be false positives. If we now move into the domain of slightly more exploratory research where even more experimental ideas are likely to be false (5 < H 0 :H 1 odds < 20; bias = 10%–30%), then FRP grows to at least 60%–91% (median = 77%). Notably, if we consider the recent estimate of 13:1 H 0 :H 1 odds [ 30 ], then FRP exceeds 50% even in the absence of bias.
It is important to note that here we use a single α = 0.05 threshold for FRP calculations because this is the rule of thumb α level used in the science fields we analyzed. That is, even if a record reports, for example, p < 0.001, it does not mean that the a priori α level was α = 0.001. Rather, most probably, the result would have been reported as statistically significant as long as the condition p ≤ (α = 0.05) would have been valid (Note that α = 0.05 is an assignment, p ≤ α is a test of inequality, and the parentheses are important for correct interpretation. This notation aims to emphasize the crucial difference between the p -value and the α level which are often confused. [ 15 ]). That is, using a single α = 0.05 threshold here provides the most accurate estimates about the lowest expected limits of FRP in the cognitive neuroscience and psychology literature.
The trustworthiness of statistically significant findings depends on power, prestudy H 0 :H 1 odds, and experimenter bias [ 5 , 7 , 13 ]. H 0 :H 1 odds are inherent to each research field, and the extent and types of biases can vary from one field to another. The distribution of the types of biases may also change within a field if focused efforts are made to reduce some types of major bias (like selective reporting), for example by preregistration of studies. However, power can in principle be easily increased by increasing sample size. Nevertheless, contrary to its importance for the economic spending of research funding, the accurate estimation of effect sizes, and minimizing FRP, our data suggest that power in cognitive neuroscience and psychology papers is stuck at an unacceptably low level. This is so because sample sizes have not increased during the past half-century [ 16 – 18 ]. Results are similar to other fields, such as behavioral ecology where power to detect small and medium effects was 0.13–0.16 and 0.4–0.47, respectively [ 31 ].
Assuming similar true effect sizes across fields, we conclude that cognitive neuroscience journals have lower power levels than more psychologically and medically oriented journals. This confirms previous similar inference asserting that FRP is likely to be high in the neuroimaging literature [ 6 , 32 ]. This phenomenon can appear for a number of reasons.
First, neuroimaging studies and other studies using complex and sophisticated measurement tools in general tend to require more expensive instrumentation than behavioral studies, and both data acquisition and analysis may need more time, investment, and resources per participant. This keeps participant numbers low. A related issue is that science funders may have reluctance to fund properly powered but expensive studies.
Second, data analysis is highly technical, can be very flexible, and many analytical choices have to be made on how exactly to analyze the results; and a large number of exploratory tests can be run on the vast amount of data collected in each brain imaging study. This allows for running a very high number of undocumented and sometimes poorly understood and difficult to replicate idiosyncratic analyses influenced by a large number of arbitrary ad hoc decisions. These, in their entirety, may be able to generate statistically significant false positive results with high frequency [ 27 , 33 – 35 ], especially when participant numbers are low. Hence, sticking to low participant numbers may facilitate finding statistically significant publishable (false positive) results. It is also important to consider that complicated instrumentation and (black box) analysis software is now more available, but training may not have caught up with this wider availability.
Third, in relation to more medical journals, the stakes at risk are probably lower in cognitive neuroscience (no patients will die, at least not immediately), which may also allow for more biased publications. That is, researchers may be more willing to publish less reliable findings if they think that these are not directly harmful. The power failure of the cognitive neuroscience literature is even more notable as neuroimaging (“brain-based”) data is often perceived as “hard” evidence, lending special authority to claims even when they are clearly spurious [ 36 ]. A related concern is the negative correlation between power and journal impact factors. This suggests that high impact factor journals should implement higher standards for pre-study power (optimally coupled with preregistration of studies) to assure the credibility of reported results. Speculatively, it is worth noting that the high FRP allowed by low power also allows for the easier production of somehow extraordinary results, which may have higher chances to be published in high impact factor journals [ 37 ].
Standardized effect sizes depend on the largeness of effects and the noise level they are embedded in (effect size is larger if signal to noise ratio is better). In behavioral psychology studies, measurement imprecision and variability (e.g., test–retest replicability and reliability, stableness of participant characteristics, etc.) introduce noise. In cognitive neuroscience studies, physiological noise (e.g., various physiological artefacts generated externally or internally to participants) will further contribute to measurement imprecision, while the physiological signals of interest are usually small. Hence, we could expect that measurable standardized effect sizes are in general smaller in cognitive neuroscience than in psychology because both behavioral and physiological noise may contribute to measurements (however, note, as explained before, that due to reliance on NHST, typically only statistically significant exaggerated effect sizes are reported in papers). Were effect sizes really smaller, power would be even worse in cognitive neuroscience relative to psychology than indicated here. Good quality cognitive neuroscience studies may try to counteract physiological noise by increasing trial numbers in individual measurements. A larger number of trials in individuals will then decrease the standard errors of means in these individuals, which may result in smaller group level standard deviations if there is an “ideal” mean measurement value not depending on individuality (but note that individual differences are usually neglected in group studies). This, in turn, will increase group-level t -values and effect sizes. Hence, consequences of individual trial numbers have already been taken into account in the calculations reported here when calculating the lower limits of FRP.
Here, we have not explicitly factored in the impact of specific questionable research practices (see, e.g., [ 26 , 38 ]). Rather, we have factored in their potential joint impact through the general “bias” parameter when calculating FRP. Nevertheless, it would be important to see the individual contribution of various data dredging techniques to increasing FRP. For example, researchers may neglect multiple testing correction [ 39 – 41 ]; post hoc select grouping variables [ 42 , 26 ]; use machine-learning techniques to explore a vast range of post hoc models, thereby effectively p -hacking their data by overfitting models ( http://dx.doi.org/10.1101/078816 ); and/or liberally reject data not supporting their favored hypotheses. Some of these techniques can easily generate 50% or more false positive results on their own while outputting some legitimate looking statistics [ 25 – 26 ]. In addition, it is also well documented that a large number of p -values are misreported, indicating statistically significant results when results are, in fact, nonsignificant [ 41 , 43 – 45 ].
With specific respect to functional magnetic resonance imaging (fMRI), a recent analysis of 1,484 resting state fMRI data sets have shown empirically that the most popular statistical analysis methods for group analysis are inadequate and may generate up to 70% false positive results in null data [ 46 , 47 ]. This result alone questions the published outcomes and interpretations of thousands of fMRI papers. Similar conclusions have been reached by the analysis of the outcome of an open international tractography challenge, which found that diffusion-weighted magnetic resonance imaging reconstructions of white matter pathways are dominated by false positive outcomes ( http://dx.doi.org/10.1101/084137 ). Hence, provided that here we conclude that FRP is very high even when only considering low power and a general bias parameter (i.e., assuming that the statistical procedures used were computationally optimal and correct), FRP is actually likely to be even higher in cognitive neuroscience than our formal analyses suggest.
Some limitations need to be mentioned for our study. First, given the large-scale automation, we cannot verify whether the extracted data reflect primary, secondary, or even trivial analyses in each paper. In the absence of preregistered protocols, however, this is extremely difficult to judge, even when full papers are examined. Evaluation of biomedical papers suggests that many reported p -values, even in the abstracts, are not pertinent to primary outcomes [ 3 ]. Second, some types of errors, such as nondifferential misclassification (measurement error that is not related to the outcome of interest), may lead to deflated effect sizes. However, in the big picture, with very small power, inflation of the statistically significant effects is likely to be more prominent than errors reducing the magnitude of the effect size. Third, given the large scale automated extraction, we did not record information about characteristics of the published studies, e.g., study design. It is likely that studies of different designs (e.g., experimental versus observational studies) may have different distribution of effect sizes, degrees of freedom, and power, even within the same subdiscipline. Hence, we could not take into account the impact of the quality of experimental design on power. Fourth, here we only estimated power for a mixture model of t -tests based on the extracted degrees of freedom. Nevertheless, it is very likely that the extracted degrees of freedom give a good indication of participant numbers in studies. These participant numbers would then be strongly correlated with the statistical power of any other analyses done besides t -tests. Fifth, we could not extract all nonsignificant relevant p -values that are often reported on their own. This biased the observed effect sizes towards larger values. However, this means that the FRPs we computed really reflect lower estimates. Finally, generalizations need to be cautious, since there can be large variability in the extent of these potential biases within a given subfield. Some teams and subfields may have superb, error-proof research practices, while others may have more frequent problems.
In all, the combination of low power, selective reporting, and other biases and errors that have been well documented suggest that high FRP can be expected in cognitive neuroscience and psychology. For example, if we consider the recent estimate of 13:1 H 0 :H 1 odds [ 30 ], then FRP exceeds 50% even in the absence of bias. The low reproducibility rate seen for psychology experimental studies in the recent Open Science Collaboration [ 1 ] is congruent with the picture that emerges from our data. Our data also suggest that cognitive neuroscience may have even higher FRP rates than psychology. This hypothesis is worth evaluating with focused reproducibility checks of published studies. Regardless, efforts to increase sample size and reduce publication and other biases and errors are likely to be beneficial for the credibility of this important literature.
Some promising avenues to resolve the current replication crisis could include the preregistration of study objectives, compulsory prestudy power calculations, enforcing minimally required power levels, raising the statistical significance threshold to p < 0.001 if NHST is used, publishing negative findings once study design and power levels justify this, and using Bayesian analysis to provide probabilities for both the null and alternative hypotheses [ 12 , 26 , 30 , 48 ].
Supporting information
S1 fig. t value distributions when all negative and positive results are published (df = 22; d = 0.75; α = 0.05 for both panels)..
(A) Illustration of effect size exaggeration due to lack of power. ±t(α) stand for the critical t values. The figure depicts the probability density of t values under a mixture model (Eq 11) assuming a 70% proportion of one-sample t-tests. The thin blue line denotes the probability density of t values if the null hypothesis is true. The thick red line denotes the probability density of t values if the alternative hypothesis is true with an effect size of D = 0.75. Note that because the mixture model assumes a mixture of both one-sample and two-sample t-tests, the probability density curve for t values (under H 1 ) is not symmetric. The dashed black line denotes the probability density of t values if in half the data the null hypothesis is true and in the other half the alternative hypothesis is true (ie. The H 0 :H 1 odds are 1). The little crosses, bars and triangles mark the expected value of absolute t values. Note that these are dramatically different in statistically significant and non-significant data irrespective of whether the null hypothesis is really true or not. Blue bars: the expected t value in data where the null hypothesis is true and the test outcome is non-significant (left bar: true negative) and when the test outcome is significant (right bar: false positive). Red triangles: the expected t value in data where the alternative hypothesis is true and the test outcome is non-significant (left triangle: false negative) and when the test outcome is significant (right triangle: true positive). Black crosses: the expected t values in non-significant (left cross) and significant (right cross) data. Signal detection decision probabilities are shown by α (false positive), 1-α (correct rejection of H 0 ), β (false negative) and Power (true positive) in the figure. (B) Expected mixture model t value distribution for various H 0 :H 1 odds(see legend).
https://doi.org/10.1371/journal.pbio.2000797.s001

S2 Fig. The extracted t-value distribution.
(A) The one dimensional probability density distribution of extracted t-values. (B) The two-dimensional t-value by degrees of freedom distribution. The significance threshold [p≤(α = 0.05)] is marked by the white curve. The density of records is shown by the colorbar on the right.
https://doi.org/10.1371/journal.pbio.2000797.s002
S1 Table. Journal information for the three subfields investigated.
5-year journal impact factors used in the study; the number of records in journals; the number of papers by journals and the average number of records per paper.
https://doi.org/10.1371/journal.pbio.2000797.s003
S1 Text. Supporting Methods.
https://doi.org/10.1371/journal.pbio.2000797.s004
S1 Data. Data in Matlab format.
https://doi.org/10.1371/journal.pbio.2000797.s005
S1 Code. Matlab code.
https://doi.org/10.1371/journal.pbio.2000797.s006
Acknowledgments
The authors thank the help of Ana Sanchez Marin in organizing PDF files and Timothy Myers in the validation process. We thank the helpful comments of Philip Dawid and Sir David Spiegelhalter (both at the Statistical Laboratory, University of Cambridge, UK) on some features of our data.
- View Article
- Google Scholar
- PubMed/NCBI
- 19. Epskamp, S. & Nuijten, M. B. statcheck: Extract statistics from articles and recompute p values. Retrieved from http://CRAN.R-project.org/package=statcheck . (R package version 1.0.0). 2014.
- 20. Hunter JE, Schmidt FL. Methods of meta-analysis: Correcting error and bias in research findings. Newbury park. Sage. 1990.
- 24. Gigerenzer G, Swijtnik Z, Porter T, Daston L, Beatty J, Kruger L. The empire of chance: How probability changed science and everyday life. Cambridge University Press. 1989.
- 35. Uttal WR. Reliability in cognitive neuroscience: A meta-meta-analysis. MIT Press. 2012.
- 39. Barnett V, Lewis T. Outliers in statistical data. Chichester,England: Wiley. 1994

Child Trauma Recovery Tied to Thoughts, Not Event Severity
Summary: A new study reveals that how children mentally process traumatic events is the most significant factor in predicting their mental health outcomes, including PTSD, anxiety, and depression. Cognitive factors—like how children remember the event and view themselves afterward—play a more critical role than the event’s objective severity.
Researchers found that children with more negative self-perceptions or distorted memories were at higher risk for long-term psychological impacts. Interestingly, the study showed that addressing these cognitive factors through trauma-focused cognitive behavioral therapy may improve recovery. This insight offers a path to better therapeutic approaches for children who have experienced trauma.
Key Facts :
- Children’s self-perceptions and memory distortions post-trauma were key predictors of mental health issues.
- Event severity was less predictive of outcomes than children’s subjective experience.
- Trauma-focused cognitive behavioral therapy may address these cognitive impacts effectively.
Source: University of East Anglia
A new study has shed light on why some children and adolescents develop mental health disorders like PTSD, anxiety, or depression after experiencing a traumatic event.
While most children recover well after a traumatic event, some go on to develop mental health disorders that may stay with them for months, years, or even into adulthood.
The University of East Anglia research found that cognitive psychological factors—such as how children remember the event and how they perceive themselves afterward—are the strongest predictors of poor mental health outcomes following a trauma.

Co-author Katie Lofthouse, of UEA’s Norwich Medical School, said: “Some children and adolescents who have experienced traumatic events like road traffic collisions or violence may develop post-traumatic stress disorder (PTSD), as well as other conditions such as anxiety and depression.
“However, we do not understand why children might develop one set of difficulties and not another.
“We found that cognitive psychological factors – like features of their memories for the trauma and how they see themselves after the trauma – were the most powerful predictors of all forms of poor mental health.
“Aspects of how severe the trauma was, or a child’s age or sex were much less important.”
The research team worked with 260 children aged between eight and 17 who had attended a hospital emergency department following a one-off traumatic incident. These included events such as car crashes, assaults, dog attacks and other medical emergencies.
These young people were assessed at two and nine weeks post-trauma using self-report questionnaires completed by the child, telephone interviews with parents, and hospital data, which was then used to develop four predictive models of risk factors for PTSD, Complex PTSD (CPTSD), depression, and Generalised Anxiety Disorder (GAD).
At nine weeks post-trauma, 64pc showed no signs of any disorder, 23.5pc met the criteria for PTSD, and 5.2pc for CPTSD. A total of 23.9pc and 10.7pc had developed clinically significant symptoms of depression and GAD, respectively.
When it came to predicting who would develop these mental health issues, a model based on how people think (a cognitive model) was the most accurate.
A model that looked at social and psychological factors was weaker in predicting later mental health symptoms.
Interestingly, a child’s personal perceptions of how severe the event was had a stronger impact on their mental health than objective, measurable facts about the severity of the event.
Miss Lofthouse added: “These findings highlight risk factors for the development of mental health disorders following trauma exposure in youth.
“Negative thoughts about the traumatic event were a major predictor of all types of mental health problems studied.
“This supports the use of treatments like trauma-focused cognitive behavioural therapy, which aims to address these negative thoughts.
“Our research also showed that poor memory of the trauma specifically predicted PTSD, suggesting that certain symptoms may help predict different mental health outcomes.”
Previous research from UEA found that children are more likely to suffer Post Traumatic Stress Disorder (PTSD) if they think their reaction to traumatic events is not ‘normal’.
This latest research goes into further depth, looking at not just PTSD, but also other mental health outcomes such as complex PTSD, depression, and anxiety.
Complex PTSD includes all the symptoms of PTSD but also has some additional, more severe emotional and psychological impacts. This can include problems managing extreme emotions, feelings of deep shame, guilt, or worthlessness and difficulty trusting others, feeling detached or isolated, or experiencing ongoing conflicts in relationships.
There have been few studies that compare how well different models can predict mental health outcomes in people who have experienced trauma, and none of these studies have focused on young people.
With the recent introduction of Complex PTSD (CPTSD) as a diagnosis, the research team wanted to see if it was possible to predict mental health issues in youth who have been through trauma.
The research team also considered factors including other life stressors and whether the child was experiencing on-going pain.
The researchers say the results back up the idea that how a person thinks about their trauma plays a big role in PTSD, but they also show that this model is not disorder-specific and applies to Complex PTSD, depression, and anxiety.
Further research could look more closely at thoughts tied to specific disorders or focus on general distress after trauma.
Funding: The work was supported by the Medical Research Council and led by the University of East Anglia in collaboration with the University of Cambridge, Addenbrooke’s Hospital, Macquarie University, Sussex Partnership NHS Foundation Trust and King’s College London.
About this child trauma and mental health research news
Author: Kimberley Powles Source: University of East Anglia Contact: Kimberley Powles – University of East Anglia Image: The image is credited to Neuroscience News
Original Research: Open access. “ Predictive models of post-traumatic stress disorder, complex post-traumatic stress disorder, depression, and anxiety in children and adolescents following a single-event Trauma ” by Katie Lofthouse et al. Psychological Medicine
Predictive models of post-traumatic stress disorder, complex post-traumatic stress disorder, depression, and anxiety in children and adolescents following a single-event Trauma
This study examined the power of theory-derived models to account for the development of PTSD, Complex PTSD (CPTSD), depression, and anxiety in children and adolescents who had experienced a single-event trauma.
Children ( n = 234, aged 8–17 years) recruited from local Emergency Departments were assessed at two and nine weeks post-trauma. Data obtained from self-report questionnaires completed by the child, telephone interviews with parents, and hospital data were used to develop four predictive models of risk factors for PTSD, CPTSD, depression, and Generalized Anxiety Disorder (GAD). ICD-11 proposed diagnostic criteria were used to generate measures for CPTSD and PTSD to assess for risk factors and identify the sample prevalence of these disorders.
At nine weeks post-trauma, 64% did not meet criteria for any disorder, 23.5% met criteria for PTSD, and 5.2% met criteria for CPTSD. 23.9% and 10.7% had developed clinically significant symptoms of depression and GAD, respectively. A cognitive model was the most powerful predictive model, a psychosocial model was weak, and subjective markers of event severity were more powerful than objective measures.
Conclusions
Youth exposed to single-incident trauma may develop different forms of psychopathology, and PTSD and CPTSD are frequently experienced alongside other conditions. The cognitive model of PTSD shows utility in identifying predictors of PTSD, CPTSD, depression, and GAD, particularly the role of trauma-related negative appraisals. This supports the application of cognitive interventions which focus upon re-appraising trauma-related beliefs in youth.
Helping Kids Fact-Check in the Age of Misinformation

Higher IQ in High School Linked to Increased Alcohol Use in Adulthood

Skin Pigmentation May Influence Drug Response
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 03 May 2022
Theories of consciousness
- Anil K. Seth ORCID: orcid.org/0000-0002-1421-6051 1 , 2 &
- Tim Bayne 2 , 3 , 4
Nature Reviews Neuroscience volume 23 , pages 439–452 ( 2022 ) Cite this article
62k Accesses
1188 Altmetric
Metrics details
- Consciousness
- Neuroscience
Recent years have seen a blossoming of theories about the biological and physical basis of consciousness. Good theories guide empirical research, allowing us to interpret data, develop new experimental techniques and expand our capacity to manipulate the phenomenon of interest. Indeed, it is only when couched in terms of a theory that empirical discoveries can ultimately deliver a satisfying understanding of a phenomenon. However, in the case of consciousness, it is unclear how current theories relate to each other, or whether they can be empirically distinguished. To clarify this complicated landscape, we review four prominent theoretical approaches to consciousness: higher-order theories, global workspace theories, re-entry and predictive processing theories and integrated information theory. We describe the key characteristics of each approach by identifying which aspects of consciousness they propose to explain, what their neurobiological commitments are and what empirical data are adduced in their support. We consider how some prominent empirical debates might distinguish among these theories, and we outline three ways in which theories need to be developed to deliver a mature regimen of theory-testing in the neuroscience of consciousness. There are good reasons to think that the iterative development, testing and comparison of theories of consciousness will lead to a deeper understanding of this most profound of mysteries.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
176,64 € per year
only 14,72 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others

Two conceptions of consciousness and why only the neo-Aristotelian one enables us to construct evolutionary explanations
The ConTraSt database for analysing and comparing empirical studies of consciousness theories
The complexity of the stream of consciousness
Crick, F. & Koch, C. Towards a neurobiological theory of consciousness. Semin. Neurosci. 2 , 263–275 (1990).
Google Scholar
Metzinger, T. (ed.) Neural Correlates of Consciousness: Empirical and Conceptual Questions (MIT Press, 2000).
Koch, C., Massimini, M., Boly, M. & Tononi, G. Neural correlates of consciousness: progress and problems. Nat. Rev. Neurosci. 17 , 307–321 (2016).
Article CAS PubMed Google Scholar
de Graaf, T. A., Hsieh, P. J. & Sack, A. T. The ‘correlates’ in neural correlates of consciousness. Neurosci. Biobehav. Rev. 36 , 191–197 (2012).
Article PubMed Google Scholar
Aru, J., Bachmann, T., Singer, W. & Melloni, L. Distilling the neural correlates of consciousness. Neurosci. Biobehav. Rev. 36 , 737–746 (2012).
Tsuchiya, N., Wilke, M., Frassle, S. & Lamme, V. A. No-report paradigms: extracting the true neural correlates of consciousness. Trends Cogn. Sci. 19 , 757–770 (2015).
Klein, C., Hohwy, J. & Bayne, T. Explanation in the science of consciousness: from the neural correlates of consciousness (NCCs) to the difference-makers of consciousness (DMCs). Philos. Mind Sci . https://doi.org/10.33735/phimisci.2020.II.60 (2020).
Article Google Scholar
Michel, M. et al. Opportunities and challenges for a maturing science of consciousness. Nat. Hum. Behav. 3 , 104–107 (2019).
Article PubMed PubMed Central Google Scholar
Seth, A. K. Consciousness: the last 50 years (and the next). Brain Neurosci. Adv. 2 , 2398212818816019 (2018).
Seth, A. K. Explanatory correlates of consciousness: theoretical and computational challenges. Cogn. Comput. 1 , 50–63 (2009).
Searle, J. The Rediscovery of the Mind (MIT Press, 1992).
Varela, F. J. Neurophenomenology: a methodological remedy for the hard problem. J. Conscious. Stud. 3 , 330–350 (1996).
Seth, A. K. Being You: A New Science of Consciousness (Faber & Faber, 2021).
Dennett, D. C. Welcome to strong illusionism. J. Conscious. Stud. 26 , 48–58 (2019).
Frankish, K. Illusionism as a Theory of Consciousness (Imprint Academic, 2017).
Wiese, W. The science of consciousness does not need another theory, it needs a minimal unifying model. Neurosci. Conscious. 2020 , niaa013 (2020).
Melloni, L., Mudrik, L., Pitts, M. & Koch, C. Making the hard problem of consciousness easier. Science 372 , 911–912 (2021). This work sets out how an adversarial collaboration is planning to arbitrate between integrated information and global workspace ToCs .
Hameroff, S. & Penrose, R. Consciousness in the universe: a review of the ‘Orch OR’ theory. Phys. Life Rev. 11 , 39–78 (2014).
Chalmers, D. J. & McQueen, K. in Quantum Mechanics and Consciousness (ed Gao, S.) (Oxford Univ. Press, 2022).
Nagel, T. What is it like to be a bat? Philos. Rev. 83 , 435–450 (1974).
Bayne, T., Hohwy, J. & Owen, A. M. Are there levels of consciousness? Trends Cogn. Sci. 20 , 405–413 (2016). This work challenges the common unidimensional notion of ‘level of consciousness’, outlining an alternative, richer, multidimensional account .
Metzinger, T. Being No-One (MIT Press, 2003).
Damasio, A. Self Comes To Mind: Constructing the Conscious Brain (William Heinemann, 2010).
Park, H. D. & Tallon-Baudry, C. The neural subjective frame: from bodily signals to perceptual consciousness. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369 , 20130208 (2014).
Bayne, T. The Unity of Consciousness (Oxford Univ. Press, 2010).
Bayne, T. & Chalmers, D. J. in The Unity of Consciousness: Binding, Integration, and Dissociation (ed Cleeremans, A.) 23–58 (Oxford Univ. Press, 2003).
Cummins, R. Functional analysis. J. Philos. 72 , 741–765 (1975).
Blake, R., Brascamp, J. & Heeger, D. J. Can binocular rivalry reveal neural correlates of consciousness? Philos. Trans. R. Soc. Lond. B Biol. Sci. 369 , 20130211 (2014).
Signorelli, C. M., Szczotka, J. & Prentner, R. Explanatory profiles of models of consciousness — towards a systematic classification. Neurosci. Conscious. 2021 , niab021 (2021).
Lau, H. & Rosenthal, D. Empirical support for higher-order theories of conscious awareness. Trends Cogn. Sci. 15 , 365–373 (2011). This work presents a summary of empirical evidence favouring higher-order ToCs .
Rosenthal, D. Consciousness and Mind (Clarendon, 2005).
Brown, R. The HOROR theory of phenomenal consciousness. Philos. Stud. 172 , 1783–1794 (2015).
Cleeremans, A. Consciousness: the radical plasticity thesis. Prog. Brain Res. 168 , 19–33 (2008).
Cleeremans, A. et al. Learning to be conscious. Trends Cogn. Sci. 24 , 112–123 (2020).
Fleming, S. M. Awareness as inference in a higher-order state space. Neurosci. Conscious. 2020 , niz020 (2020).
Lau, H. Consciousness, metacognition, and perceptual reality monitoring. Preprint at ArXiv https://doi.org/10.31234/osf.io/ckbyf (2020).
Gershman, S. J. The generative adversarial brain. Front. Artif. Intell. https://doi.org/10.3389/frai.2019.00018 (2019).
Cohen, M. A., Dennett, D. C. & Kanwisher, N. What is the bandwidth of perceptual experience? Trends Cogn. Sci. 20 , 324–335 (2016).
Haun, A. M., Tononi, G., Koch, C. & Tsuchiya, N. Are we underestimating the richness of visual experiences? Neurosci. Conscious. 3 , 1–4 (2017).
Odegaard, B., Chang, M. Y., Lau, H. & Cheung, S. H. Inflation versus filling-in: why we feel we see more than we actually do in peripheral vision. Philos. Trans. R. Soc. Lond. B Biol. Sci. https://doi.org/10.1098/rstb.2017.0345 (2018).
LeDoux, J. E. & Brown, R. A higher-order theory of emotional consciousness. Proc. Natl Acad. Sci. USA 114 , E2016–E2025 (2017).
Article CAS PubMed PubMed Central Google Scholar
Morrison, J. Perceptual confidence. Anal. Philos. 78 , 99–147 (2016).
Peters, M. A. K. Towards characterizing the canonical computations generating phenomenal experience. Preprint at PsyArXiv https://doi.org/10.31234/osf.io/bqfr6 (2021).
Rosenthal, D. Consciousness and its function. Neuropsychologia 46 , 829–840 (2008).
Charles, L., Van Opstal, F., Marti, S. & Dehaene, S. Distinct brain mechanisms for conscious versus subliminal error detection. Neuroimage 73 , 80–94 (2013).
Brown, R., Lau, H. & LeDoux, J. E. Understanding the higher-order approach to consciousness. Trends Cogn. Sci. 23 , 754–768 (2019).
Baars, B. J. A Cognitive Theory of Consciousness (Cambridge Univ. Press, 1988).
Dehaene, S. & Changeux, J. P. Experimental and theoretical approaches to conscious processing. Neuron 70 , 200–227 (2011).
Mashour, G. A., Roelfsema, P., Changeux, J. P. & Dehaene, S. Conscious processing and the global neuronal workspace hypothesis. Neuron 105 , 776–798 (2020). This work presents a summary of the neuronal GWT and its supporting evidence .
Dehaene, S., Sergent, C. & Changeux, J. P. A neuronal network model linking subjective reports and objective physiological data during conscious perception. Proc. Natl Acad. Sci. USA 100 , 8520–8525 (2003).
Naccache, L. Why and how access consciousness can account for phenomenal consciousness. Philos. Trans. R. Soc. Lond. B Biol. Sci. https://doi.org/10.1098/rstb.2017.0357 (2018).
Mashour, G. A. Cognitive unbinding: a neuroscientific paradigm of general anesthesia and related states of unconsciousness. Neurosci. Biobehav. Rev. 37 , 2751–2759 (2013).
Demertzi, A. et al. Human consciousness is supported by dynamic complex patterns of brain signal coordination. Sci. Adv. 5 , eaat7603 (2019). This large empirical study of functional connectivity patterns across different global states of consciousness focuses on how these patterns relate to underlying structural connectivity .
Barttfeld, P. et al. Signature of consciousness in the dynamics of resting-state brain activity. Proc. Natl Acad. Sci. USA 112 , 887–892 (2015).
Uhrig, L. et al. Resting-state dynamics as a cortical signature of anesthesia in monkeys. Anesthesiology 129 , 942–958 (2018).
Carruthers, P. Human and Animal Minds: The Consciousness Questions Laid to Rest (Oxford Univ. Press, 2019).
Tononi, G. Consciousness as integrated information: a provisional manifesto. Biol. Bull. 215 , 216–242 (2008).
Tononi, G. Integrated information theory of consciousness: an updated account. Arch. Ital. Biol. 150 , 293–329 (2012).
CAS PubMed Google Scholar
Tononi, G., Boly, M., Massimini, M. & Koch, C. Integrated information theory: from consciousness to its physical substrate. Nat. Rev. Neurosci. 17 , 450–461 (2016). This work presents an account of the core claims and concepts of the integrated information ToC .
Oizumi, M., Albantakis, L. & Tononi, G. From the phenomenology to the mechanisms of consciousness: integrated information theory 3.0. PLoS Comput. Biol. 10 , e1003588 (2014).
Article PubMed PubMed Central CAS Google Scholar
Tononi, G. & Koch, C. Consciousness: here, there and everywhere? Philos. Trans. R. Soc. Lond. B Biol. Sci. https://doi.org/10.1098/rstb.2014.0167 (2015).
Haun, A. M. & Tononi, G. Why does space feel the way it does? Towards a principled account of spatial experienc. Entropy 21 , 1160 (2019).
Article PubMed Central Google Scholar
Albantakis, L., Hintze, A., Koch, C., Adami, C. & Tononi, G. Evolution of integrated causal structures in animats exposed to environments of increasing complexity. PLoS Comput. Biol. 10 , e1003966 (2014).
Marshall, W., Gomez-Ramirez, J. & Tononi, G. Integrated information and state differentiation. Front. Psychol. 7 , 926 (2016).
Lamme, V. A. Towards a true neural stance on consciousness. Trends Cogn. Sci. 10 , 494–501 (2006).
Lamme, V. A. & Roelfsema, P. R. The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci. 23 , 571–579 (2000).
Hohwy, J. & Seth, A. K. Predictive processing as a systematic basis for identifying the neural correlates of consciousness. Philos. Mind Sci. 1 , 3 (2020).
Lamme, V. A., Super, H., Landman, R., Roelfsema, P. R. & Spekreijse, H. The role of primary visual cortex (V1) in visual awareness. Vis. Res. 40 , 1507–1521 (2000).
Pascual-Leone, A. & Walsh, V. Fast backprojections from the motion to the primary visual area necessary for visual awareness. Science 292 , 510–512 (2001). This early study uses transcranial magnetic stimulation to reveal a role for re-entrant activity in conscious visual perception in humans .
Boehler, C. N., Schoenfeld, M. A., Heinze, H. J. & Hopf, J. M. Rapid recurrent processing gates awareness in primary visual cortex. Proc. Natl Acad. Sci. USA 105 , 8742–8747 (2008).
Lamme, V. A. How neuroscience will change our view on consciousness. Cogn. Neurosci. 1 , 204–220 (2010).
von Helmholtz, H. Handbuch der Phsyiologischen Optik [German] (Voss, 1867).
Clark, A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav. Brain Sci. 36 , 181–204 (2013). This work presents a classic exposition of predictive processing and its relevance for perception, cognition and action .
Friston, K. J. The free-energy principle: a unified brain theory? Nat. Rev. Neurosci. 11 , 127–138 (2010).
Seth, A. K. in Open MIND (eds Windt, J. M. & Metzinger, T.) (MIND Group, 2015).
Friston, K. J. Am I self-conscious? (Or does self-organization entail self-consciousness?). Front. Psychol. 9 , 579 (2018).
Seth, A. K. & Tsakiris, M. Being a beast machine: the somatic basis of selfhood. Trends Cogn. Sci. 22 , 969–981 (2018).
Bruineberg, J., Dolega, K., Dewhurst, J. & Baltieri, M. The Emperor’s new Markov blankets. Behav. Brain Sci. https://doi.org/10.1017/S0140525X21002351 (2021).
Hohwy, J. The Predictive Mind (Oxford Univ. Press, 2013).
Rao, R. P. & Ballard, D. H. Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat. Neurosci. 2 , 79–87 (1999).
Teufel, C. & Fletcher, P. C. Forms of prediction in the nervous system. Nat. Rev. Neurosci. 21 , 231–242 (2020).
Friston, K. J., Daunizeau, J., Kilner, J. & Kiebel, S. J. Action and behavior: a free-energy formulation. Biol. Cybern. 102 , 227–260 (2010).
Parr, T. & Friston, K. J. Generalised free energy and active inference. Biol. Cybern. 113 , 495–513 (2019).
Pennartz, C. M. A. Consciousness, representation, action: the importance of being goal-directed. Trends Cogn. Sci. 22 , 137–153 (2018).
Williford, K., Bennequin, D., Friston, K. & Rudrauf, D. The projective consciousness model and phenomenal selfhood. Front. Psychol. 9 , 2571 (2018).
Hohwy, J. New directions in predictive processing. Mind Lang. 35 , 209–223 (2020).
Seth, A. K. A predictive processing theory of sensorimotor contingencies: explaining the puzzle of perceptual presence and its absence in synesthesia. Cogn. Neurosci. 5 , 97–118 (2014).
O’Regan, J. K. & Noë, A. A sensorimotor account of vision and visual consciousness. Behav. Brain Sci. 24 , 939–973; discussion 973–1031 (2001). This primary description of the sensorimotor ToC argues that conscious perception is intimately related to action .
Seth, A. K. Interoceptive inference, emotion, and the embodied self. Trends Cogn. Sci. 17 , 565–573 (2013). This work presents a theoretical application of predictive processing to interoception and physiological regulation, relating this to experiences of emotion and selfhood .
Barrett, L. F. The theory of constructed emotion: an active inference account of interoception and categorization. Soc. Cogn. Affect. Neurosci. 12 , 1833 (2017).
Solms, M. The hard problem of consciousness and the free energy principle. Front. Psychol. 9 , 2714 (2018).
Hohwy, J., Roepstorff, A. & Friston, K. Predictive coding explains binocular rivalry: an epistemological review. Cognition 108 , 687–701 (2008).
Parr, T., Corcoran, A. W., Friston, K. J. & Hohwy, J. Perceptual awareness and active inference. Neurosci. Conscious. 2019 , niz012 (2019).
Friston, K. J., FitzGerald, T., Rigoli, F., Schwartenbeck, P. & Pezzulo, G. Active inference: a process theory. Neural Comput. 29 , 1–49 (2017).
Boly, M. et al. Preserved feedforward but impaired top-down processes in the vegetative state. Science 332 , 858–862 (2011). This neuroimaging study uses dynamic causal modelling to show that loss of consciousness in the vegetative state is associated with impaired top-down connectivity from frontal to temporal cortices .
Parr, T. & Friston, K. J. Working memory, attention, and salience in active inference. Sci. Rep. 7 , 14678 (2017).
Chalmers, A. What is This Thing Called Science? (Queensland Univ. Press, 2013).
Godfrey-Smith, P. G. Theory and Reality: An Introduction to the Philosophy of Science 2nd edn (Univ. Chicago Press, 2021).
Lipton, P. Inference to the Best Explanation (Routledge, 2004).
Lau, H. & Passingham, R. E. Relative blindsight in normal observers and the neural correlate of visual consciousness. Proc. Natl Acad. Sci. USA 103 , 18763–18768 (2006). This empirical study compares conscious and unconscious visual perception in humans, controlling for performance, and reveals differences in prefrontal activation .
van Vugt, B. et al. The threshold for conscious report: signal loss and response bias in visual and frontal cortex. Science 360 , 537–542 (2018). This empirical study tracks the time course of neural signals in primate frontal cortex, showing that perceived stimuli elicit sustained activity, when compared with non-perceived stimuli .
Article PubMed CAS Google Scholar
Gaillard, R. et al. Converging intracranial markers of conscious access. PLoS Biol. 7 , e61 (2009).
Panagiotaropoulos, T. I., Deco, G., Kapoor, V. & Logothetis, N. K. Neuronal discharges and gamma oscillations explicitly reflect visual consciousness in the lateral prefrontal cortex. Neuron 74 , 924–935 (2012).
Kapoor, V. et al. Decoding internally generated transitions of conscious contents in the prefrontal cortex without subjective reports. Nat. Comm. 13 , 1535 (2022).
Article CAS Google Scholar
Bellet, J. et al. Decoding rapidly presented visual stimuli from prefrontal ensembles without report nor post-perceptual processing. Neurosci. Conscious. 2022 , niac005 (2022).
Levinson, M., Podvalny, E., Baete, S. H. & He, B. J. Cortical and subcortical signatures of conscious object recognition. Nat. Commun. 12 , 2930 (2021).
Boly, M. et al. Are the neural correlates of consciousness in the front or in the back of the cerebral cortex? Clinical and neuroimaging evidence. J. Neurosci. 37 , 9603–9613 (2017).
Raccah, O., Block, N. & Fox, K. C. R. Does the prefrontal cortex play an essential role in consciousness? Insights from intracranial electrical stimulation of the human brain. J. Neurosci. 41 , 2076–2087 (2021).
Odegaard, B., Knight, R. T. & Lau, H. Should a few null findings falsify prefrontal theories of conscious perception? J. Neurosci. 37 , 9593–9602 (2017).
Brascamp, J., Blake, R. & Knapen, T. Negligible fronto-parietal BOLD activity accompanying unreportable switches in bistable perception. Nat. Neurosci. 18 , 1672–1678 (2015). This empirical ‘no-report’ study shows that fronto-parietal activity does not track switches in perceptual dominance when subjective reports are not required .
Sergent, C. et al. Bifurcation in brain dynamics reveals a signature of conscious processing independent of report. Nat. Commun. 12 , 1149 (2021).
Siclari, F. et al. The neural correlates of dreaming. Nat. Neurosci. 20 , 872–878 (2017).
Wong, W. et al. The Dream Catcher experiment: blinded analyses failed to detect markers of dreaming consciousness in EEG spectral power. Neurosci. Conscious. 2020 , niaa006 (2020).
Block, N. Consciousness, accessibility, and the mesh between psychology and neuroscience. Behav. Brain Sci. 30 , 481–548 (2007). This work argues that research in psychology and neuroscience shows that there is a real and not merely conceptual distinction between phenomenal consciousness (that is, experience) and cognitive access to phenomenal consciousness .
Musgrave, A. in Relativism and Realism in Science (ed Nola, R.) 229–252 (Kluwer, 1988).
Song, C., Haun, A. M. & Tononi, G. Plasticity in the structure of visual space. eNeuro https://doi.org/10.1523/ENEURO.0080-17.2017 (2017).
Marshel, J. H. et al. Cortical layer-specific critical dynamics triggering perception. Science https://doi.org/10.1126/science.aaw5202 (2019).
Dembski, C., Koch, C. & Pitts, M. Perceptual awareness negativity: a physiological correlate of sensory consciousness. Trends Cogn. Sci. 25 , 660–670 (2021).
Sanchez, G., Hartmann, T., Fusca, M., Demarchi, G. & Weisz, N. Decoding across sensory modalities reveals common supramodal signatures of conscious perception. Proc. Natl Acad. Sci. USA 117 , 7437–7446 (2020).
Sergent, C. The offline stream of conscious representations. Philos. Trans. R. Soc. Lond. B Biol. Sci. https://doi.org/10.1098/rstb.2017.0349 (2018).
Michel, M. & Doerig, A. A new empirical challenge for local theories of consciousness. Mind Lang. https://doi.org/10.1111/mila.12319 (2021).
Sergent, C. et al. Cueing attention after the stimulus is gone can retrospectively trigger conscious perception. Curr. Biol. 23 , 150–155 (2013). This empirical study reveals that conscious perception of a stimulus can be influenced by events happening (hundreds of milliseconds) after the stimulus appeared (‘retro-perception’) .
Roseboom, W. et al. Activity in perceptual classification networks as a basis for human subjective time perception. Nat. Commun. 10 , 267 (2019).
Kent, L. & Wittmann, M. Special Issue: Consciousness science and its theories. Time consciousness: the missing link in theories of consciousness. Neurosci. Conscious. 2021 , niab011 (2021).
Husserl, E. Ideas: A General Introduction to Pure Phenomenology (Collier Books, 1963).
Yaron, I., Melloni, L., Pitts, M. & Mudrik, L. The ConTraSt database for analyzing and comparing empirical studies of consciousness theories. Nat. Hum. Behav. https://doi.org/10.1038/s41562-021-01284-5 (2022). This work presents an online resource of empirical studies of consciousness, organized with respect to different ToCs .
Joglekar, M. R., Mejias, J. F., Yang, G. R. & Wang, X. J. Inter-areal balanced amplification enhances signal propagation in a large-scale circuit model of the primate cortex. Neuron 98 , 222–234.e8 (2018).
VanRullen, R. & Kanai, R. Deep learning and the global workspace theory. Trends Neurosci. 44 , 692–704 (2021).
Shea, N. & Frith, C. D. The global workspace needs metacognition. Trends Cogn. Sci. 23 , 560–571 (2019).
Suzuki, K., Roseboom, W., Schwartzman, D. J. & Seth, A. K. A deep-dream virtual reality platform for studying altered perceptual phenomenology. Sci. Rep. 7 , 15982 (2017).
Vilas, M. G., Auksztulewicz, R. & Melloni, L. Active inference as a computational framework for consciousness. Rev. Philos. Psychol. https://doi.org/10.1007/s13164-021-00579-w (2021).
Browning, H. & Veit, W. The measurement problem in consciousness. Philos. Top. 48 , 85–108 (2020).
Seth, A. K., Dienes, Z., Cleeremans, A., Overgaard, M. & Pessoa, L. Measuring consciousness: relating behavioural and neurophysiological approaches. Trends Cogn. Sci. 12 , 314–321 (2008).
Michel, M. Calibration in consciousness science. Erkenntnis https://doi.org/10.1007/s10670-021-00383-z (2021).
Birch, J., Schnell, A. K. & Clayton, N. S. Dimensions of animal consciousness. Trends Cogn. Sci. 24 , 789–801 (2020).
Bayne, T., Seth, A. K. & Massimini, M. Are there islands of awareness? Trends Neurosci. 43 , 6–16 (2020). This work presents an examination of the possibility of consciousness in isolated neural systems such as brain organoids, disconnected cortical hemispheres and ex cranio brains .
Dehaene, S., Lau, H. & Kouider, S. What is consciousness, and could machines have it? Science 358 , 486–492 (2017).
Hu, H., Cusack, R. & Naci, L. Typical and disrupted brain circuitry for conscious awareness in full-term and pre-term infants. (2021).
Owen, A. M. & Coleman, M. R. Detecting awareness in the vegetative state. Ann. N Y Acad. Sci. 9 , 130–138 (2008).
Cleeremans, A. The radical plasticity thesis: how the brain learns to be conscious. Front. Psychol. 2 , 86 (2011).
Jackendoff, R. Consciousness and the Computational Mind (MIT Press, 1987).
Prinz, J. The Conscious Brain: How Attention Engenders Experience (Oxford Univ. Press, 2012).
Chang, A. Y. C., Biehl, M., Yu, Y. & Kanai, R. Information closure theory of consciousness. Front. Psychol. 11 , 1504 (2020).
Tononi, G. & Edelman, G. M. Consciousness and complexity. Science 282 , 1846–1851 (1998). This work presents an early proposal of how measures of neural complexity might relate to phenomenological properties of (all) conscious experiences .
Edelman, G. M. Neural Darwinism: The Theory of Neuronal Group Selection (Basic Books 1987).
Edelman, G. M. The Remembered Present (Basic Books, 1989).
Damasio, A. The Feeling of What Happens: Body and Emotion in the Making of Consciousness (Harvest Books, 2000).
Graziano, M. S. A. The attention schema theory: a foundation for engineering artificial consciousness. Front. Robot. AI 4 , 60 (2017).
Dennett, D. C. Consciousness Explained (Little, Brown, 1991).
Ginsburg, S. & Jablonka, E. The Evolution of the Sensitive Soul: Learning and the Origins of Consciousness (MIT Press, 2019).
Aru, J., Suzuki, M. & Larkum, M. E. Cellular mechanisms of conscious processing. Trends Cogn. Sci. 24 , 814–825 (2020).
McFadden, J. Integrating information in the brain’s EM field: the cemi field theory of consciousness. Neurosci. Conscious. 2020 , niaa016 (2020).
Fleming, S. M., Ryu, J., Golfinos, J. G. & Blackmon, K. E. Domain-specific impairment in metacognitive accuracy following anterior prefrontal lesions. Brain 137 , 2811–2822 (2014).
Fox, K. C. R. et al. Intrinsic network architecture predicts the effects elicited by intracranial electrical stimulation of the human brain. Nat. Hum. Behav. 4 , 1039–1052 (2020).
Dehaene, S. & Naccache, L. Towards a cognitive neuroscience of consciousness: basic evidence and a workspace framework. Cognition 79 , 1–37 (2001).
Sergent, C., Baillet, S. & Dehaene, S. Timing of the brain events underlying access to consciousness during the attentional blink. Nat. Neurosci. 8 , 1391–1400 (2005).
Mediano, P. A. M., Seth, A. K. & Barrett, A. B. Measuring integrated information: comparison of candidate measures in theory and simulation. Entropy 21 , 17 (2019).
Casali, A. G. et al. A theoretically based index of consciousness independent of sensory processing and behavior. Sci. Transl. Med. 5 , 198ra105 (2013). This empirical study shows that a measure of the complexity of the cortical response to transcranial magnetic stimulation distinguishes between a range of global conscious states, including disorders of consciousness .
Luppi, A. I. et al. Consciousness-specific dynamic interactions of brain integration and functional diversity. Nat. Commun. 10 , 4616 (2019).
Hardstone, R. et al. Long-term priors influence visual perception through recruitment of long-range feedback. Nat. Commun. 12 , 6288 (2021).
de Lange, F. P., Heilbron, M. & Kok, P. How do expectations shape perception? Trends Cogn. Sci. 22 , 764–779 (2018).
Melloni, L., Schwiedrzik, C. M., Muller, N., Rodriguez, E. & Singer, W. Expectations change the signatures and timing of electrophysiological correlates of perceptual awareness. J. Neurosci. 31 , 1386–1396 (2011). This empirical study uses a perceptual hysteresis paradigm to show that expectations enhance and accelerate conscious perception .
Pinto, Y., van Gaal, S., de Lange, F. P., Lamme, V. A. & Seth, A. K. Expectations accelerate entry of visual stimuli into awareness. J. Vis. 15 , 13 (2015).
Chalmers, D. J. Facing up to the problem of consciousness. J. Conscious. Stud. 23 , 200–219 (1995). This work presents the classic statement of the philosophical distinction between the ‘hard’ and ‘easy’ problems of consciousness .
Levine, J. Materialism and qualia: the explanatory gap. Pac. Philos. Q. 64 , 354–361 (1983).
Seth, A. K. The Real Problem (Aeon, 2016).
Balog, K. in The Oxford Handbook of Philosophy of Mind (eds Beckermann, A., McLaughlin, B. P., & Walter S.) 292–312 (Oxford Univ. Press, 2009).
Perry, J. Knowledge, Possibility, and Consciousness (MIT Press, 2001).
Varela, F. J., Thompson, E. & Rosch, E. The Embodied Mind: Cognitive Science and Human Experience (MIT Press, 1993).
Carvalho, G. B. & Damasio, A. Interoception and the origin of feelings: a new synthesis. Bioessays 43 , e2000261 (2021).
Solms, M. The Hidden Spring: A Journey to the Source of Consciousness (Profile Books, 2021).
Merker, B. Consciousness without a cerebral cortex: a challenge for neuroscience and medicine. Behav. Brain Sci. 30 , 63–81; discussion 81–134 (2007).
Parvizi, J. & Damasio, A. Consciousness and the brainstem. Cognition 79 , 135–160 (2001).
Naber, M., Frassle, S. & Einhauser, W. Perceptual rivalry: reflexes reveal the gradual nature of visual awareness. PLoS ONE 6 , e20910 (2011).
Casarotto, S. et al. Stratification of unresponsive patients by an independently validated index of brain complexity. Ann. Neurol. 80 , 718–729 (2016).
Shea, N. & Bayne, T. The vegetative state and the science of consciousness. Br. J. Philos. Sci. 61 , 459–484 (2010).
Birch, J. The search for invertebrate consciousness. Noûs 56 , 133–153 (2020).
Phillips, I. The methodological puzzle of phenomenal consciousness. Philos. Trans. R. Soc. Lond. B Biol. Sci. https://doi.org/10.1098/rstb.2017.0347 (2018).
Download references
Acknowledgements
A.K.S. is Co-Director of, and T.B. is a Fellow in, the CIFAR Program on Brain, Mind, and Consciousness. A.K.S. is additionally grateful to the European Research Council (Advanced Investigator Grant 101019254) and the Dr. Mortimer and Theresa Sackler Foundation.
Author information
Authors and affiliations.
Department of Informatics and Sackler Centre for Consciousness Science, University of Sussex, Brighton, UK
Anil K. Seth
Program on Brain, Mind, and Consciousness, Canadian Institute for Advanced Research (CIFAR), Toronto, Ontario, Canada
Anil K. Seth & Tim Bayne
School of Philosophical, Historical, and International Studies, Monash University, Melbourne, Victoria, Australia
Monash Centre for Consciousness and Contemplative Studies, Monash University, Melbourne, Victoria, Australia
You can also search for this author in PubMed Google Scholar
Contributions
The authors both contributed to all aspects of preparing the article.
Corresponding author
Correspondence to Anil K. Seth .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Reviews Neuroscience thanks L. Melloni, C. Sergent and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
(NCCs). The minimal set of neural events that is jointly sufficient for a conscious state.
Intuitions that there is no prospect of a fully satisfying explanation of consciousness in physical, mechanistic terms.
Research projects in which proponents of different theories together design an experiment to distinguish their preferred theories, and agree in advance about how the outcome will favour one theory over the other(s).
Relating to an organism’s overall state of consciousness, usually linked to arousal and behavioural responsiveness, and associated with the ‘level’ of consciousness.
Relating to particular conscious mental states, such as a conscious perception, emotion or thought. Local states are also often called conscious contents.
A phenomenon in which different images are presented to each eye, and conscious perception alternates between the two images.
The experiential nature of a local state, such as the ‘redness’ of an experience of red or the pain of a toothache — sometimes also called qualia.
A mental representation that has as its target another mental representation
Behavioural experiments in which participants do not provide subjective (verbal, behavioural) reports.
The amount of information specified by a system that is irreducible to that specified by its parts. There are many variations of Φ, each calculated differently and making different assumptions.
A range of brain regions towards the rear of the cortex, including parietal, temporal and occipital areas, as well as regions such as the precuneus.
In integrated information theory (IIT), a subset of a physical system that underpins a maximum of irreducible integrated information.
Predictions about the causes of sensory signals originating from within the body (interoception refers to perception of the body ‘from within’).
The fact that that the experiences that a single agent has at a time seem always to occur as the components of a single complex experience.
A functional property whereby a mental state has access to a wide range of cognitive processes, usually including verbal and/or behavioural report.
The use of computational models to account for the phenomenal character of a conscious state in terms of (neural) mechanisms.
The problem of identifying whether a particular mental state is conscious, or determining whether an organism or other system is, or has the capacity to be, conscious.
Laboratory-grown neural structures that self-organize into systems with cellular and network features resembling aspects of the developing human brain.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Seth, A.K., Bayne, T. Theories of consciousness. Nat Rev Neurosci 23 , 439–452 (2022). https://doi.org/10.1038/s41583-022-00587-4
Download citation
Accepted : 22 March 2022
Published : 03 May 2022
Issue Date : July 2022
DOI : https://doi.org/10.1038/s41583-022-00587-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
University of Rochester brain research featured at Society for Neuroscience conference in Chicago
Several dozen Del Monte Institute for Neuroscience at the University of Rochester faculty, researchers, and trainees from programs including Neuroscience, Brain and Cognitive Science, Biomedical Genetics, Biomedical Engineering, Neurosurgery, Toxicology, and Pharmacology & Physiology, traveled to Chicago for the annual Society for Neuroscience (SfN) Conference. According to SfN, more than 30,000 people regularly attend the annual conference.
Neuroscience Diversity Commission hosts programs’ scholars at SfN
The Del Monte Institute for Neuroscience Diversity Commission also brought scholars from its outreach programs. Along with attending the conference the NE UR OEAST scholars, high school students from the Rochester City School District, also went on college tours and celebrated their accomplishments with lunch at Pizano’s Pizza and Pasta.
Undergraduate students who participate in the NE UR OCITY program had the opportunity to present posters on the research they conducted while working in labs at the University of Rochester.

Faculty, postdocs, and students gave talks and served as symposium chairs during the five-day event.

Graduate Fair

More than 60 Rochester related research posters were presented.
Neuroscience networking
The annual Neuroscience Graduate Program Social at SfN brought together faculty, students, alumni, and friends to network and celebrate achievements over the past year.
- Del Monte Institute for Neuroscience
- students and trainees

Skip Navigation

- Individual Member Benefits
- Institutional Program Member Benefits
- Sustaining Associate Member Benefits
- Get Involved at SfN
- Sponsorship Information for New Members
- Information for Members in Developing Countries
- Automatic Renewals
- Frequently Asked Questions
- Member Obituaries
- Individual Member Directory
- Start or Reactivate a Chapter
- Resources for Chapters
- Submit Annual Report
- Chapter Directory
- Meetings Overview
- Dates and Deadlines
- SfN Virtual Events
- Neuroscience 2024
- Neuroscience 2023
- Search Past Annual Meeting Abstracts
- Attendance Statistics
- Code of Conduct at SfN Events
- Guidelines for Participating in SfN Events
- Photography & Recording Policy
- Presenter Guidelines and Policies for SfN Events
- Trainee Professional Development Award
- FENS Member Awards to SfN Annual Meeting
- IBRO Member Awards to SfN Annual Meeting
- JNS Member Awards to SfN Annual Meeting
- FENS Forum Awards
- IBRO World Congress Awards
- JNS Meeting Awards
- Careers Overview
- Institutional Program (IP) Directory
- Job Seekers
- 2024 Graduate School Fair
- Neurobiology of Disease Workshop
- Scientific Short Courses
- Responsible Conduct of Research Short Courses
- Global Funding Sources
- Core Competencies
- Neuroscience Training Program Survey
- Outstanding Career and Research Achievements
- Early Career
- Initiatives Overview
- 2024 Award Recipients
- Awards and Prizes FAQ
- Scientific Rigor and Reproducibility
- SfN Zoom Backgrounds
- Neuroscience Scholars Program
- Increasing Women in Neuroscience (IWiN) Courses & Toolkit
- Celebration of Women in Neuroscience Event
- Support for Members and Institutions
- Tools and Resources
- Resources for Medical Students
- Resources for Educators
- Brain Awareness Video Contest
- Life of a Neuron Exhibit
- Advocacy Overview
- The NeuroAdvocate Challenge
- Advocacy Action Center
- Advocacy Best Practices
- Advocacy Network Newsletter
- Advocacy Training Seminars
- Capitol Hill Day
- Connect with Policymakers
- Early Career Policy Ambassadors
- Partner with a Local Chapter
- Engage the Media
- Global Neuroscience Initiatives
- Global Funding
- North American Programs
- Advocacy Videos
- Advocacy Resources
- US Neuroscience Initiatives
- Funding Priorities and Processes
- Statements and Testimony
- Sign-On Letters
- Outreach Overview
- BrainFacts.org
- Find a Neuroscientist
- Webinar: The ABC's of BAW
- How to Get Involved
- Award for Education in Neuroscience
- Next Generation Award
- Science Educator Award
- Publications Overview
- Neuroscience Quarterly
- Annual Report
- History of Neuroscience Autobiographical Chapters
- About Overview
- Mission and Strategic Plan
- Professional Conduct
- Resolutions to the Bylaws
- Strategic Partners
- SfN 50th Anniversary Celebration
- NIH Public Health Service-Supported Funding Financial Conflict of Interest Policy
- History of SfN
- Environmental Commitment
- SfN Council
- SfN Presidents
- Call for Nominations
- SfN Ethics Policy
- Guidelines for Responsible Conduct Regarding Scientific Communication
- Neuronline Digital Learning Community Guidelines
- Autobiographical Chapters
- Autobiographical Videos of Prominent Neuroscientists
- Classic Papers
- Neuroscience History Resources
- Robert Doty's Chapter on Neuroscience

- We're aware there is currently a technical issue with abstract submission. We're hoping to have this resolved shortly.
- Neuroscience 2025
Neuroscience 2025 See You in San Diego!
Join Us in San Diego for Neuroscience 2025!

Meeting Dates: Saturday, November 15–Wednesday, November 19
Location: San Diego Convention Center
Each year, scientists from around the world congregate to discover new ideas, share their research, and experience the best the field has to offer. Attend so you can: present research, network with scientists, attend session and events, and browse the exhibit hall.
Join the nearly half a million neuroscientists from around the world who have propelled their careers by presenting an abstract at an SfN annual meeting — the premier global neuroscience event.
Join our email list to receive annual meeting updates.
Quick Links

- Join or Renew Membership
- Visa Information
- On-Site Advertising and Sponsored Products
- Virtual Component
- Late-Breaking Abstracts
- Call for Abstracts
- Scientific Program
- Registration
SfN Event Policies
- Code of Conduct Pertaining to SfN Events
- Presenter Guidelines and Policies
- Resources for Finding Diverse Speakers
Contact us if you have questions
Email [email protected] or call (202) 962-4000

Engage with SfN

- Global Events
- Code of Conduct
- Jobs at SfN

- Accessibility Policy
- Privacy Notice
- Manage Cookies

Dementia: Causes, Treatments and Research (Neuroscience) MSc
London, Bloomsbury
This is the programme information for 2024 entry
If you require details of this year's programme, Dementia: Causes, Treatments and Research (Neuroscience) MSc (2025), click here
The Dementia: Causes, Treatments and Research (Neuroscience) MSc, offered by the UCL Queen Square Institute of Neurology, tackles one of the biggest global health problems facing society today. It provides research-oriented and cutting-edge training in the study of dementia and its scientific basis, delivered by international leaders in the science and practice of dementia.
UK tuition fees (2024/25)
Overseas tuition fees (2024/25), programme starts, applications accepted.
Applications closed
- Entry requirements
A degree in medicine or a minimum of an upper second-class Bachelor's degree from a UK university of an overseas qualification of an equivalent standard in psychology, biological sciences, biology, neuroscience, biomedical sciences, anatomy and physiology. Graduates from other scientific disciplines will be considered on an individual basis.
The English language level for this programme is: Level 1
UCL Pre-Master's and Pre-sessional English courses are for international students who are aiming to study for a postgraduate degree at UCL. The courses will develop your academic English and academic skills required to succeed at postgraduate level.
Further information can be found on our English language requirements page.
Equivalent qualifications
Country-specific information, including details of when UCL representatives are visiting your part of the world, can be obtained from the International Students website .
International applicants can find out the equivalent qualification for their country by selecting from the list below. Please note that the equivalency will correspond to the broad UK degree classification stated on this page (e.g. upper second-class). Where a specific overall percentage is required in the UK qualification, the international equivalency will be higher than that stated below. Please contact Graduate Admissions should you require further advice.
About this degree
The programme brings the latest bench-top research findings to the bedside, develops and integrates basic and clinical neuroscience skills, and equips students for future careers in the clinical practice or basic neuroscience of dementia.
Who this course is for
This course is ideally suited to lab scientists who want to appreciate the clinical and human resonance of neurodegenerative diseases and current concepts and challenges in their diagnosis and management; and equally suited to psychologists and clinicians who want to explore the neurobiological foundations of dementia practice and cutting-edge research. A major mission of the course is to inspire dialogue and understanding between the many disciplines that are shaping the future of dementia research and treatment.
What this course will give you
This programme is unique in linking neuroscientific foundations with specialist clinical skills and knowledge in dementia.
The programme integrates the expertise of UCL Queen Square Institute of Neurology with affiliated departments at the forefront of the global mission to defeat dementia, and is taught by international leaders working closely together to link bench and bedside as part of UCL's Dementia Strategy .
The programme builds on UCL's global perspective, targeting students in developing as well as developed countries to drive future training opportunities. The programme emphasises active student participation and enquiry, develops practical skills, and offers unparalleled exposure to laboratories and patient-based teaching.
The foundation of your career
This unique programme will equip graduates with in-depth knowledge of dementia diseases and their treatments; strong, practical research skills that could facilitate doctoral or postdoctoral research in the field; and transferable scientific communication skills. This experience could support further doctoral studies and applications for fellowships. .
Employability
For scientists and psychologists, the programme can lead to future placements in clinically oriented research environments or clinical training. For clinicians, this is an excellent opportunity to gain a higher qualification at a world-leading centre of excellence in neurodegeneration research, which could be tailored to a variety of future roles in clinical, research and management fields. Many students have gone on to pursue PhDs and research careers in the fields of dementia and neurodegeneration.
The Queen Square Institute of Neurology is home to some of the world's most influential academics. By studying with us, students will have the opportunity to be part of the next generation of experts in neuroscience. We are home to some of the world's most influential researchers and offer a wealth of scientific and clinical expertise, from laboratory benches through to patient care.
Many of our lecturers are also NHS consultants working at The National Hospital for Neurology and Neurosurgery which is a leading centre for the diagnosis, treatment and care of patients with a wide range of neurological conditions.
Alumni networking events are provided for current and former students to discuss their experiences and career options after completing their degree. Students also take part in a research project symposium to share their work with peers and early career researchers from UCL, and some students may be able to present their work at local or international conferences depending upon the research project chosen.
Teaching and learning
This programme is delivered through a combination of lectures, seminars, tutorials, journal clubs, and patient-based teaching sessions supplemented by self-directed learning. Students are encouraged to actively contribute to teaching sessions and learn through enquiry. The research project provides students with an authentic research experience to develop their scientific planning, reporting and critical analysis skills as well as project-specific practical skills. This programme uniquely provides extracurricular opportunities for students to develop a range of communication skills during journal club discussions, project symposium oral presentations and discussions with people with lived experience of a rare dementia.
Assessment is through ongoing formative assessments (for example interactive discussions and presentations), timetabled summative assessments (including unseen short-answer examinations, multiple choice question (MCQ) examinations, essays, and presentations) as well as a 10,000 word written research project dissertation.
For each 15 credit module there will be approximately 20-25 hours of contact hours, with around 120 hours of self directed study.
The programme consists of five compulsory modules (75 credits), two or three optional modules (total value of 45 credits) and a research project (60 credits).
Students must complete four of the compulsory modules (60 credits) and up to 30 credits of optional modules in Year One. In Year Two, students are required to complete the remaining optional modules (total 45 credits across both years), the final core module Research Methods and Introduction to Statistics (15 credits), and the research project (60 credits).
Compulsory modules
Optional modules.
Please note that the list of modules given here is indicative. This information is published a long time in advance of enrolment and module content and availability are subject to change. Modules that are in use for the current academic year are linked for further information. Where no link is present, further information is not yet available.
Students undertake modules to the value of 180 credits. Upon successful completion of 180 credits, you will be awarded an MSc in Dementia: Causes, Treatments and Research (Neuroscience).
Accessibility
Details of the accessibility of UCL buildings can be obtained from AccessAble accessable.co.uk . Further information can also be obtained from the UCL Student Support and Wellbeing Services team .
Fees and funding
Fees for this course.
| Fee description | Full-time | Part-time |
|---|---|---|
| Tuition fees (2024/25) | £15,100 | £7,550 |
| Tuition fees (2024/25) | £37,500 | £18,750 |
Programme also available on a modular (flexible) basis .
The tuition fees shown are for the year indicated above. Fees for subsequent years may increase or otherwise vary. Where the programme is offered on a flexible/modular basis, fees are charged pro-rata to the appropriate full-time Master's fee taken in an academic session. Further information on fee status, fee increases and the fee schedule can be viewed on the UCL Students website: ucl.ac.uk/students/fees .
Additional costs
International Students will bear any costs incurred in acquiring certification equivalent to DBS (Police check) in their home country. These checks are neccessary for any student who undertakes a clinical project based at University College London Hospitals.
For more information on additional costs for prospective students please go to our estimated cost of essential expenditure at Accommodation and living costs .
Funding your studies
UCL Queen Square Institute of Neurology International Excellence Scholarships - we are delighted to announce four fee reduction scholarships (worth £6,000 each) for overseas fee-paying students.
These are offered in celebration of Queen Square’s rich history of welcoming students from across the world who have contributed much to our community and wider society. In 2021-22 we had students from over 50 countries enrolled.
These scholarships are offered based on academic merit and are available for all our eligible postgraduate programmes (except for the MSc/Dip/Cert in Clinical Neurology).
For a comprehensive list of the funding opportunities available at UCL, including funding relevant to your nationality, please visit the Scholarships and Funding website .
Queen Square IoN International Excellence Scholarship
Deadline: 8 April 2024 Value: £6,000 towards tuition fees (1yr) Criteria Based on academic merit Eligibility: EU, Overseas
UCL Queen Square Institute of Neurology BAME Scholarship
Deadline: 8 April 2024 Value: £4,000 towards fees (1 year) Criteria Based on academic merit Eligibility: UK
UCL Queen Square Institute of Neurology Stroke Academic Excellence Scholarship
Deadline: 8 April 2024 Value: £4,000 towards fees (1 year) Criteria Based on academic merit Eligibility: UK, EU, Overseas
UCLFAA MSc in Dementia Scholarship (Neuroscience or Mental Health)
Deadline: 21 June 2024 Value: $20,000 (1 year) Criteria Based on both academic merit and financial need Eligibility: EU, Overseas
There is an application processing fee for this programme of £90 for online applications and £115 for paper applications. Further information can be found at Application fees .
When we assess your application we would like to learn:
- why you want to study Dementia Neuroscience at graduate level?
- why you want to study Dementia Neuroscience at UCL?
- what particularly attracts you to the chosen programme
- how do your academic and professional background and skills meet the demands of this challenging programme?
- where would you like to go professionally with your degree?
Together with essential academic requirements, the personal statement is your opportunity to illustrate whether your reasons for applying to this programme match what the programme will deliver.
Students are advised to apply as early as possible due to competition for places. Those applying for scholarship funding (particularly overseas applicants) should take note of application deadlines. The academic merit of the CV and application will be considered in awarding bursaries, which are potentially available to any student applying for this degree.
Please note that you may submit applications for a maximum of two graduate programmes (or one application for the Law LLM) in any application cycle.
Got questions? Get in touch

UCL Queen Square Institute of Neurology
UCL is regulated by the Office for Students .
Prospective Students Graduate
- Graduate degrees
- Taught degrees
- Taught Degrees
- Applying for Graduate Taught Study at UCL
- Research degrees
- Research Degrees
- Funded Research Opportunities
- Doctoral School
- Funded Doctoral Training Programmes
- Applying for Graduate Research Study at UCL
- Teacher training
- Teacher Training
- Early Years PGCE courses
- Primary PGCE courses
- Secondary PGCE courses
- Further Education PGCE programme
- How to apply
- The IOE approach
- Teacher training in the heart of London
- Why choose UCL?
- Entrepreneurship
- Inspiring facilities and resources
- Careers and employability
- Your global alumni community
- Your wellbeing
- Postgraduate Students' Association
- Your life in London
- Accommodation
- Funding your Master's
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Dreaming and the brain: from phenomenology to neurophysiology
1 Department of Psychiatry, University of Wisconsin, Madison, WI 53719
Giulio Tononi
Associated data.
Dreams are a most remarkable experiment in psychology and neuroscience, conducted every night in every sleeping person. They show that our brain, disconnected from the environment, can generate by itself an entire world of conscious experiences. Content analysis and developmental studies have furthered our understanding of dream phenomenology. In parallel, brain lesion studies, functional imaging, and neurophysiology have advanced our knowledge of the neural basis of dreaming. It is now possible to start integrating these two strands of research in order to address some fundamental questions that dreams pose for cognitive neuroscience: how conscious experiences in sleep relate to underlying brain activity; why the dreamer is largely disconnected from the environment; and whether dreaming is more closely related to mental imagery or to perception.
Contemporary dream research
Although dreams have fascinated us since the dawn of time, their rigorous, scientific study is a recent development[ 1 – 4 ] ( Supplementary Fig. 1 ). In The interpretation of dreams [ 5 ] Freud predicted that “Deeper research will one day trace the path further and discover an organic basis for the mental event.” Recent work, which we review in this article, begins to fulfill Freud s prediction.
The study of dreams is a formidable task, because dream consciousness is only accessible via report rather than direct observation ( Box 1 ) and because it is difficult to manipulate dream content experimentally, whether by exposure to stimuli before[ 6 , 7 ] or during sleep[ 7 , 8 ]. Therefore, it is difficult to predict the contents of specific dreams[ 9 ], and most modern dream research tries to relate neuronal activity retrospectively to dream form rather than dream content, i.e. to focus on properties of all dreams rather than to investigate the neural correlates of a particular dream. Yet, as we shall see, encouraging progress has been made in relating the phenomenology of dreams to underlying brain activity, and to studies of brain damage and development.
BOX 1Can reports be trusted to accurately convey internal experiences in sleep?
Do dream reports obtained by awakening a sleeping subject accurately convey subjective experiences in sleep? At one extreme, we could be fully conscious throughout sleep but remember dreams well, little, or not at all depending on the brain state when we are awakened. Indeed, we know that dreaming often goes unreported – some people claim they rarely dream, but systematic awakenings in sleep labs have revealed that we greatly underestimate how often and how much we are conscious during sleep. On the other hand, neurological patients who report loss of dreaming are no more likely to have memory disorders than those who report dreaming[ 22 ], suggesting that lack of dream reports indeed reflects lack of experience rather than changes in memory alone. Further studies may illuminate this issue since, for example, memory-related regions in the medial temporal lobe are highly active in REM sleep ( Fig. 1 ).

Meta-analysis of relative increases and decreases in neuronal activity during REM sleep as seen with PET imaging using H2 15 O measurements of regional cerebral blood flow (rCBF) [ 15 , 16 , 19 ] or [ 18 F]-flurodeoxyglucose measurements of glucose metabolism[ 17 ]. Top row: cortical surface, lateral view. Middle row: cortical surface, medial view. Bottom row: subcortical foci (left) and ventral view of cortical surface (right). Analysis is based on published Talairach coordinates of foci whose activity was significant at p<0.001 corrected (Z-score > 3.09). Circles, squares, triangles, and stars denote activity foci as reported by [ 15 ] (Maquet 96), [ 16 ] (Braun 97), [ 17 ] (Nofzinger 97), and [ 19 ] (Maquet 2000), respectively. Each symbol marks a region’s center-of-mass regardless of its spatial extent. Yellow symbols denote increased regional activity in the (1) mesopontine tegmentum and midbrain nuclei, (2) thalamus, (3) basal forebrain and diencephalic structures, (4) limbic MTL structures including amygdala and hippocampus, (5) medial prefrontal cortex, (6) occipito-temporal visual cortex, and (7) anterior cingulate cortex. Cyan symbols denote decreased activity in the (8) orbitofrontal cortex, (9) posterior cingulate and precuneus, (10) dorsolateral prefrontal cortex, and (11) inferior parietal cortex.
At the other extreme, one could claim that we are unconscious throughout sleep and merely have a tendency to confabulate during the transition into wakefulness. While such a claim is hard to refute conclusively (just as it is hard to prove conclusively that one is not a zombie when awake), it seems highly implausible; when one has just experienced a vivid dream, it seems hard to believe that it was made up in a flash during an awakening. Indeed, (a) the estimated time in dream report correlates well with the time elapsed in REM sleep before awakening[ 62 ]; and (b) in REM sleep behavior disorder (where muscle atonia is disrupted), movements seem to match the reported dream[ 113 ].
Reports obtained upon awakenings from deep NREM sleep are more difficult to evaluate because of disorientation associated with increased sleep inertia[ 114 ]. However, some evidence indicates that indeed dream consciousness can occur in NREM sleep and does not merely reflect recalls of earlier REM sleep dreams[ 59 ]: (a) It is sometimes possible to influence dream content by sounds delivered in NREM sleep, and to “tag” NREM reports[ 59 ], (b) Some NREM parasomnias (sleep talking, sleep terrors) correspond to reported dream experiences[ 115 ], and (c) “Full-fledged” dreams are sometimes reported upon awakening from the first NREM episode, before any REM sleep occurred [ 59 , 66 ], and even in naps consisting of only NREM sleep[ 67 ].
Nevertheless, it is worth keeping in mind that several factors may render dream reports less trustworthy when compared to reports of waking experience, including: (a) a dramatic state change, since we report about a sleep experience when awake; (b) considerable time delay, since dream reports are obtained after the experience, possibly leading to passive forgetting and interference; (c) difficulties in verbally describing experiences that are mainly visual and emotional; and (d) censorship of embarrassing, immoral, sexual and aggressive material.
Phenomenology of dreams and their relation to brain activity
The level and nature of our conscious experience varies dramatically in sleep. During slow wave sleep (SWS) early in the night, consciousness can nearly vanish despite persistent neural activity in the thalamocortical system[ 10 ]. Subjects awakened from other phases of sleep, especially but not exclusively during REM sleep, report “typical”, full-fledged dreams - vivid, sensorimotor hallucinatory experiences that follow a narrative structure[ 3 , 11 ]. The dreamer is highly conscious (she has vivid experiences), is disconnected from the environment (she is asleep), but somehow her brain is creating a story, filling it with actors and scenarios, and generating hallucinatory images. How does the brain accomplish this remarkable feat? And, conversely, what do dreams tell us about the organization and working of the brain?
Since awakenings from REM sleep regularly yield reports of typical dreams, we will first focus on neural activity during REM sleep, to gain insight into brain states that are compatible with dreaming. It should be emphasized at the outset, however, that dreams can occur in other brain states, such as late NREM sleep, as will be discussed below.
Similarities between dreaming and waking
In order to gain insight into the phenomenology and neural basis of dreams, it is useful to consider both similarities and differences between waking consciousness and dreaming consciousness, and to relate these differences to changes in brain activity and organization[ 11 ]. Perhaps the most striking feature of conscious experiences in sleep is how altogether similar the inner world of dreams is to the real world of wakefulness. Indeed, at times the dreamer may be uncertain whether he is awake or asleep. Certainly, dreams are not created in a vacuum but closely reflect the organization and functions of our brain.
In most dreams, perceptual modalities and submodalities that dominate in wakefulness are heavily represented. Dreams are highly visual, in full color, rich in shapes, full of movement, and incorporate typical wakefulness categories such as people, faces, places, objects, and animals[ 3 ]. Dreams also contain sounds (including speech and conversation), and more rarely tactile percepts, smells and tastes, as well as pleasure and pain[ 4 , 12 – 14 ]. Experiences in typical dreams have a clear sensory character (i.e. they are seen, heard, and felt) and are not mere thoughts or abstractions.
These phenomenological similarities are reflected in neurophysiological similarities between waking and dreaming. For historical and methodological reasons, most electroencephalogram (EEG) and neuroimaging studies have contrasted brain activity during quiet wakefulness with that observed during REM sleep, when subjects are most likely to report dreams[ 15 – 20 ]. At least superficially, the EEG looks remarkably similar in active waking and REM sleep. Positron emission tomography (PET) studies have shown that global brain metabolism is comparable between wakefulness and REM sleep[ 11 , 20 ]. Such studies have also revealed a strong activation of high-order occipito-temporal visual cortex in REM sleep, consistent with the vivid visual imagery during dreams ( Fig. 1 )[ 16 , 17 , 19 ].
There is also remarkable consistency between a subject s cognitive and neural organization in dreaming and waking[ 13 , 14 ]. For instance, children studies demonstrate that dream features show a gradual development that parallels their cognitive development when awake[ 21 ] ( Box 2 ). Patients with brain lesions that impair their waking cognition show corresponding deficits in dreams. For example, subjects with impaired face perception also do not dream of faces[ 22 , 23 ] ( Box 3 ).
BOX 2The development of dreams in children
When do children start dreaming, and what kind of dreams do they have? Since children often show signs of emotion in sleep, many assume they dream a great deal. However, a series of studies by David Foulkes showed that children under the age of 7 reported dreaming only 20% of the time when awakened from REM sleep, compared with 80–90% in adults[ 21 ].
Preschoolers dreams are often static and plain, such as seeing an animal or thinking about eating. There are no characters that move, no social interactions, very little feeling, and they do not include the dreamer as an active character. There are also no autobiographic, episodic memories, perhaps because children have trouble with conscious episodic recollection in general, as suggested by the phenomenon of infantile amnesia. Preschoolers do not report fear in dreams, and there are few aggressions, misfortunes, and negative emotions. Note that children who have night terrors , in which they awaken early in the night from SWS and display intense fear and agitation, are probably terrorized by disorientation due to incomplete awakening rather than by a dream[ 116 ]. Thus, although children of age 2–5 can obviously see and speak of everyday people, objects and events, apparently they cannot dream of them.
Between ages 5 to 7 dream reports become longer, although still infrequent. Dreams may contain sequences of events in which characters move about and interact, but narratives are not well developed. At around age 7, dream reports become longer and more frequent, contain thoughts and feelings, the child s self becomes an actual participant in the dream, and dreams begin to acquire a narrative structure and to reflect autobiographic, episodic memories.
It could be argued that perhaps all children dream, but some do not yet realize that they are dreaming, do not remember their dreams, or cannot report them because of poor verbal skills. Contrary to these intuitive suggestions, dream recall was found to correlate best with abilities of mental imagery rather than language proficiency. Mental imagery in children is assessed by the Block Design Test of the Wechsler intelligence test battery[ 117 ]. In this task, children look at models or pictures of red and white patterns, and then recreate those patterns with blocks. Critically, scores on this test are the one parameter that correlates best with dream report in children. Put simply, it is children with the most developed mental imagery and visuo-spatial skills (rather than verbal or memory capabilities) that report the most dreams, suggesting a real difference in dream experience. Visuo-spatial skills are known to depend on the parietal lobes, which are not fully myelinated until age 7. Thus, linking visuo-spatial cognitive development with brain maturation studies[ 118 ] is an important field of further research.
The static nature of preschoolers dreams is also in accord with the notion that preoperational children can’t imagine continuous visual transformations[ 119 ]. In the “mental rotation” test[ 120 ] a subject is asked to determine whether two figures are the same or different. In adults, reaction times (which are used as the score) increase linearly with the degree of rotation, but children do not show this relationship and do not seem to be mentally imagining movement using visuo-spatial imagery. This is consistent with their dream reports lacking movement[ 21 ].
Along the same line, people who are blinded after the age of 5–7 seem to have visual imagination and dream with visual imagery throughout life, while blinding at an earlier age leads to absence of visualization in both waking and dreaming[ 121 , 122 ], though dreaming in blind individuals is a subject of debate[ 123 – 125 ]. Overall, dreaming appears to be a gradual cognitive development that is tightly linked to the development of visual imagination.
The slow development of full-fledged dreams and their intimate relation with imagination cast doubts on whether animals can dream as we do. It is likely that animals, too, can be conscious during sleep. For instance, lesions in parts of the brainstem that control movements cause cats to seemingly act out their dreams[ 126 ], very much like humans with REM sleep behavior disorder [ 113 ]. However, while a cat may experience images and emotions in sleep, it is less likely that these experiences are tied together by a narrative as is the case in our typical dreams[ 127 ]. Altogether, what kind of dreaming consciousness an animal has may reflect the extent to which it is conscious in general, and both waking and dreaming consciousness are best viewed as graded phenomena[ 80 ].
BOX 3Lesion studies of dreaming
The primary source on neuropsychology of dreaming is a study by Solms[ 22 ] who examined 361 neurological patients and asked them in detail about their dreaming. Overall, lesion studies indicate that dreaming depends on specific forebrain regions rather than on the brainstem REM sleep generator[ 22 , 128 , 129 ]. In most cases, global cessation of dreaming follows damage in or near the temporo-parieto-occipital junction (around Brodmann’s Area 40), more often unilaterally than bilaterally[ 23 , 128 ]. This region supports various cognitive processes that are essential for mental imagery[ 130 ]. Accordingly, patients with such damage typically show a parallel decline in waking visuo-spatial abilities[ 109 ]. These results strongly suggest that mental imagery is the cognitive ability most related to dreaming (though a link between loss of dreaming and aphasia has also been suggested[ 131 ]).
Less frequently, global cessation of dreaming follows bilateral lesions of white matter tracts surrounding the frontal horns of the lateral ventricles, underlying ventromedial prefrontal cortex[ 22 ]. Many of these nerve fibers originate or terminate in limbic areas, in line with increased limbic activity in REM sleep as revealed by functional imaging[ 15 , 16 , 18 ]. The ventromedial white matter contains dopaminergic projections to the frontal lobe which were severed in prefrontal leucotomy, once performed on many schizophrenic patients[ 53 ]. Most leucotomized patients (70–90%) complained of global cessation of dreaming as well as of lack in initiative, curiosity, and fantasy in waking life[ 23 ]. Since dopamine can instigate goal-seeking behavior, these data have been interpreted as supporting the classical psychodynamic view of dreams as fulfillment of unconscious wishes related to egoistic impulses[ 132 ].
Apart from global cessation of dreaming, more restricted lesions produce the cessation of visual dreaming [ 22 , 109 ], or the disruption of particular visual dimensions in dreams. For example, lesions in specific regions that underlie visual perception of color or motion are associated with corresponding deficits in dreaming[ 23 , 109 ]. In general, it seems that lesions leading to impairments in waking have parallel deficits in dreaming.
Some lesions, especially those in medial prefrontal cortex, the anterior cingulate cortex, and the basal forebrain, are associated with increased frequency and vividness of dreams and their intrusion into waking life[ 22 ]. Importantly, many brain-damaged patients report no changes in dreaming, indicating that the neural network supporting dreaming has considerable specificity. For example, lesions of dorsolateral prefrontal cortex, sensorimotor cortex, and V1 do not seem to affect dreaming at all[ 22 ]. The fact that patients with V1 lesions report vivid dreaming argues against the notion that reentry to early retinotopic cortex is a necessary condition for visual awareness[ 133 ].
Dreams also reflect our interests and personality, just like mental activity during wakefulness. Formal content analysis has revealed that mood, imaginativeness, individuals of interest, and predominant concerns are correlated between our waking and dreaming selves[ 12 – 14 ]. Personal anxieties we experience in wake, such as being inappropriately dressed, being lost, or being late for an examination, can appear in dreams that involve social interactions[ 24 ]. Dreams, like our personality in general, are quite stable over time in adulthood[ 12 – 14 ], and share many characteristics across cultures[ 12 – 14 ]. In addition, we feel we are personally participating in many dream events.
Despite these remarkable similarities, what makes dream consciousness so fascinating are the ways in which it differs from our waking experience. Some of these phenomenological differences are accompanied by consistent neurophysiological differences.
Reduced voluntary control and volition
We are generally surprised on awakening from a dream (“it was only a dream”) mainly because we didn’t consciously will that we would dream it. In fact, during dreaming there is a prominent reduction of voluntary control of action and thought. We cannot pursue goals, and have no control over the dream’s content. The fact that we are so surprised, excited and even skeptical about lucid dreaming – possibly a way to control some dreams[ 25 ] - illustrates how dreams normally lack voluntary control[ 9 ]. Interestingly, recent evidence points to the role of the right inferior parietal cortex (Brodmann’s Area 40) in waking volition[ 26 , 27 ], an area that is deactivated during REM sleep[ 15 , 16 ] ( Fig. 1 ).
Reduced self-awareness and altered reflective thought
Our dreaming consciousness consists of a single “track”: we are not contextually aware of where we are (in bed) or of what we are doing (sleeping, dreaming). There is a strong tendency for a distinct narrative of thoughts and images to persist without disruption (“single-mindedness”[ 28 ]). Indeed, reports of mental activity in REM sleep are longer than reports obtained from awake subjects[ 28 ]. Dreaming is almost always delusional since events and characters are taken for real. Reflective thought is altered in that holding contradictory beliefs is common, and a dreamer easily accepts impossible events such as flying, inconsistent scene switches, sudden transformations and impossible objects[ 29 ] such as a pink elephant. There is often uncertainty about space, time, and personal identities[ 30 ]. For example, a character may have the name, clothes and hairstyle of a male friend, but have mother’s face. Reduced self-monitoring in dreams may be related to the deactivation of brain regions such as posterior cingulate cortex, inferior parietal cortex, orbitofrontal cortex, and dorsolateral prefrontal cortex[ 15 , 16 ] ( Fig 1 ). Indeed, deactivation of prefrontal cortex has been shown to accompany reduced self-awareness during highly engaging sensory perception in wakefulness[ 31 ]. However, some dreams may have conserved reflective thought processes such as thoughtful puzzlement about impossible events[ 32 ], contemplating alternatives in decision-making[ 32 ], reflecting during social interactions[ 32 ], and “theory of mind”[ 33 ], demonstrating that individual dreams can differ from each other substantially.
Emotionality
Some dreams are characterized by a high degree of emotional involvement, including joy, surprise, anger, fear, and anxiety[ 34 – 36 ]. Interestingly, sadness, guilt, and depressed affect are rare[ 11 ], possibly due to reduced self-reflection. Some claim that fear and anxiety are enhanced in dreams to a degree rare in waking life[ 37 ], in line with Freud’s suggestion that dream narratives originate in perceived threats or conflicts[ 5 ]. Whether or not this interpretation has merits, REM sleep is in fact associated with a marked activation of limbic and paralimbic structures such as the amygdala, the anterior cingulate cortex, and the insula[ 15 , 17 , 19 ] ( Fig. 1 ). However, emotions are feeble in other dreams, and are absent altogether in 25–30% of REM sleep reports[ 34 – 36 ], including in situations where emotions would likely be present in waking[ 34 ], once again highlighting the variability in dream phenomenology.
Altered mnemonic processes
Memory is drastically altered for the dream and within the dream. Unless the dreamer wakes up, most dreams are forever lost. Upon awakening, memory for the dream often vanishes rapidly unless written down or recorded, even for intense emotional dreams. It is not clear why this is the case since from a neuroimaging perspective, limbic circuits in the medial temporal lobe that are implicated in memory processes, are highly active during REM sleep[ 15 – 18 ] ( Fig. 1 ). Perhaps the hypoactivity of prefrontal cortex, also implicated in mnemonic processes, plays an important role in dream amnesia. Contemporary theories of dreaming ( Table 1 ) offer different accounts of dream amnesia. For example, according to psychodynamic models, dream amnesia is due to processes of active repression[ 5 ]. According to Hobson s Activation-Input-Mode [AIM] model, dream amnesia is related to a state-change involving inactivity of monoaminergic systems (“aminergic de-modulation”) and deactivation of dorsolateral prefrontal cortex[ 11 ]. The neurocognitive model claims that dreams are usually forgotten because they are internal narratives; unless internal experiences are tied to external cues such as times and places they are bound to be forgotten[ 13 ].
Contemporary theories of dreaming
| Psychodynamic (Freud, Solms) | Activation-Input-Modulation [AIM] Model (Hobson) | Neurocognitive (Foulkes, Domhoff) | |
|---|---|---|---|
| General | Dreams represent fulfillment of unconscious wishes related to egoistic (often infantile sexual) impulses [ ]. unconscious content is disguised via censorship creating the bizarre dream content [ ]. More recently, the drive for dreaming has been associated with dopaminergic systems and “appetitive interests” [ ]. | Our conscious state is determined by three factors: (a) - total and regional brain activity levels, (b) - activation generated internally or externally, (c) - the ratio of aminergic to cholinergic neuromodulators. REM sleep and dreaming are characterized by high levels of activation, internal input, and cholinergic modulation [ ]. | Dreaming is what occurs when the mature brain is adequately activated, disconnected from external stimuli and without self-reflection. Once instigated, dreaming actively draws on memory schemas, general knowledge, and episodic information to produce simulations of the world [ , ]. |
| Dream amnesia | Since unconscious wishes are noxious to our consciousness, they are via censorship processes [ ]. Dream amnesia is anything but arbitrary: “our memory reproduces the dream not only incompletely but also untruthfully, in a falsifying manner”[ ]. | Dream amnesia largely stems from a Aminergic de-modulation and deactivation of dorsolateral prefrontal cortex in REM sleep create a brain state which is not favorable for subsequent memory [ ]. This also explains why we forget moments of brief awakenings during sleep. | Dream amnesia is primarily related to a and lack of context. To remember, we need an external narrative to which internal events can be tied [ , ]. Dream amnesia cannot be explained by a state- change since dreaming can occur at any state (NREM sleep and wake). |
| Signal propagation in dreaming | dreams originate from psychic motives which are later instantiated as sensory percepts: “a thought... is objectified in the dream, and represented as a scene”[ ]. | dreams originate from activation of sensory cortex by the brainstem (e.g. PGO waves), later to be interpreted and synthesized by mnemonic and high-order modules [ , ]. | Dreams originate in abstract knowledge and figurative thought which are processed back into “imaginal copies” of perceptual phenomena [ ]. |
| Is REM sleep a good model for dreaming? | REM sleep and dreaming can occur one without the other [ , ], for example in neurological patients. Dream- like experiences are related to forebrain mechanisms rather than to REM sleep generators in brainstem [ , ]. | Because REM sleep provides the most favorable brain conditions for dreaming, we can focus on its neurophysiology in our attempt to model the neuronal basis of dreaming [ , ]. | Dream-like experiences can occur also in NREM sleep, sleep onset, and wakefulness [ , ]. Children studies show that REM sleep may be an important condition for dreaming but not sufficient [ , ]. |
| Is dreaming largely similar to waking consciousness? | The apparent (manifest) aspect of dreams is bizarre and includes nonsensical changes in time and place, as well as incongruities of plot, character, and action [ ]. This is because the true (latent) dream content is disguised by the censor [ ]. Dreaming may be closely akin to mental illness [ , ]. | Dreaming is altogether comparable to delirium (acute confusional state) that can occur upon alcohol withdrawal [ ]; REM sleep shares its physiological substrate with psycho-pathological conditions such as schizophrenia (limbic hyper-activation and frontal hypo-activation) [ , ]. | Dreams are “a remarkably faithful replica of waking life” [ ]. Dreams are largely coherent and internally plausible narrative sequences rather than the stereotypical illogical sequences of bizarre images. Content analysis indicate a strong continuity between dream content and waking life[ ]. Evidence linking dreams to psychosis is limited[ ]: REM sleep deprivation does not alter schizophrenic pathology, aminergic agonists suppress REM sleep with no psychopathological effects. |
| Neurochemistry of dreaming | Dreaming is driven by the ‘wanting’ system: evidence from prefrontal leucotomies & effects of l-DOPA on dreaming [ , ]. | Primarily a role for REM sleep and dreaming [ , ]. Administration of cholinergic agonists (e.g. pilocarpine) can induce an artificial REM sleep period associated with dream reports [ ]. | Dreaming is unlikely to be driven by a specific chemical or brain region. It is most likely related to a where serotonin, norepinephrine, and histamine are absent while both acetylcholine and dopamine are present [ , ]. |
| The function of dreaming | According to Freud, dreams preserve sleep in the face of unconscious needs for excitement [ ]. More recently (Solms): “the biological function of dreaming remains unknown” [ ]. | Dreams may serve a creative function by providing a virtual reality model (protoconsciousness). The brain is preparing itself for integrative functions including learning and secondary consciousness [ ]. | Dreams probably have no function, but they do have coherence and meaning, which is often conflated with function[ ]: “dreaming is a spandrel of the mind, a by- product of the evolution of sleep and consciousness.” [ ] |
| What is the psychological meaning of dreams? | This theory emphasizes : individual dreams carry meaningful information about the dreamer. This theory lacks in power with regards to explaining dreams shared by all people [ ] (e.g. flying, teeth falling). | Dreaming is an attempt to best interpret activating signals in a coherent manner, and contents of individual dreams are nearly random. Nevertheless, the process of interpretation may carry some psychological meaning[ ]. | This theory emphasizes : dreams are based on stored memory representations and therefore reflect individual ways of abstracting knowledge, but specific dreams are not traceable back to particular episodes in our life[ ]. |
| Are dreams directly related to previous experience? | Dream content is related to daytime experience ( ) that triggers the emergence of related memories. “All the material making up the content of a dream is in some way derived from experience” [ ] | Dream content is largely unrelated to the preceding day’s experiences[ ] and in general does not accurately represent episodic memories which are available during wakefulness[ , ]. | Familiar settings and people are sometimes incorporated into dreams but dreams are not a recollection of everyday life [ ]. |
Episodic memory is also impaired within the dream. Indeed, a dream is not like an episode of life being “replayed”. In one example in which subjects had intensively played the computer game Tetris, there was no episodic memory in subsequent dreams that subjects had indeed played Tetris. In fact, dreams of healthy subjects were indistinguishable from those of profoundly amnesic subjects, who could not remember having played Tetris at all. In contrast, both normal and amnesic subjects often reported perceptual fragments, such as falling blocks on a computer screen, at sleep onset[ 38 ]. While ‘residues’ from waking experience are incorporated in about 50% of dreams[ 39 – 41 ], they do so in new and unrelated contexts, and verified memories for episodes of recent life are only found in about 1.5% of dreams[ 42 ]. Such residual recollections have been interpreted by some to suggest that dreaming may have an active role in forgetting[ 5 , 43 ]. Finally, many have the impression that the network of associations stored in our memory may become looser than in wake[ 44 , 45 ], perhaps favoring creativity, divergent thinking, and problem resolution[ 4 , 46 ].
In summary, dream consciousness is remarkably similar to waking consciousness, though there are several intriguing differences. These include reduced attention and voluntary control, lack in self-awareness, altered reflective thought, occasional hyperemotionality, and impaired memory. Traditionally, dream phenomenology has often been compared to madness or psychosis[ 3 , 11 , 47 ], but in fact the hallucinations, disorientation, and subsequent amnesia of some bizarre dreams may be more akin to the acute confusional state – also known as delirium - which occurs after withdrawal from alcohol and drugs[ 48 ]. However, most dreams are less bizarre, perhaps more similar to mind wandering or stimulus independent thoughts[ 14 , 49 , 50 ]. Waking thoughts jump around and drift into bizarre daydreaming, rumination, and worrying far more than stereotypes of rational linear thinking suggest[ 51 ]. Importantly, individual dreams are highly variable in their phenomenology, and only some conform to the typical monolithic template that is often portrayed. Thus, just like diverse waking experiences, “Not all dreams are created equal” , and future studies should consider different kinds of dreams and their neural correlates separately.
What mechanisms are responsible for regional differences in brain activity between waking and REM sleep, and thus presumably for some of the cognitive differences between waking and dreaming? Single-unit physiology indicates that generally, cortical activity in REM sleep reaches similar levels as found in active wake ( Fig. 2 ), but variability between brain areas remains poorly explored. Regional differences may likely stem from changes in the activity of neuromodulatory systems ( Fig. 2 ). During REM sleep, acetylcholine is alone in maintaining brain activation, whereas monoaminergic systems are silent, an observation that could explain many features of dreams[ 11 ]. For example, consistent with imaging results, cholinergic innervation is stronger in limbic and paralimbic areas than in dorsolateral prefrontal cortex[ 52 ], which may explain why limbic regions are highly active in REM sleep while dorsolateral prefrontal cortex is deactivated ( Fig. 1 ). Dopaminergic modulation may also play a role[ 23 ], since dreaming is decreased by prefrontal leucotomies that cut dopaminergic fibers[ 53 ] and is increased by dopaminergic agonists[ 23 ] ( Table 1 and Fig. 2 ).

A comparison of cortical activity (upper panel) and neuromodulator activity (bottom panel) in wake, early NREM (when sleep pressure is high and dream reports are rare), late NREM (when sleep pressure dissipates, and dream reports are more frequent), and REM sleep (when dreams are most common).
(a) Intracellular studies. The membrane potential of cortical neurons in both wake and REM sleep is depolarized and fluctuates around −63mV and −61mV, respectively [ 77 ]. In REM sleep, whenever phasic events such as rapid eye movements and PGO waves occur (gray arrows, events not shown), neurons increase their firing rates to levels that surpass those found in wake [ 77 , 146 ]. In early NREM sleep, neurons alternate between two distinct states, each lasting tens/hundreds of milliseconds: UP states (red arrow) are associated with depolarization and increased firing, while in DOWN states (blue arrow) the membrane potential is hyperpolarized around −75mV, and neuronal firing fades[ 78 , 147 ]. Intracellular studies focusing specifically on late NREM sleep are not available (N.A.).
(b) Extracellular studies. Spiking of individual neurons in REM sleep reaches similar levels as in active wake. In both wake and REM sleep, neurons exhibit tonic irregular asynchronous activity [ 77 , 148 – 151 ]. Sustained activity in wake and REM sleep can be viewed as a continuous UP state [ 78 ] (red bars). In early NREM sleep, UP states are short and synchronous across neuronal populations, and are frequently interrupted by long DOWN states (blue bars). In late NREM sleep, UP states are longer and less synchronized [ 79 ].
(3) Polysomnography. Waking is characterized by low-amplitude, high-frequency EEG activity (above 7Hz), occasional saccadic eye movements, and elevated muscle tone. In early NREM sleep, high-amplitude slow waves (below 4Hz) dominate the EEG. Neuronal UP (red) and DOWN (blue) states correspond to positive and negative peaks in the surface EEG, respectively [ 79 ]. Eye movements are largely absent and muscle tone is decreased. In late NREM sleep, slow waves are less frequent, while spindles (related to UP states and surface EEG positivity) become more common. Eye movements and muscle tone are largely similar to early NREM sleep [ 152 ]. In REM sleep, theta activity (4–7 Hz) prevails, rapid eye movements occur, and muscle tone is dramatically reduced.
(d) Neuromodulator activity. Subcortical cholinergic modulation is highly active in wake and REM sleep (green arrows) and leads to sustained depolarization in cortical neurons and EEG activation [ 77 ]. Wake is further maintained by activity of monoamines, histamine, and hypocretin/orexin (green arrows). In sleep, monoaminergic systems including norepinephrine and serotonin reduce their activity (pink arrows), and are silent in REM sleep (red arrows). While dopamine levels do not change dramatically across the sleep-wake cycle (asterisks), phasic events and regional profiles may differ[ 153 ].
Data are pooled across different species for illustration purposes. Intracellular cat data adapted with permission from Ref [ 77 ]; extracellular and EEG rat data obtained from V. Vyazovskiy (personal communication).
On the whole, relating typical dreams to the neurophysiology of REM sleep has proven to be a useful starting point for revealing the neural basis of dreaming. However, dream consciousness can not be reduced to brain activity in REM sleep. Indeed, some fundamental questions concerning the relationship between the brain and dreaming linger on. We shall discuss three in turn: i) what determines the level of consciousness during sleep; ii) why the dreamer is disconnected from the environment; and iii) whether dreams are more akin to perception or to imagination.
What determines the level of consciousness during sleep?
In principle, studying mental experiences during sleep offers a unique opportunity to explain how changes in brain activity relate to changes in consciousness[ 3 , 54 ]. In fact, if it were not for sleep, when consciousness fades in and out on a regular basis, it might be hard to imagine that consciousness is not a given, but depends somehow on the way our brain is functioning. Traditionally, studies have focused on differences among reports obtained after awakenings from different sleep stages or at different times of night. When REM sleep was initially distinguished from NREM sleep[ 55 ], it was reported that 74–80% of REM sleep awakenings produced vivid dream recall, compared to only 7–9% of awakenings from NREM sleep[ 56 , 57 ]. It was only natural to conclude that, compared to NREM sleep, the distinct physiology of REM sleep, and especially its fast, low-voltage EEG resembling that of wakefulness, was the reason why we are conscious and dream in REM sleep, and not in NREM sleep[ 29 ]. Indeed, for some time, reports of mental activity upon awakenings from NREM sleep were assumed to be recalls of earlier REM sleep dreams, or considered analogous to sleep talking[ 3 ], or treated as confabulations made up by subjects confused upon awakening[ 9 ] ( Box 1 ). However, when changing the question from “tell me if you had a dream” to “tell me anything that was going through your mind just before you woke up,” reports of conscious experiences in NREM sleep ranged between 23% and 74%[ 9 ]. Subsequent studies demonstrated clearly that NREM sleep awakenings yielded reports of mental activity[ 58 , 59 ].
Specifically, reports from sleep stage N1 are extremely frequent (80–90% of the time), though they are very short[ 60 ]. Usually people report vivid hallucinatory experiences, so-called hypnagogic hallucinations . In contrast to typical dreams, hypnagogic hallucinations are often static - like single snapshots[ 11 , 47 ], and usually do not include a self character[ 14 ]. Some activities performed before sleep (e.g. video games) may influence the content of hypnagogic dreams[ 38 , 61 ]. Awakenings from NREM sleep stages N2 and N3 yield reports about some experienced content 50–70% of the time[ 59 ], although there is great variability throughout the night and between subjects. Early in the night, when stage N3 is prevalent and many large slow waves dominate the EEG, awakenings yield few reports[ 62 ]. Moreover, these reports are often qualitatively different than typical REM sleep reports, being usually short, thought-like, less vivid, less visual and more conceptual, less motorically animated, under greater volitional control, more plausible, more concerned with current issues, less emotional and less pleasant[ 9 , 11 , 63 ]. Also, the average length of REM sleep reports increases with the duration of the REM sleep episode while this is not true for NREM sleep reports[ 62 ]. However, late in the night NREM sleep reports are considerably longer and more hallucinatory. Indeed, 10–30% of all NREM sleep reports are indistinguishable by any criteria from those obtained from REM sleep[ 64 , 65 ]. Since NREM sleep accounts for 75% of total sleep time, this means that full-fledged NREM sleep dreams actually account for a significant portion of all typical dreams.
Thus, the initial equation of a physiological state (REM sleep) with a mental state (dreaming) was incorrect, or at best, an oversimplification. Moreover, neuropsychological evidence indicates that dreaming and REM sleep can be dissociated: forebrain lesions may abolish dreaming and spare REM sleep, whereas brainstem lesions may nearly eliminate overt features of REM sleep without abolishing dreams[ 23 ] ( Box 3 ). But if dream reports can be elicited during any stage of sleep[ 11 , 47 , 59 , 66 , 67 ], and conversely some awakenings may yield no report, no matter in which sleep stage they were obtained[ 59 ], where do we stand today with respect to the relationship between brain activity and consciousness during sleep?
The one thing that seems clear is that we need to move beyond the REM/NREM sleep dichotomy and beyond traditional sleep staging. Though staging is useful, it treats brain activity as uniform in space (only a few electrodes are used) and in time (for 30 sec epochs). Inevitably, subtler features of brain activity, which may well influence the presence, degree, and reportability of consciousness, are missed both in space and in time.
In the spatial domain, increasing evidence suggests that different brain regions may be in different states at the same time. For example, preliminary findings suggest that during sleepwalking, thalamocingulate pathways may be active as in wake, while the rest of the cerebral cortex is in NREM sleep[ 68 ]. A related notion of dissociated states is derived from the study of parasomnias, where wake-like behaviors occur during sleep[ 69 ]. For instance, the study of REM sleep behavior disorder shows that, contrary to common assumptions, wakefulness, REM sleep and NREM sleep may not be mutually exclusive states[ 69 ]. In the current context, it has been suggested that dreaming in NREM sleep is related to ‘covert’ REM processes that occur locally[ 59 ]. Thus, refined spatial analysis using fMRI or high-density EEG (hd-EEG) could potentially identify regionally-specific predictors of dreaming, and possibly indicate, in real time, whether dream reports will be obtained.
In the temporal domain, some attempts have been made to relate transient, phasic activities[ 70 ] to dreaming. For example, various studies have tried to link dream recall to eye movements[ 71 , 72 ], PGO waves[ 73 ], and EEG power bouts in specific frequency bands[ 74 ] but limited success has been achieved, and little has been done for NREM sleep[ 11 , 75 , 76 ]. We now know that slow waves in NREM sleep reflect a slow oscillation of cortical neurons between UP and DOWN states ( Fig. 2 )[ 77 , 78 ]. Perhaps long UP states are necessary for dreaming to occur. This is normally the case in REM sleep since slow waves are absent. As for NREM sleep, we would expect that higher occurrence of recalls, and especially of typical dreams in the morning hours, would reflect longer UP periods upon dissipation of sleep pressure ( Fig. 2 )[ 79 ]. In general, focusing on (rather than avoiding) “gray zones” where it is more difficult to predict whether a dream report will be obtained, for example in early REM sleep or late NREM sleep, may be a promising strategy for identifying psychophysiological correlates that go beyond traditional staging.
Finally, theoretical considerations suggest that the level of consciousness may depend on the brain s ability to integrate information[ 80 ]. Indeed, during wakefulness external perturbations such as TMS pulses (transcranial magnetic stimulation) cause changing patterns of activation across distant interconnected brain regions[ 10 ]. In REM sleep, evoked activity propagates much like it does in wakefulness[ 81 ]. By contrast, in deep SWS early in the night, when consciousness is most likely to fade, the response evoked by TMS remains either local (loss of integration), or spreads nonspecifically (loss of information). Apparently, the brain s capacity for information integration is reduced whenever neurons become bistable between UP and DOWN states. Intriguingly, the brain s response to a TMS pulse may offer a more sensitive measure of the inner state than spontaneous EEG. For example, such perturbations can uncover inherent bistability in short stretches of NREM sleep even when the EEG shows a wake-like low-voltage pattern[ 82 ].
Why is the dreamer disconnected from the environment?
The most obvious difference between dreaming and waking consciousness is the profound disconnection of the dreamer from his current environment. Such disconnection, of course, is a key feature of sleep: by definition a sleeping person shows no meaningful responses to external stimuli, unless they are strong enough to cause an awakening. This feature is known as “high arousal threshold”, and it persists in REM sleep despite its wake-like low-voltage EEG[ 83 ]. Moreover, stimuli not only fail to elicit a behavioral response, but also largely fail to be incorporated in the content of the dream[ 8 , 84 – 86 ] (though some stimuli, such as a spray of water, pressure on the limbs, and meaningful words have a slightly higher chance of incorporation[ 84 , 85 ]). This striking disconnection occurs even when subjects sleep with their eyes taped open and objects are illuminated in front of them[ 8 ]. Surely just before awakening, stimuli such as the sound of an alarm clock can enter our dreams, but when sleep is preserved, such relations are by and large surprisingly weak and dream consciousness is remarkably disconnected from the external environment.
The disconnection of the dreamer poses an intriguing paradox, especially if one considers that dreams involve vivid sensory experiences, and that they can occur upon a state of strong cortical activation. Several possibilities come to mind. For example, it has been suggested that during sleep a thalamic “gate” may close and sensory inputs may not reach the cortex effectively[ 87 ]. However, evoked responses in primary sensory cortices are largely preserved during REM sleep[ 88 , 89 ]. Also, olfactory stimuli are not directly incorporated in dreams[ 90 ], though they are not routed through the thalamus (their emotional valence, however, may affect dreams). A related notion is that of a cortical “gate” leading to diminished inter-cortical propagation[ 91 ], as seems to be the case in the dissociation of primary visual cortex (V1) from high-order visual cortex in REM sleep[ 18 ]. It would be interesting to establish whether direct activation of cortical areas can overcome the disconnection from the environment. For example, can TMS over V1 or area MT bypass thalamic or cortical “gates” and produce sensations of phosphenes or movement in dream consciousness?
An intriguing possibility concerns the putative antagonism between externally oriented cortical networks and internally oriented, default-mode networks[ 92 , 93 ]. Perhaps in dreams intrinsic activity dominates, as it does during stimulus-independent thoughts in wake[ 50 ]. This may occur at the expense of the processing of external stimuli, leading to disconnection from the environment. Indeed, both PET and magnetoencephalography (MEG) suggest that medial prefrontal cortex, a part of the default network, is highly active in REM sleep[ 16 , 17 , 94 ] as it is during wakeful rest ( Fig. 1 ). Conversely, other components of the default network, including posterior cingulate and inferior parietal cortices, are deactivated in REM sleep[ 15 , 16 ], as in highly-engaging waking tasks ( Fig. 1 ). The exact cognitive task associated with the default-mode network is still not well understood[ 95 ] and it may be primarily driven by self-related introspective processes rather than general mind wandering[ 31 , 96 , 97 ]. Indeed, since most nodes of this network are deactivated in REM dreaming and mental imagery[ 98 ], cognitive states that are oriented internally but away from the self do not seem sufficient to elicit activity in this network.
Another possibility is that dreams may be analogous to altered states of consciousness in which attention is profoundly altered, as may be the case in extreme absorption, hypnosis, neglect[ 99 ], and Balint s syndrome, when visual experience may persist for single but unlocalizable objects (simultanagnosia)[ 100 , 101 ]. The reticular thalamic nucleus has been implicated in redirecting attention across modalities[ 102 , 103 ] and its activity in sleep may underlie some aspects of disconnection. It would also be interesting to determine whether neuronal correlates of momentary lapses of attention[ 104 ] occur regularly while dreaming.
Finally, as we have seen, the neuromodulatory milieu changes drastically in sleep ( Fig. 2 ). Specifically, the levels of norepinephrine, serotonin, histamine, and hypocretin are greatly reduced in REM sleep compared to wake, so the presence of one or more of these neuromodulators may be necessary for external stimuli to be incorporated into our stream of consciousness. This search can be narrowed down by considering cataplexy, which affects people with narcolepsy[ 105 ]. Cataplexy is a transient episode of muscle tone loss in which humans report that awareness of external stimuli is preserved, and presumably animals are likewise aware of their environment during cataplectic attacks. Neuromodulatory activity in cataplectic dogs is largely similar to that in REM sleep except that levels of histamine are high, much like during wakefulness[ 105 ]. It thus seems that levels of histamine are correlated with our ability to incorporate sensory stimuli into conscious experience. It would be important to establish whether histamine is indeed necessary for such incorporation, and how it may do so. For instance, could it be that in wakefulness histaminergic tone facilitates transmission of feed-forward sensory inputs in cortical layer 4, at the expense of backward signal propagation?
Are dreams more like perception or imagination?
Whether dreams are generated in a “bottom-up” or a “top-down” manner is a question that has been asked since at least Aristotle[ 106 ]. To put the question in a modern context, do dreams start from activity in low-level sensory areas, which is then interpreted and synthesized by higher-order areas, as is presumably the case in waking perception? Or do they begin as wishes, abstract thoughts, and memories deep in the brain, which are then enriched with perceptual and sensory aspects, as in imagination? Of course, it is possible that such a dichotomy is misguided, and dreams may be best conceptualized as global attractors that emerge simultaneously over many brain areas. However, as we shall see, the available data do indeed suggest that there may be a privileged direction of dream generation.
In the 19th century, sensory experience was often regarded as the source of dreams, which were considered to be an attempt of the mind to interpret somatic nerve-stimuli ( Supplementary Fig. 1 ). A similar notion was later adopted by Henri Beaunis, and recently championed by Allan Hobson ( Table 1 )[ 4 , 11 , 47 ]. According to his AIM model, internally generated signals originating in the brainstem during REM sleep, such as PGO waves, excite visual cortex and are later processed and synthesized by higher-order areas. High levels of acetylcholine in the absence of aminergic neuromodulation may enhance feed-forward transmission and suppress back-propagation[ 3 , 107 ]. By contrast, Freud and some of his followers asserted that dreams originate from psychic motives that are later instantiated as sensory percepts, much like mental imagery[ 5 ].
Deciding between these alternative views will most likely require difficult experiments in which the direction of signal flow during dreaming sleep is evaluated and compared to that during waking perception and imagery[ 108 ] ( Box 4 ). However, various lines of evidence already suggest that dreaming may be more closely related to imagination than to perception. From lesion studies ( Box 3 ) we know that dreaming requires an intact temporo-parieto-occipital junction[ 22 , 23 ] and lesions in this region also affect mental imagery in wakefulness[ 109 ]. Cognitive studies indicate that the skill that maximally correlates with dream recall in adults is visuo-spatial imagery[ 110 ]. In children, dream recall develops hand in hand with visuo-spatial imagery ( Box 2 ). In epileptic patients, direct electrical stimulation in high-order regions such as the medial temporal lobe, rather than in visual cortex, can elicit “dream-like” experiences[ 111 ], although such patients are simultaneously aware of their surroundings. Other evidence comes from lucid dreamers[ 25 ] who report that it is impossible to focus on fine-grain details of visual objects, as is the case in mental imagery[ 112 ]. Perhaps top-down connections lack the anatomical specificity to support detailed representations. The rare occurrences of smells or pain in dreams may also be related to our difficulty in imagining them vividly when awake. However, one important difference between dreaming and mental imagery is that while imagining we are aware that the images are internally generated (preserved reflective thought).
Box 4Future directions
1. Signal propagation in dreams
During wakefulness, sensory responses precede responses in higher-order areas by more than 100ms[ 134 , 135 ]. Does neural activity during dreaming sleep show a similar feed-forward progression as in perception? Or does neural activity propagate backwards, from higher to lower areas, as it is thought to do during imagery? This issue, which is crucial to our understanding of dream generation, could be resolved by examining unit and field potential recordings from the same neuronal populations in wake and REM (or late NREM) sleep in both animals and humans[ 135 ]. One can also apply directional measures of signal propagation (e.g. Granger causality) to hd-EEG data, and check whether the main direction of signal flow inverts between wake and sleep. Finally, one could use TMS with concurrent hd-EEG during both wake and REM sleep, and examine whether there may be a preferential direction of the brain s response to perturbations depending on behavioral state[ 10 ].
2. Functional networks underlying dreaming
So far, most regional studies of brain activity during sleep have employed PET. While PET allows for quantification of cerebral blood flow and comparison across vigilance states, functional MRI (fMRI) offers superior spatial and temporal resolutions. Event-related fMRI has been already used to map brain activity associated with phasic events such as slow waves[ 136 ] and eye movements[ 137 , 138 ]. Studies of functional and effective connectivity[ 139 ] may be especially well suited to map the functional networks underlying dreaming. Notably, perceptual awareness is associated with specific functional connectivity patterns within sensory modalities[ 140 ], between modalities[ 141 ], and with a striking segregation between sensory systems and the default-mode/intrinsic system[ 31 , 93 , 104 ]. Are such connectivity patterns also a hallmark of activity in the dreaming brain? What regional brain activity underlies dreaming in NREM sleep? How do functional networks of mental imagery and dreaming compare in the same subjects? Finally, hd-EEG may be particularly suited for sleep imaging since it (a) allows for relatively undisturbed sleep, (b) upon source modeling can provide a spatial resolution roughly comparable to PET, (c) offers high temporal resolution suitable for evaluating signal propagation, and (d) can be combined with TMS during sleep.
3. Initial steps towards studying dream content
Progress in signal decoding may ultimately enable us to investigate the neural correlates not only of dream form – what is common to all dreams – but also of dream content – what is specific to a particular dream. This can be done, for instance, by using classification techniques applied to fMRI or hd-EEG data[ 142 ]. At least initially, it may be worthwhile to consider some coarse properties of individual dreams, such as the frequency of occurrence of faces or places in a dream report, the amount of movement, or the dominant affective valence. In principle, it should be possible to predict not only the likelihood of a report upon awakening, but also the likelihood of specific features based on preceding brain activity. An important step in this direction would be to identify the contents of internally generated mental imagery using the same approach[ 143 ]. Furthermore, some patients with epilepsy or post-traumatic stress disorder who experience recurring dream contents[ 144 , 145 ] may provide a unique opportunity to relate specific dream content to its neural basis.
If the flow of brain activity during dreaming were shown to be largely backwards, as one would expect in imagery, rather than forwards, as in perception, many of the seemingly bizarre properties of dreams, such as blended characters and scene switches, would be easier to explain, as they are standard features of our imagination. Such a top-down mode may disrupt the encoding of new memories, and thus underlie dream amnesia. In addition, top-down mental imagery could obstruct the processing of incoming stimuli and disconnect us from the environment. If this view is correct, waking consciousness is more like watching the news in real time, while dreaming is more like watching a movie created by an imaginative director[ 81 ]. As in some B-movies, the director is not particularly choosey and any actor, dress, means of transportation, or object that is readily available will do. Albert Einstein said that “imagination points to all we might yet discover and create”, and indeed, dreaming may turn out to be the purest form of our imagination.
Concluding remarks
In summary, dream consciousness is remarkably similar to waking consciousness, though there are several intriguing differences in volition, self-awareness and reflection, affect, and memory, and there is great variability between individual dreams. The neurophysiology of REM sleep, and in particular recent insights into its regional activity patterns, offers a useful starting point for relating dream phenomenology to underlying brain activity. However, the initial equation of REM sleep with dreaming has been shown to be inaccurate. Thus, it is time we moved beyond sleep stages when trying to link dream consciousness to neuronal events, and focused on more subtle features of brain activity in space and time. Our profound disconnection from the external environment when dreaming poses a central unsolved paradox, the answer to which may be instrumental for understanding dreams. Converging evidence from multiple fields of study, including phenomenology, development, neuropsychology, functional imaging, and neurophysiology, support the notion that dreaming may be closely related to imagination, where brain activity presumably flows in a “top-down” manner. Viewing dreams as a powerful form of imagination can help explain many of their unique features, such as sudden transitions, uncertainty about people and places, poor subsequent recall, disconnection from the environment, and offers testable predictions for future studies.
Supplementary Material
Acknowledgments.
We apologize to those whose work was not cited because of space constraints. We thank Michal Harel, Lior Fisch, and Vlad Vyazovskiy for help with figures; Chiara Cirelli, Rafi Malach, Simone Sarasso, Brady Riedner, and Fabio Ferrarelli for helpful discussions and comments; our anonymous reviewers for valuable suggestions. Y.N. is supported by an EMBO long term fellowship and the Brainpower for Israel Fund. G.T is supported by an NIH Director’s Award DP1 OD000579 and NIH Conte Center Award P20 MH077967.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- Yuzhno-Sakhalinsk Tourism
- Yuzhno-Sakhalinsk Hotels
- Yuzhno-Sakhalinsk Bed and Breakfast
- Flights to Yuzhno-Sakhalinsk
- Yuzhno-Sakhalinsk Restaurants
- Things to Do in Yuzhno-Sakhalinsk
- Yuzhno-Sakhalinsk Travel Forum
- Yuzhno-Sakhalinsk Photos
- Yuzhno-Sakhalinsk Map
- All Yuzhno-Sakhalinsk Hotels
- Yuzhno-Sakhalinsk Hotel Deals
Kuril Islands - Yuzhno-Sakhalinsk Forum
- Europe
- Russia
- Far Eastern District
- Sakhalin Oblast
- Sakhalin
- Yuzhno-Sakhalinsk
Kuril Islands
- United States Forums
- Europe Forums
- Canada Forums
- Asia Forums
- Central America Forums
- Africa Forums
- Caribbean Forums
- Mexico Forums
- South Pacific Forums
- South America Forums
- Middle East Forums
- Honeymoons and Romance
- Business Travel
- Train Travel
- Traveling With Disabilities
- Tripadvisor Support
- Solo Travel
- Bargain Travel
- Timeshares / Vacation Rentals
- Sakhalin forums
- Yuzhno-Sakhalinsk forum

I'm going to organize in August 207 independent expedition to Kuril Islands (Kunashir and Iturup) with access to the islands and escape with a m/v from Korsakov (Yuzhno-Sakhalinsk).
Is it possible?

I know several persons that have traveled from Sakhalin to the Kurils . Note that :
- You might need a Border Pass
- Logistics to travel to Kuril are nor simple. You can stay longer than expected over there due to logistics.
- You might check several of the travel agencies in Yuzho that offer support for this expeditions at : http://www.sakh.com
This topic has been closed to new posts due to inactivity.
- Day trip from cruise ship korsakov Nov 03, 2019
- Is it really a hassle for a foreigner to do anything... Jun 11, 2018
- Kurilian Bobtail Cats Aug 05, 2009
- Kuril Islands Apr 29, 2007
- Tourist visas May 30, 2006
- Kurilian Bobtail Cats 2 replies
- Tourist visas 5 replies
Yuzhno-Sakhalinsk Hotels and Places to Stay
- GreenLeaders
Yuzhno-Sakhalinsk
| Overview | Map | Directions | Satellite | Photo Map |
| Overview | Map | Directions |
| Satellite | Photo Map |
| Tap on the map to travel |
Notable Places in the Area
Sakhalin regional museum.

Anton Chekov Theatre Center

Yuzhno-Sakhalinsk railway station

- Type: City with 180,000 residents
- Description: city in far-eastern Russia
- Categories: administrative territorial entity of Russia , city or town , big city , village and locality
- Location: Sakhalin Oblast , Russian Far East , Russia , Eastern Europe , Europe
- View on OpenStreetMap
Yuzhno-Sakhalinsk Satellite Map
Popular Destinations in Russian Far East
Explore these curated destinations.

IMAGES
VIDEO
COMMENTS
Neuroscience research has the potential to support and refine models of motivation and cognitive skill. It may play a pivotal role in developing classroom interventions and understanding non-cognitive skills (e.g., mindset). ... Most empirical research on growth mindset and intrinsic motivation has focused on behavioral methods and self-reports ...
Research data. Cognitive neuroscience seeks generalizable theories explaining the relationship between behavioral, physiological and mental states. In pursuit of such theories, we propose a ...
The theory of multiple intelligences (MI) has motivated the present investigation. Gardner [3], [4] redefined intelligence as the ability to solve problems or create products of value in a culture or community. Using this broad, common sense definition and eight criteria* that cover a range of empirical evidence (e.g., neuroscience, psychometric and evolutionary evidence, and atypical ...
Intrinsic motivation would seem to be an especially ripe topic for neuroscience precisely because of the large body of empirical data that has already been garnered at the experiential and behavioral levels of analysis. Our purpose of this review article is to survey the progress of neuroscience research on intrinsic motivation. Because ...
Read the latest Research articles from Nature Neuroscience. Skip to main content. ... Nature Neuroscience (Nat Neurosci) ISSN 1546-1726 (online) ISSN 1097-6256 (print) nature.com sitemap ...
Educational neuroscience is an interdisciplinary field exploring the effects of education on the human brain and promotes the translation of research findings to brain-based pedagogies and policies . The brain is the target organ of education. Education is thought to influence brain development [2,3] and health, even as the brain ages [4,5 ...
Cognitive neuroscience research on conceptual knowledge often is discussed with respect to "embodiment" or "grounding." We tried to disentangle at least three distinct claims made using these terms. One of these, the view that concepts are entirely reducible to sensory-motor representations, is untenable and diminishing in the literature.
The primary mission of Behavioral Neuroscience® is to publish original research articles as well as reviews in the broad field of the neural bases of behavior. We seek empirical papers reporting novel results that provide insight into the mechanisms by which nervous systems produce and are affected by behavior.
Top 100 in Neuroscience. This collection highlights our most downloaded* neuroscience papers published in 2020. Featuring authors from around the world, these papers showcase valuable research ...
Clark makes a convincing case for the merits of conceptualizing brains as hierarchical prediction machines. This perspective has the potential to provide an elegant and powerful general theory of brain function, but it will ultimately stand or fall with evidence from basic neuroscience research. Her …
Investigating the relationship between brain structure and function is a central endeavor for neuroscience research. Yet, the mechanisms shaping this relationship largely remain to be elucidated and are highly debated. ... Empirical and simulated windowed FC were computed on individual subjects using sliding time-windows (increment of 20 s) of ...
A major thrust of cognitive neuroscience is the elucidation of structure-function relationships in the human brain. Over the last several years, functional neuroimaging has risen in prominence relative to the lesion studies that formed the historical core of work in this field. ... an empirical study of impact in cognitive neuroscience J Cogn ...
Neurophenomenology presents a methodological strategy that takes a vantage point from phenomenology, that is the conscious experience of the first-person-perspective, to especially research consciousness and its connection to the neuronal level of the third-person-perspective of the observational-experimental empirical research of neuroscience ...
Consciousness is now a well-established field of empirical research. A large body of experimental results has been accumulated and is steadily growing. In parallel, many Theories of Consciousness (ToCs) have been proposed. These theories are diverse in nature, ranging from computational to neurophysiological and quantum theoretical approaches.
Author summary Biomedical science, psychology, and many other fields may be suffering from a serious replication crisis. In order to gain insight into some factors behind this crisis, we have analyzed statistical information extracted from thousands of cognitive neuroscience and psychology research papers. We established that the statistical power to discover existing relationships has not ...
Neuroscience News posts science research news from labs, universities, hospitals and news departments around the world. Science articles cover neuroscience, psychology, AI, robotics, neurology, brain cancer, mental health, machine learning, autism, Parkinson's, Alzheimer's, brain research, depression and other sciences.
Recent years have seen a blossoming of theories about the biological and physical basis of consciousness. Good theories guide empirical research, allowing us to interpret data, develop new ...
Several dozen Del Monte Institute for Neuroscience at the University of Rochester faculty, researchers, and trainees from programs including Neuroscience, Brain and Cognitive Science, and Toxicology traveled to Chicago for the annual Society for Neuroscience (SfN) Conference. According to SfN, more than 30,000 people regularly attend the annual conference.
Abstract. This paper explores memory from a cognitive neuroscience perspective and examines associated neural mechanisms. It examines the different types of memory: working, declarative, and non-declarative, and the brain regions involved in each type. The paper highlights the role of different brain regions, such as the prefrontal cortex in ...
Join Us in San Diego for Neuroscience 2025! Meeting Dates: Saturday, November 15-Wednesday, November 19. Location: San Diego Convention Center. Each year, scientists from around the world congregate to discover new ideas, share their research, and experience the best the field has to offer. Attend so you can: present research, network with ...
The Clinical Neuroscience MSc is a pioneering Master's programme taught at the UCL Queen Square Institute of Neurology. UCL is the top-ranked university in the UK for research power in Psychology, Psychiatry and Neuroscience according to the UK's Research Excellence Framework 2021.
The Dementia: Causes, Treatments and Research (Neuroscience) MSc, offered by the UCL Queen Square Institute of Neurology, tackles one of the biggest global health problems facing society today. It provides research-oriented and cutting-edge training in the study of dementia and its scientific basis, delivered by international leaders in the science and practice of dementia.
Empirical research in the US often focuses on IC deficiencies, suggesting a need for empirical indicators of robust IC (Chen et al., Citation 2016). ICQ measurement methods include disclosure indices and content analysis, which have limitations such as self-construction and potential bias (Hassan & Marston, Citation 2010 ; Chalmers et al ...
Sakhalin (Russian: Сахали́н, IPA: [səxɐˈlʲin]) is an island in Northeast Asia.Its north coast lies 6.5 km (4.0 mi) off the southeastern coast of Khabarovsk Krai in Russia, while its southern tip lies 40 kilometres (25 mi) north of Japan's Hokkaido.An island of the West Pacific, Sakhalin divides the Sea of Okhotsk to its east from the Sea of Japan to its southwest.
Yuzhno-Sakhalinsk began as a small Russian settlement called Vladimirovka, founded by convicts in 1882. [2] The Treaty of Portsmouth in 1905, which brought an end to the Russo-Japanese War of 1904-1905, awarded the southern half of the Sakhalin Island to Japan.Vladimirovka was renamed Toyohara (meaning "bountiful plain"), and was the prefect capital of the Japanese Karafuto Prefecture.
Contemporary dream research. Although dreams have fascinated us since the dawn of time, their rigorous, scientific study is a recent development[1-4] (Supplementary Fig. 1).In The interpretation of dreams [] Freud predicted that "Deeper research will one day trace the path further and discover an organic basis for the mental event."Recent work, which we review in this article, begins to ...
Answer 1 of 2: I'm going to organize in August 207 independent expedition to Kuril Islands (Kunashir and Iturup) with access to the islands and escape with a m/v from Korsakov (Yuzhno-Sakhalinsk). Is it possible?
Yuzhno-Sakhalinsk, also spelled Uzno-Sakhalinsk and previously known in Japanese as Toyohara, is the largest city and capital of Sakhalin Oblast, in the Russian Far East, with a population of around 173,000. Photo: Alkhimov Maxim, CC BY-SA 4.0. Photo: Wikimedia, CC BY-SA 2.0. Photo: Maarten, CC BY 2.0. Ukraine is facing shortages in its brave ...