An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Portland Press Opt2Pay


Mesenchymal stem cells from biology to therapy
David kuntin.
Department of Biology, University of York, York YO10 5DD, U.K.
Paul Genever
Mesenchymal stem cells are as fascinating as they are enigmatic. They appear capable of performing a wide array of functions that cross skeletal biology, immunology and haematology. As therapeutics, mesenchymal stem cells or even just their secreted products may be used to regenerate tissue lost through injury or disease and suppress damaging immune reactions. However, these cells lack unique markers and are hard to identify and isolate as pure cell populations. They are often grown in laboratories using basic and undefined culture conditions. We cannot even agree on their name. While mesenchymal stem cells may lack the developmental understanding and defined differentiation hierarchies of their more illustrious stem cell cousins, they offer a compelling scientific challenge. In depth understanding of mesenchymal stem cell biology will enable us to exploit fully one of the most clinically valuable cell sources.
MSC discovery and biology
In 1966, Friedenstein et al. [ 1 ] demonstrated that cells derived from mouse bone marrow, as well as other blood-forming organs, contain a subpopulation of stem-like cells that give rise to bone cell precursors. In this seminal paper, these cells were named osteogenic stem cells, although with further study, Friedenstein realised their greater potential to differentiate into fat and cartilage precursors, too [ 2 , 3 ]. In 1991, Caplan [ 4 ] coined the term ‘mesenchymal stem cell’ and the abbreviation ‘MSC’, which has since remained the most commonly used moniker. The notion that MSCs have trilineage potential, i.e. the capacity to differentiate into bone, cartilage and fat cells, was developed further in the 1999 report by Pittenger et al. [ 5 ], where bone marrow cells isolated from iliac crest aspirates were shown to differentiate into these lineages in vitro with the addition of differentiation-specific stimuli. Many further studies have since reproduced these methods and built on them.
Expanding research activity and evolution of the field made clear the more complex nature of these cells and that technically limited isolation techniques often failed to select a homogeneous stem cell population. It was thought that the name should reflect this, with proposed MSC expansions including ‘multipotent stromal cells’, ‘mesenchymal stromal cells’ [ 6 ] and even ‘medicinal signalling cells’ [ 7 ]. Some of this nomenclature actually refers to specific subpopulations of cells isolated from tissues by plastic adherence, while others are an attempt at broadening the term. In either case, it can confuse discourse and conflate smaller, more specialised subpopulations, with the overall, heterogeneous cell population. Some authors use the term ‘skeletal stem cell (SSC)’, recognising that a stem cell population exists in adult bone marrow, capable of forming bone, cartilage, fat, and haematopoietic supporting tissue [ 8 , 9 ]. The SSC term also removes reference to embryonic mesenchyme, which implies the capacity to differentiate in all mesenchyme-derived cells and tissues including blood cells. The naming of these cells continues to be debated [ 10 ]. For the remainder of this review, the term ‘mesenchymal stromal cells’ will be used for MSCs, to refer to the broader population of cells, and to acknowledge their heterogeneity and the fact that not all plastic-adherent cells isolated from sources such as bone marrow and fat have multipotent differentiation capability. This view is in line with the International Society for Cellular Therapy (ISCT) position paper first published in 2005 [ 6 ], where MSCs were defined to be plastic-adherent cells, derived from several tissues, such as bone marrow, umbilical cord or fat, with the potential to differentiate into bone, cartilage, and fat cells. They should also express the cell surface proteins CD105, CD73 and CD90, and lack CD45, CD34, CD14 or CD11b, CD79 or CD19 and HLA-DR [ 11 ], though it is important to note that this statement specifies that these are minimal criteria and a starting point for further study. This publication is often referred to in MSC-related literature to assure the reader that the MSCs used in the study in question met the ‘ISCT criteria’, though there are pitfalls with this approach, which we discuss later in this review. The ISCT criteria were later expanded [ 12 ] to recommend the inclusion of tissue source when referring to particular MSC populations used in experimental work, alongside a robust body of evidence clarifying whether stem- like cells or stromal- like cells are being presented, with emphasis on the fact that mesenchymal stem cells represent self-renewing, multipotent cells, while mesenchymal stromal cells describe bulk, unfractionated cells.
While most of this work attempted to define an in vitro expanded MSC population, there has been some progress in identifying the in vivo location of MSCs, or ‘niche’, focusing mainly on murine skeletal tissues. The niche is a specialised tissue microenvironment that houses and regulates the function of an adult stem cell [ 13 , 14 ]. Stem and progenitor cells that give rise to osteogenic and chondrogenic lineages have been identified primarily around blood vessels in bone marrow [ 15–19 ] and more recently, the outer bone surface [ 20 ] and the growth plate of cartilage [ 21–23 ]. Much of the work on bone marrow MSCs has analysed stromal cells as in vivo regulators of the haematopoietic stem cell (HSC) niche [ 18 , 24 ]. Through these and related studies, MSCs have been identified by their production of HSC-niche regulatory factors, such as CXCL12 and stem cell factor (SCF) and the expression of leptin receptor (LEPR), Nestin and CD146, amongst others [ 17 , 19 , 25 , 26 ]. Using in-depth gene profiling techniques, up to 17 different subtypes of related stromal cells have been identified [ 27 ]. A clear picture of the in vivo ‘MSC map’ is still developing, which will be aided by the emergence of advanced spatial profiling techniques; see Dolgalev and Tikhonova [ 28 ] for a recent extensive review. Further studies of MSCs in vivo using human tissues are needed, particularly due to the differences in postnatal mouse and human long bone development [ 29 ]. In situ analyses of MSCs in different tissues will also provide better biological understanding and more appropriate terminology linked to tissue-specific subtypes. Effects of factors such as oxygen tension [ 30 ] and cell–cell interactions will be of particular interest, as this could shed light on the nature of the in vivo MSC environment, which may inform bioengineering approaches to maintaining MSCs ex vivo in as natural a state as possible [ 30 , 31 ].
The issue of MSC identity is complicated further as MSC- like cells have been isolated from a myriad of tissues, though most commonly from bone marrow and adipose tissue from adults. The umbilical cord and placenta are also accessible sources of MSCs, as these are often considered medical waste. To achieve relevant cell numbers, MSCs are usually culture expanded for both research and clinical applications, which is an easily reproducible procedure in the laboratory. Simple MSC isolation and expansion procedures and their clinically appealing regenerative potential underlie the steady increase in the number of publications and clinical trials using MSCs, especially since the year 2000 ( Figure 1 ).

( A ) Number of publications listed on PubMed database (search term: ‘mesenchymal stem cells’ OR ‘mesenchymal stromal cells’) by year from 2000. ( B ) Clinical trials first posted (search term on ClinicalTrials.gov: ‘mesenchymal stem cells’ OR ‘mesenchymal stromal cells’) by year from 2000.
However, it is important to bear in mind that cultured MSCs differ substantially from their native physiological state, due to the vastly different environmental conditions. Culture expansion removes tissue-specific biological cues of the niche, including the presence of different cell types, extracellular matrix (ECM) components and oxygen gradients, and may disguise true in vivo function. The question of whether the therapeutic potential we observe in the laboratory is a product of the process these cells undergo when they are isolated from their tissues and expanded, or in fact reflects their natural function in the organism is a matter of further research. Indeed, if considering the bone marrow niche as an example, which appears varied and complex based on the evidence from single cell and spatial profiling studies described above, tissue culture conditions differ substantially. The presence of animal serum allows for colony formation and expansion of cells, with abundant nutrition and stimulation to remain in culture for extended periods of time. It is, however, understood that within the bone marrow, MSCs maintain their stem-like properties, at least in part, through specific cell–cell interactions. These are comparatively less abundant once the cells are introduced into a culture vessel. The serum contains ECM proteins, such as fibronectin and collagens, which prompt the formation of extensive cytoskeletal networks as cells attach and spread on a rigid, flattened surface. The cells interact with their substrate through integrin-mediated focal adhesions, which is thought to influence their fate [ 32 , 33 ].
Integrin-based interactions are also involved in directing MSC function through substrate stiffness. On stiff surfaces, MSCs were shown to exhibit a tendency for osteogenic differentiation, based on alkaline phosphatase activity, osteogenic gene marker expression, and calcium staining. In addition to increased expression of several integrins, increased activation of downstream signalling events, for example via focal adhesion kinase (FAK), phosphorylated extracellular signalling regulated kinase (pERK), phosphorylated Akt, glycogen synthase kinase 3β (GSK3β), and β-catenin, have been observed, indicating a complex mechanotransduction cascade mediating the effect of substrate stiffness on cell fate [ 34–37 ]. Soft surfaces, on the other hand, have been shown to maintain MSC self-renewal capacity and appear to promote adipogenic differentiation [ 38 , 39 ]. This property of soft hydrogels, thought to be via Yes-associated protein-1 (YAP) signalling, is being investigated as a strategy for maintaining MSC surface marker expression patterns associated with their regenerative properties, which are lost over time in culture [ 40 ]. In general, material stiffnesses mimicking those of certain tissues tends to condition MSCs to adapt to this and induces gene expression patterns consistent with corresponding MSC niches (reviewed in [ 41 ]), explaining to some extent the propensity for certain lineages on particular substrates.
While in vitro analyses may offer only an interpretation of the true biological nature of MSCs, it is clear from this work that MSCs have substantial clinical potential and that there are opportunities to use these cells as therapeutics in a broad range of applications.
MSC therapeutic approaches
Autologous and allogeneic sources of MSCs have been used as cell therapies for many years and form the vast majority of clinical trials identified in Figure 1B . Recently, interest in the use of MSC-derived bioactive products — those secreted by MSCs into the extracellular environment — has increased markedly. We will cover both these approaches under ‘Cell-based therapies’ and ‘Cell-derived therapies’ below (see also Figure 2 ).

MSCs can be applied by direct injection of cell suspensions or seeded onto biomaterial scaffolds as adhesion sites for local administration. MSC-derived EVs can be used in their naïve, unaltered state or engineered to carry specific cargos and/or cell-targeting motifs. Both modalities are applicable in tissue regeneration or immunomodulatory therapies.
Cell-based therapies
Therapeutic approaches exploiting MSC biology focus on their ability to differentiate into new tissues and act as modulators of the immune system. Early work investigating MSCs for their therapeutic utility demonstrated that MSCs have certain immunomodulatory characteristics that allow them to persist in a xenogeneic environment. To demonstrate this, Liechty et al. [ 42 ] introduced human MSCs into sheep foetuses both before and after the foetuses were expected to develop immune-competence. The cells successfully engrafted in both cases and integrated into the developing tissues, undergoing site-specific differentiation. This immunomodulatory capability, coupled with their tissue-forming capacity, provides MSCs with their unique therapeutic value. Clinical targets for MSC therapies include inflammatory indications such as graft versus host disease (GvHD) [ 43 ] and rheumatoid arthritis [ 44 ], as well as for the purposes of tissue regeneration, such as osteogenesis imperfecta [ 45 , 46 ] and large bone defects [ 47 , 48 ]. In our recent analysis of all published clinical trials (2009–2019) using MSCs, we identified 35 different indications, most commonly those affecting the nervous, cardiovascular and musculoskeletal systems [ 49 ].
Many MSC-based interventions rely on MSCs homing to the target site following systemic injection. MSC homing is thought to be cytokine and surface antigen regulated, and refers to the idea that MSCs, when injected systemically into the bloodstream or administered locally, preferentially migrate toward sites of injury [ 50 ]. While systemic administration has its benefits, such as being the least invasive means of delivering MSCs, it has been shown that homing to the desired tissue can be very inefficient [ 51–53 ], resulting in low levels of engraftment, mainly due to entrapment in the lung microvasculature [ 54 ]. Many strategies are being investigated to improve MSC homing, focusing on making patients more receptive to MSCs [ 55 , 56 ] or engineering the MSCs to avoid problems related to patient responses to systemically administered cells [ 57 , 58 ]. It should be noted that in order to translate this approach into viable therapies, scale-up must be addressed. MSC doses are generally in the region of 1–2 million cells/Kg [ 59 ], which poses a particular challenge related to culture-associated loss of specific MSC markers used as critical quality attributes in the manufacture of MSC therapeutics [ 60 ].
MSCs can be administered in a more targeted manner by local administration using scaffolds ( Figure 2 ). To address the issue of low engraftment, a biomaterial scaffold is often used to provide a three-dimensional (3D) structure with a high surface area for cell adhesion, especially when large areas of damaged tissue need to be replaced and/or mechanical strength is required, for example in bone and cartilage replacements, where much of the activity in this area has focused. The aim of this approach is to mimic the tissue microenvironment. Biomimetic scaffolds can range from simply imitating the stiffness or general architecture of the tissue in question, to being doped with specific growth factors and coated with matrix proteins to coax MSCs into a particular lineage. For example, this concept can be applied to critical size bone defects, where a physical structure is required to administer MSCs. Persson and colleagues describe an 80 : 20 mixture of polylactic acid (PLA) and hydroxyapatite (HA), which was used to fabricate a woven scaffold with specific porosity and pore size. These scaffolds were shown to promote MSC proliferation, as well as supporting osteoblastic differentiation and mineralised bone matrix formation in critical size defects [ 61 ]. Scaffolds can also be more complex composites, and even can be personalised, by combining state-of-the-art engineering techniques with current knowledge of MSC biology. An example of this was demonstrated by Kuss et al., where a 3D-printed polycaprolactone (PCL)/HA composite scaffold was constructed, then coated with a complex, cell-laden hydrogel, with the aim of improving vascularisation. The hydrogel contained a mixture of adipose-derived MSCs and human umbilical vein endothelial cells. This essentially prevascularized the construct, demonstrating the possibility of creating an already vascularised scaffold, made to fit unique anatomical structures [ 62 ]. For further information on the use of 3D scaffolds for MSC delivery, tissue regeneration, directing cell function, immunomodulation and genetic modification, please refer to recent reviews [ 63–66 ].
These studies are promising steps toward regenerative solutions to tissue repair by effectively engaging in multidisciplinary research to advance our understanding of how materials integrate into and interact with tissues to achieve optimal regeneration.
Cell-derived therapies
While cell-based therapies are proving encouraging, there has been growing interest in the use of cell-derived material for therapeutic purposes. Bioactive factors produced by cells, extracellular vesicles (EVs) in particular, can reflect the functions of the cell from which they originate. EVs are nanoscale, membranous particles secreted from cells, containing diverse cargo including nucleic acids, such as miRNA, and proteins. It has been shown that EVs mediate cell-to-cell communication by shuttling biomolecules to influence the microenvironment [ 67–70 ]. Given that the function of EVs is to act as signalling particles for surrounding cells, it follows that the signals carried by the EVs could be harnessed to deliver desirable biological factors to target cells, affording them innate therapeutic utility ( Figure 2 ). The EV field has grown hugely in recent years and several recent reviews describe in more detail EV biogenesis, function and clinical possibilities [ 71–73 ].
EVs are also being viewed as delivery vehicles (see Figure 2 ). Engineering EVs to transport specific cargo is an attractive prospect as they carry surface molecules which could aid in targeted delivery [ 74 , 75 ]. EVs can be loaded with proteins, nucleic acids, or small molecules by either modifying the producing cell or by directly loading the EVs, making this a versatile platform for drug delivery [ 76 ].
MSCs seem to be a particularly good source of EVs. Studies have shown that EVs derived from MSCs are more stable than those derived from other cell types [ 77 ], and the capabilities that MSCs exhibit in terms of their differentiation and immunomodulation potential leads to naturally clinically potent EVs. It could also be more efficient to use EVs from MSCs over the MSCs themselves, as EVs are produced constantly, so could be harvested as MSCs are expanded. Cell therapy on the other hand, would generally require cells to be expanded up to the point where they are used in that therapy. EV production can be assisted by MSC immortalisation, which has already been demonstrated by some groups [ 78–80 ], which gives rise to an inexhaustible source of therapeutically useful MSC-EVs, effectively eliminating batch variability; a problem inherent to the use of primary donor cells.
While there are currently no approved treatments available using EVs, there is an increasing body of published data pointing toward the clinical utility of EVs for many indications. The function of EVs in fracture healing, for example, was demonstrated in CD9 knockout mice, which were shown to have impaired EV biogenesis [ 81 ], as well as lowered rates of bone repair, as exhibited by retardation of callous formation [ 82 ], compared with wild type. Furuta and colleagues showed that this retardation was rescued by injection of EVs isolated from the conditioned medium of bone marrow-derived MSCs, but not from EV-free conditioned medium. Work by Qin et al. [ 83 ] further demonstrated that EVs from bone marrow-derived MSCs could enhance bone formation in calvarial defects of Sprague Dawley rats, with miR-196a identified as critical in regulating osteoblastic differentiation and osteogenic gene expression.
There has also been a lot of interest in the use of EVs for the attenuation of the after-effects of COVID-19, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The mechanism(s) of action is yet to be fully understood but revolve around dampening the aggravated inflammatory effects of the respiratory system and repairing tissue damage. It has been suggested in work completed before the onset of this novel coronavirus that inflammation in the lungs could be controlled using MSC-derived EVs by immune cell modulation [ 84 ], the notion of which has been put under more intense investigation as a result of the COVID-19 pandemic, though appropriately controlled trials are required [ 85 ].
Current challenges
It is an accepted truth that cultured cells termed ‘MSCs’ are vastly heterogeneous, as MSCs differ depending on donor/tissue source, isolation/culture technique, and inherent heterogeneity. With the ISCT position statement in 2006, there has been an attempt at harmonisation across groups, which is a positive step forward, but the lack of standardised criteria for the identification and classification of MSC subpopulations presents a substantial obstacle to the development of MSC therapies. More work is required to further our understanding of MSC identity to move the field forward effectively.
A further challenge in the current approach to MSC therapy is the reliance on donor-derived cells for MSC-based therapy scale-up. Whether relying on MSCs themselves to deliver a therapeutic effect, or harvesting MSC-derived factors, MSCs will have to be culture expanded ex vivo to produce clinically usable doses. Using donor-derived cells, which will differ from donor-to-donor, introduces an extra quality control step into production, where there is the potential for many batches to be rejected. Additionally, there is an overwhelming reliance on animal-derived culture additives to produce the quantity of cells required for therapeutic use, which is both ethically and scientifically challenging. The most commonly used additive, foetal bovine serum (FBS), is unsustainably sourced, with the global demand of FBS increasing and the supply struggling to keep up [ 86 , 87 ]. FBS is a complex, undefined mixture, suffering from batch variability. Sources of variability cause major problems in the development of therapeutics, where consistency is key to overcoming regulatory burdens and successfully scaling up production. Xeno-free medium solutions are available, but there is a tendency for life science companies to develop proprietary formulations to protect commercial interests. As far as the research community is concerned, commercially available media are still undefined while being very costly. A chemically defined, non-proprietary medium would aid standardisation across MSC research groups and assist the development and manufacture of MSCs, and their secreted products, for clinical use.
The field of EV therapeutics is an emerging one and we still find ourselves in the early stages of developing and determining a gold standard set of processes by which EVs can be produced, harvested, isolated, and characterised. The problems are similar to those plaguing MSCs currently, as EVs are broadly characterised based on their size and how they were formed, often using marker expression and imaging as readouts. One example of how this problem becomes evident is the fact that the method by which EVs are isolated generally determines the identity of the resulting EV preparation. Currently, the most commonly used isolation technique involves differential ultracentrifugation, which is effective, but fairly crude and time-consuming. To address this, many researchers have developed other isolation techniques and the EV size distribution and yield, quality, and function differs between techniques [ 88 , 89 ].
The International Society for Extracellular Vesicles (ISEV) has published a position statement, similar to the ISCT in 2006, outlining a list of suggested protocols and recommendations on specific criteria to be reported in order to aid in the advancement of the field as a whole with a unified vocabulary. These guidelines also point out that it is an evolving document, and that new technologies are arising regularly, and that the aim is to enhance communication between researchers [ 90 ]. Communication is key.
With regard to EV functionality and their use to address the COVID-19 pandemic, ISCT and ISEV issued a joint statement encouraging investigations into MSC-derived EVs, as well as possibly other cell sources, as treatments for COVID-19, recognising their potential in this area, but stressing that they do not currently endorse their use without sufficient evidence of their safety and efficacy, alongside several more provisions related to clinical studies, manufacture, and regulation [ 85 ]. EVs are a rapidly growing, exciting field of research but careful consideration needs to be given to their mechanisms of action to ensure that these are used in a targeted manner, for maximal efficacy. Our currently limited understanding of factors underlying COVID-19 complications, as well as the complex mechanisms of action of EV interventions are an obstacle to good clinical trial design [ 91 ]. Further work into understanding the very nature of EVs is required to effectively design EV therapeutics.
Conclusions and future directions
MSCs are an exciting cell population. A vast amount of work is attempting to translate MSCs and related technologies into viable therapeutics for an enormous range of applications. In this review, we touched on some of the key target tissues, bone in particular, but the research is being developed in many more areas, including nerve, heart, cartilage, liver, kidney and, as we discussed above, virally induced inflammatory lung disorders. There are new and improved delivery methods in the pipeline, such as hydrogels for cells [ 92 ] and intranasal aerosols for EVs [ 93 ]. The emergence of EVs as a therapeutic modality has opened the doors to cell-free regenerative medicines, with great versatility and utility. That is not to say that cell therapies will be surpassed by EVs, but EVs are a powerful offshoot of traditional cell therapies with the potential to disrupt the regenerative medicine space. It is important to remember that while excitement continues to grow for MSC-based therapies, clinical development must always follow scientific understanding. There is much we still need to do in order to decipher the enigmatic MSC.
- Mesenchymal stem cells are frequently studied for research and clinical use as heterogeneous cell populations, giving rise to the term mesenchymal stromal cells (MSCs).
- MSCs have wide-ranging therapeutic applications but aspects of MSC biology require further work in order to maximise their potential.
- MSC-derived EVs are an emerging therapeutic modality.
- A harmonised approach to defining and analysing MSCs and MSC-EVs is essential for effective communication within the research community to facilitate progression within the field.
Acknowledgements
P.G. is part of the Tissue Engineering and Regenerative Therapies Centre Versus Arthritis (21156). Figure 2 was created with BioRender.com.
Abbreviations
Conflicts of interest.
D.K. and P.G. have no conflicts of interest.
D.K. is funded by the Wellcome Trust (204829) through the Centre for Future Health (CFH) at the University of York.
Open Access Statement
Open access for this article was enabled by the participation of University of York in an all-inclusive Read & Publish pilot with Portland Press and the Biochemical Society under a transformative agreement with JISC.
Author Contributions
D.K. and P.G. wrote and revised the manuscript, and approved the final version.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 06 August 2022
Stem cell-based therapy for human diseases
- Duc M. Hoang ORCID: orcid.org/0000-0001-5444-561X 1 ,
- Phuong T. Pham 2 ,
- Trung Q. Bach 1 ,
- Anh T. L. Ngo 2 ,
- Quyen T. Nguyen 1 ,
- Trang T. K. Phan 1 ,
- Giang H. Nguyen 1 ,
- Phuong T. T. Le 1 ,
- Van T. Hoang 1 ,
- Nicholas R. Forsyth 3 ,
- Michael Heke 4 &
- Liem Thanh Nguyen 1
Signal Transduction and Targeted Therapy volume 7 , Article number: 272 ( 2022 ) Cite this article
72k Accesses
243 Citations
66 Altmetric
Metrics details
- Mesenchymal stem cells
- Stem-cell research
Recent advancements in stem cell technology open a new door for patients suffering from diseases and disorders that have yet to be treated. Stem cell-based therapy, including human pluripotent stem cells (hPSCs) and multipotent mesenchymal stem cells (MSCs), has recently emerged as a key player in regenerative medicine. hPSCs are defined as self-renewable cell types conferring the ability to differentiate into various cellular phenotypes of the human body, including three germ layers. MSCs are multipotent progenitor cells possessing self-renewal ability (limited in vitro) and differentiation potential into mesenchymal lineages, according to the International Society for Cell and Gene Therapy (ISCT). This review provides an update on recent clinical applications using either hPSCs or MSCs derived from bone marrow (BM), adipose tissue (AT), or the umbilical cord (UC) for the treatment of human diseases, including neurological disorders, pulmonary dysfunctions, metabolic/endocrine-related diseases, reproductive disorders, skin burns, and cardiovascular conditions. Moreover, we discuss our own clinical trial experiences on targeted therapies using MSCs in a clinical setting, and we propose and discuss the MSC tissue origin concept and how MSC origin may contribute to the role of MSCs in downstream applications, with the ultimate objective of facilitating translational research in regenerative medicine into clinical applications. The mechanisms discussed here support the proposed hypothesis that BM-MSCs are potentially good candidates for brain and spinal cord injury treatment, AT-MSCs are potentially good candidates for reproductive disorder treatment and skin regeneration, and UC-MSCs are potentially good candidates for pulmonary disease and acute respiratory distress syndrome treatment.
Similar content being viewed by others
Mesenchymal stem cell perspective: cell biology to clinical progress
Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: a next generation therapeutic tool?
Next-generation stem cells — ushering in a new era of cell-based therapies
Introduction.
The successful approval of cancer immunotherapies in the US and mesenchymal stem cell (MSC)-based therapies in Europe have turned the wheel of regenerative medicine to become prominent treatment modalities. 1 , 2 , 3 Cell-based therapy, especially stem cells, provides new hope for patients suffering from incurable diseases where treatment approaches focus on management of the disease not treat it. Stem cell-based therapy is an important branch of regenerative medicine with the ultimate goal of enhancing the body repair machinery via stimulation, modulation, and regulation of the endogenous stem cell population and/or replenishing the cell pool toward tissue homeostasis and regeneration. 4 Since the stem cell definition was introduced with their unique properties of self-renewal and differentiation, they have been subjected to numerous basic research and clinical studies and are defined as potential therapeutic agents. As the main agenda of regenerative medicine is related to tissue regeneration and cellular replacement and to achieve these targets, different types of stem cells have been used, including human pluripotent stem cells (hPSCs), multipotent stem cells and progenitor cells. 5 However, the emergence of private and unproven clinics that claim the effectiveness of stem cell therapy as “magic cells” has raised highly publicized concerns about the safety of stem cell therapy. The most notable case involved the injection of a cell population derived from fractionated lipoaspirate into the eyes of three patients diagnosed with macular degeneration, resulting in the loss of vision for these patients. 6 Thus, as regenerative medicine continues to progress and evolve and to clear the myth of the “magic” cells, this review provides a brief overview of stem cell-based therapy for the treatment of human diseases.
Stem cell therapy is a novel therapeutic approach that utilizes the unique properties of stem cells, including self-renewal and differentiation, to regenerate damaged cells and tissues in the human body or replace these cells with new, healthy and fully functional cells by delivering exogenous cells into a patient. 7 Stem cells for cell-based therapy can be of (1) autologous, also known as self-to-self therapy, an approach using the patient’s own cells, and (2) allogeneic sources, which use cells from a healthy donor for the treatment. 8 The term “stem cell” were first used by the eminent German biologist Ernst Haeckel to describe the properties of fertilized egg to give rise to all cells of the organism in 1868. 9 The history of stem cell therapy started in 1888, when the definition of stem cell was first coined by two German zoologists Theodor Heinrich Boveri and Valentin Haecker, 9 who set out to identify the distinct cell population in the embryo capable of differentiating to more specialized cells (Fig. 1a ). In 1902, studies carried out by the histologist Franz Ernst Christian Neumann, who was working on bone marrow research, and Alexander Alexandrowitsch Maximov demonstrated the presence of common progenitor cells that give rise to mature blood cells, a process also known as haematopoiesis. 10 From this study, Maximov proposed the concept of polyblasts, which later were named stem cells based on their proliferation and differentiation by Ernst Haeckel. 11 Maximov described a hematopoietic population presented in the bone marrow. In 1939, the first case report described the transplantation of human bone marrow for a patient diagnosed with aplastic anemia. Twenty years later, in 1958, the first stem cell transplantation was performed by the French oncologist George Mathe to treat six nuclear researchers who were accidentally exposed to radioactive substances using bone marrow transplantation. 12 Another study by George Mathe in 1963 shed light on the scientific community, as he successfully conducted bone marrow transplantation in a patient with leukemia. The first allogeneic hematopoietic stem cell transplantation (HSCT) was pioneered by Dr. E. Donnall Thomas in 1957. 13 In this initial study, all six patients died, and only two patients showed evidence of transient engraftment due to the unknown quantities and potential hazards of bone marrow transplantation at that time. In 1969, Dr. E. Donnall Thomas conducted the first bone marrow transplantation in the US, although the success of the allogeneic treatment remained exclusive. In 1972, the year marked the discovery of cyclosporine (the immune suppressive drug), 14 the first successes of allogeneic transplantation for aplastic anemia and acute myeloid leukemia were reported in a 16-year-old girl. 15 From the 1960s to the 1970s, series of works conducted by Friendenstein and coworkers on bone marrow aspirates demonstrated the relationship between osteogenic differentiation and a minor subpopulation of cells derived from bone marrow. 16 These cells were later proven to be distinguishable from the hematopoietic population and to be able to proliferate rapidly as adherent cells in tissue culture vessels. Another important breakthrough from Friendenstein’s team was the discovery that these cells could form the colony-forming unit when bone marrow was seeded as suspension culture following by differentiation into osteoblasts, adipocytes, and chondrocytes, suggesting that these cells confer the ability to proliferate and differentiate into different cell types. 17 In 1991, combined with the discovery of human embryonic stem cells (hESCs), which will be discussed in the next section, the term “mesenchymal stem cells”, previously known as stromal stem cells or “osteogenic” stem cells, was first coined in Caplan and widely used to date. 18 Starting with bone marrow transplantation 60 years ago, the journey of stem cell therapy has developed throughout the years to become a novel therapeutic agent of regenerative medicine to treat numerous incurable diseases, which will be reviewed and discussed in this review, including neurological disorders, pulmonary dysfunctions, metabolic/endocrine-related diseases, reproductive disorders, skin burns, and cardiovascular conditions).
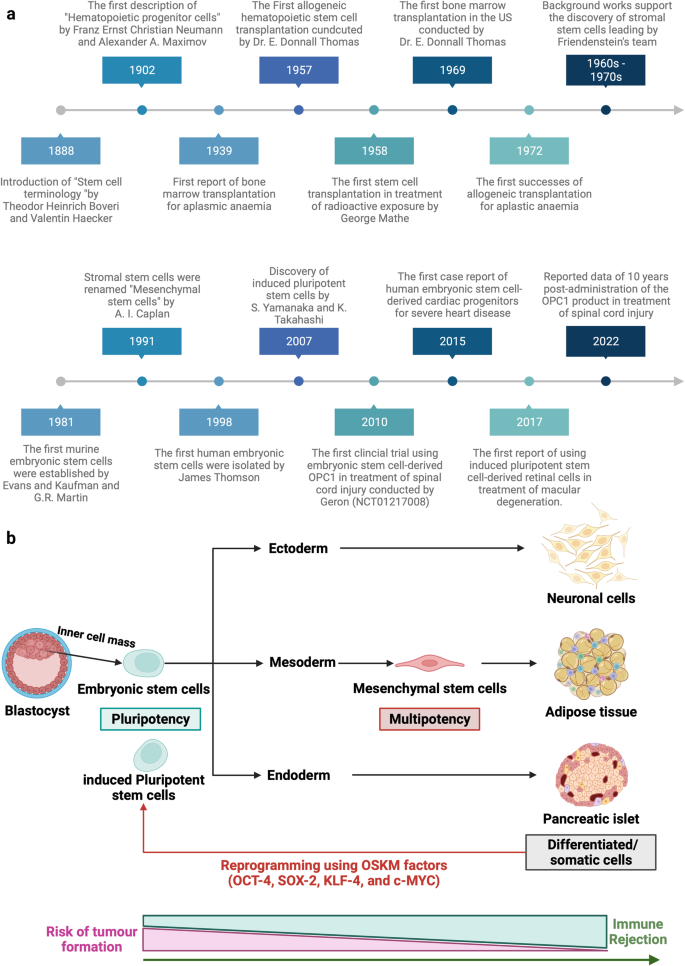
Stem cell-based therapy: the history and cell source. a The timeline of major discoveries and advances in basic research and clinical applications of stem cell-based therapy. The term “stem cells” was first described in 1888, setting the first milestone in regenerative medicine. The hematopoietic progenitor cells were first discovered in 1902. In 1939, the first bone marrow transplantation was conducted in the treatment of aplasmic anemia. Since then, the translation of basic research to preclinical studies to clinical trials has driven the development of stem cell-based therapy by many discoveries and milestones. The isolations of “mesenchymal stem cells” in 1991 following by the discovery of human pluripotent stem cells have recently contributed to the progress of stem cell-based therapy in the treatment of human diseases. b Schematic of the different cell sources that can be used in stem cell-based therapy. (1) Human pluripotent stem cells, including embryonic stem cells (derived from inner cell mass of blastocyst) and induced pluripotent stem cells confer the ability to proliferate indefinitely in vitro and differentiate into numerous cell types of the human body, including three germ layers. (2) Mesenchymal stem cells are multipotent stem cells derived from mesoderm possessing self-renewal ability (limited in vitro) and differentiation potential into mesenchymal lineages. The differentiated/somatic cells can be reprogrammed back to the pluripotent stage using OSKM factors to generate induced pluripotent stem cells. It is important to note that stem cells show a relatively higher risk of tumor formation and lower risk of immune rejection (in the case of mesenchymal stem cells) when compared to that of somatic cells. The figure was created with BioRender.com
In this review, we described the different types of stem cell-based therapies (Fig. 1b ), including hPSCs and MSCs, and provided an overview of their definition, history, and outstanding clinical applications. In addition, we further created the first literature portfolio for the “targeted therapy” of MSCs based on their origin, delineating their different tissue origins and downstream applications with an in-depth discussion of their mechanism of action. Finally, we provide our perspective on why the tissue origin of MSCs could contribute greatly to their downstream applications as a proposed hypothesis that needs to be proven or disproven in the future to further enhance the safety and effectiveness of stem cell-based therapy.
Stem cell-based therapy: an overview of current clinical applications
Cardiovascular diseases.
The clinical applications of stem cell-based therapies for heart diseases have been recently discussed comprehensively in the reviews 19 , 20 and therefore will be elaborated in this study as the focus discussions related to hPSCs and MSCs in the following sections. In general, the safety profiles of stem cell-based therapies are supported by a large body of preclinical and clinical studies, especially adult stem cell therapy (such as MSC-based products). However, clinical trials have not yet yielded data supporting the efficacy of the treatment, as numerous studies have shown paradoxical results and no statistically significant differences in infarct size, cardiac function, or clinical outcomes, even in phase III trials. 21 The results of a meta-analysis showed that stem cells derived from different sources did not exhibit any therapeutic effects on the improvement of myocardial contractility, cardiovascular remodeling, or clinical outcomes. 22 The disappointing results obtained from the clinical trials thus far could be explained by the fact that the administered cells may exert their therapeutic effects via an immune modulation rather than regenerative function. Thus, well-designed, randomized and placebo-controlled phase III trials with appropriate cell-preparation methods, patient selection, follow-up schedules and suitable clinical measurements need to be conducted to determine the efficacy of the treatments. In addition, concerns related to optimum cell source and dose, delivery route and timing of administration, cell distribution post administration and the mechanism of action also need to be addressed. In the following section of this review, we present clinical trials related to MSC-based therapy in cardiovascular disease with the aim of discussing the contradictory results of these trials and analyzing the potential challenges underlying the current approaches.
Digestive system diseases
Gastrointestinal diseases are among the most diagnosed conditions in the developed world, altering the life of one-third of individuals in Western countries. The gastrointestinal tract is protected from adverse substances in the gut environment by a single layer of epithelial cells that are known to have great regenerative ability in response to injuries and normal cell turnover. 23 These epithelial cells have a rapid turnover rate of every 2–7 days under normal conditions and even more rapidly following tissue damage and inflammation. This rapid proliferation ability is possible owing to the presence of a specific stem cell population that is strictly compartmentalized in the intestinal crypts. 24 The gastrointestinal tract is highly vulnerable to damage, tissue inflammation and diseases once the degradation of the mucosal lining layer occurs. The exposure of intestinal stem cells to the surrounding environment of the gut might result in the direct destruction of the stem cell layer or disruption of intestinal functions and lead to overt clinical symptoms. 25 In addition, the accumulation of stem cell defects as well as the presence of chronic inflammation and stress also contributes to the reduction of intestinal stem cell quality.
In terms of digestive disorders, Crohn’s disease (CD) and ulcerative colitis are the two major forms of inflammatory bowel disease (IBD) and represent a significant burden on the healthcare system. The former is a chronic, uncontrolled inflammatory condition of the intestinal mucosa characterized by segmental transmural mucosal inflammation and granulomatous changes. 26 The latter is a chronic inflammatory bowel disease affecting the colon and rectum, characterized by mucosal inflammation initiating in the rectum and extending proximal to the colon in a continuous fashion. 27 Cellular therapy in the treatment of CD can be divided into haematopoietic stem cell-based therapy and MSC-based therapy. The indication and recommendation of using HSCs for the treatment of IBD were proposed in 1995 by an international committee with four important criteria: (1) refractory to immunosuppressive treatment; (2) persistence of the disease conditions indicated via endoscopy, colonoscopy or magnetic resonance enterography; (3) patients who underwent an imminent surgical procedure with a high risk of short bowel syndromes or refractory colonic disease; and (4) patients who refused to treat persistent perianal lesions using coloproctectomy with a definitive stroma implant. 28 In the standard operation procedure, patents’ HSCs were recruited using cyclophosphamide, which is associated with granulocyte colony-stimulating factor (G-CSF), at two different administered concentrations (4 g/m 2 and 2 g/m 2 ). Recently, it was reported that high doses of cyclophosphamide do not improve the number of recruited HSCs but increase the risk of cardiac and bladder toxicity. An interest in using HSCTs in CD originated from case reports that autologous HSCTs can induce sustained disease remission in some 29 , 30 but not all patients 31 , 32 , 33 with CD. The first phase I trial was conducted in Chicago and recruited 12 patients with active moderate to severe CD refractory to conventional therapies. Eleven of 12 patients demonstrated sustained remission after a median follow-up of 18.5 months, and one patient developed recurrence of active CD. 31 The ASTIC trial (the Autologous Stem Cell Transplantation International Crohn Disease) was the first randomized clinical trial with the largest cohort of patients undergoing HSCT for refractory CD in 2015. 34 The early report of the trial showed no statistically significant improvement in clinical outcomes of mobilization and autologous HSCT compared with mobilization followed by conventional therapy. In addition, the procedure was associated with significant toxicity, leading to the suggestion that HSCT for patients with refractory CD should not be widespread. Interestingly, by using conventional assessments for clinical trials for CD, a group reassessed the outcomes of patients enrolled in the ASTIC trial showing clinical and endoscopic benefits, although a high number of adverse events were also detected. 35 A recent systematic review evaluated 18 human studies including 360 patients diagnosed with CD and showed that eleven studies confirmed the improvement of Crohn’s disease activity index between HSCT groups compared to the control group. 36 Towards the cell sources, HSCs are the better sources as they afforded more stable outcomes when compared to that of MSC-based therapy. 37 Moreover, autologous stem cells were better than their allogeneic counterparts. 36 The safety of stem cell-based therapy in the treatment of CD has attracted our attention, as the risk of infection in patients with CD was relatively higher than that in those undergoing administration to treat cancer or other diseases. During the stem cell mobilization process, patient immunity is significantly compromised, leading to a high risk of infection, and requires carefully nursed and suitable antibiotic treatment to reduce the development of adverse events. Taken together, stem cell-based therapy for digestive disease reduced inflammation and improved the patient’s quality of life as well as bowel functions, although the high risk of adverse events needs to be carefully monitored to further improve patient safety and treatment outcomes.
Liver diseases
The liver is the largest vital organ in the human body and performs essential biological functions, including detoxification of the organism, metabolism, supporting digestion, vitamin storage, and other functions. 38 The disruption of liver homeostasis and function might lead to the development of pathological conditions such as liver failure, cirrhosis, cancer, alcoholic liver disease, nonalcoholic fatty liver disease (NAFLD), and autoimmune liver disease (ALD). Orthotropic liver transplantation is the only effective treatment for severe liver diseases, but the number of available and suitable donor organs is very limited. Currently, stem cell-based therapies in the treatment of liver disease are associated with HSCs, MSCs, hPSCs, and liver progenitor cells.
Liver failure is a critical condition characterized by severe liver dysfunctions or decompensation caused by numerous factors with a relatively high mortality rate. Stem cell-based therapy is a novel alternative approach in the treatment of liver failure, as it is believed to participate in the enhancement of liver regeneration and recovery. The results of a meta-analysis including four randomized controlled trials and six nonrandomized controlled trials in the treatment of acute-on-chronic liver failure (ACLF) demonstrated that clinical outcomes of stem cell therapy were achieved in the short term, requiring multiple doses of stem cells to prolong the therapeutic effects. 39 , 40 Interestingly, although MSC-based therapies improved liver functions, including the model of end-stage liver disease score, albumin level, total bilirubin, and coagulation, beneficial effects on survival rate and aminotransferase level were not observed. 41 A randomized controlled trial illustrated the improvement of liver functions and reduction of severe infections in patients with hepatitis B virus-related ACLF receiving allogeneic bone marrow-derived MSCs (BM-MSCs) via peripheral infusion. 42 HSCs from peripheral blood after the G-CSF mobilization process were used in a phase I clinical trial and exhibited an improvement in serum bilirubin and albumin in patients with chronic liver failure without any specific adverse events related to the administration. 43 Taken together, an overview of stem cell-based therapy in the treatment of liver failure indicates the potential therapeutic effects on liver functions with a strong safety profile, although larger randomized controlled trials are still needed to assure the conclusions.
Liver cirrhosis is one of the major causes of morbidity and mortality worldwide and is characterized by diffuse nodular regeneration with dense fibrotic septa and subsequent parenchymal extinction leading to the collapse of liver vascular structure. 44 In fact, liver cirrhosis is considered the end-stage of liver disease that eventually leads to death unless liver transplantation is performed. Stem cell-based therapy, especially MSCs, currently emerges as a potential treatment with encouraging results for treating liver cirrhosis. In a clinical trial using umbilical cord-derived MSCs (UC-MSCs), 45 chronic hepatitis B patients with decompensated liver cirrhosis were divided into two groups: the MSC group ( n = 30) and the control group ( n = 15). 45 The results showed a significant reduction in ascites volume in the MSC group compared with the control. Liver function was also significantly improved in the MSC groups, as indicated by the increase in serum albumin concentration, reduction in total serum bilirubin levels, and decrease in the sodium model for end-stage liver disease score. 45 Similar results were also reported from a phase II trial using BM-MSCs in 25 patients with HCV-induced liver cirrhosis. 46 Consistent with these studies, three other clinical trials targeting liver cirrhosis caused by hepatitis B and alcoholic cirrhosis were conducted and confirmed that MSC administration enhanced and recovered liver functions. 47 , 48 , 49 With the large cohort study as the clinical trial conducted by Fang, the safety and potential therapeutic effects of MSC-based therapies could be further strengthened and confirmed the feasibility of the treatment in virus-related liver cirrhosis. 49 In terms of delivery route, a randomized controlled phase 2 trial suggested that systemic delivery of BM-MSCs does not show therapeutic effects on patients with liver cirrhosis. 50 MSCs are not the only cell source for liver cirrhosis. Recently, an open-label clinical trial conducted in 19 children with liver cirrhosis due to biliary atresia after the Kasai operation illustrated the safety and feasibility of the approach by showing the improvement of liver function after bone marrow mononuclear cell (BMNC) administration assessed by biochemical tests and pediatric end-stage liver disease (PELD) scores. 51 Another study using BMNCs in 32 decompensated liver cirrhosis patients illustrated the safety and effectiveness of BMNC administration in comparison with the control group. 52 Recently, a long-term analysis of patients receiving peripheral blood-derived stem cells indicated a significant improvement in the long-term survival rate when compared to the control group, and the risk of hepatocellular carcinoma formation did not increase. 53 CD133 + HSC infusion was performed in a multicentre, open, randomized controlled phase 2 trial in patients with liver cirrhosis; the results did not support the improvement of liver conditions, and cirrhosis persisted. 54 Notably, these results are in line with a previous randomized controlled study, which also reported that G-CSF and bone marrow-derived stem cells delivered via the hepatic artery did not introduce therapeutic potential as expected. 55 Thus, stem cell-based therapy for liver cirrhosis is still in its immature stage and requires larger trials with well-designed experiments to confirm the efficacy of the treatment.
Nonalcoholic fatty liver disease (NAFLD) is the most common medical condition caused by genetic and lifestyle factors and results in a severe liver condition and increased cardiovascular risk. 56 NAFLD is the hidden enemy, as most patients are asymptomatic for a long time, and their routine life is unaffected. Thus, the detection, identification, and management of NAFLD conditions are challenging tasks, as patients diagnosed with NAFLD often develop nonalcoholic steatohepatitis, cirrhosis, and hepatocellular carcinoma. 57 Although preclinical studies have shown that stem cell administration could enhance liver function in NAFLD models, a limited number of clinical trials were performed in human subjects. Recently, a multi-institutional clinical trial using freshly isolated autologous adipose tissue-derived regenerative cells was performed in Japan to treat seven NAFLD patients. 58 The results illustrated the improvement in the serum albumin level of six patients and prothrombin activity of five patients, and no treatment-related adverse events or severe adverse events were observed. This study illustrates the therapeutic potential of stem cell-based therapy in the treatment of NAFLD.
Autoimmune liver disease (ALD) is a severe liver condition affecting children and adults worldwide, with a female predominance. 59 The condition occurs in genetically predisposed patients when a stimulator, such as virus infection, leads to a T-cell-mediated autoimmune response directed against liver autoantigens. As a result, patients with ALD might develop liver cirrhosis, hepatocellular carcinoma, and, in severe cases, death. To date, HSCT and bone marrow transplantation are the two common stem cell-based therapies exhibiting therapeutic potential for ALD in clinical trials. An interesting report illustrated that haploidentical HSCTs could cure ALD in patients with sickle cells. 60 This report is particularly important, as it illustrates the potential therapeutic approach of using haploidentical HSCTs to treat patients with both sickle cells and ALD. Another case report described a 19-year-old man with a 4-year history of ALD who developed acute lymphoblastic leukemia and required allogeneic bone marrow transplantation from this wholesome brother. 61 The clinical data showed that immunosuppressive therapy for transplantation generated ALD remission in the patient. 62 However, the data also provided valid information related to the sustained remission and the normalization of ASGPR-specific suppressor-inducer T-cell activity following bone marrow transplantation, suggesting that these suppressor functions originated from donor T cells. 61 Thus, it was suggested that if standard immunosuppressive treatment fails, alternative cellular immunotherapy would be a viable option for patients with ALD. Primary biliary cholangitis (PBC), usually known as primary biliary cirrhosis, is a type of ALD characterized by a slow, progressive destruction of small bile ducts of the liver leading to the formation of cirrhosis and accumulation of bile and other toxins in the liver. A pilot, single-arm trial from China recruited seven patents with PBC who had a suboptimal response to ursodeoxycholic acid (UDCA) treatment. 63 These patients received UDCA treatment in combination with three rounds of allogeneic UC-MSCs at 4-week intervals with a dose of 0.5 × 10 6 cells/kg of patient body weight via the peripheral vein. No treatment-related adverse events or severe adverse events were observed throughout the course of the study. The clinical data indicated significant improvement in liver function, including reduction of serum ALP and gamma-glutamyltransferase (GGT) at 48 weeks post administration. The common symptoms of PBC, including fatigue, pruritus, and hypogastric ascites volume, were also reduced, supporting the feasibility of MSC-based therapy in the treatment of PBC, although major limitations of the study were nonrandomized, no control group and small sample size. Another study was conducted in China with ten PBC patients who underwent incompetent UDCA treatment for more than 1 year. These patients received a range of 3–5 allogeneic BM-MSCs/kg body weight by intravenous infusion. 64 Although these early studies have several limitations, such as small sample size, nonrandomization, and no control group, their preliminary data related to safety and efficacy herald the prospects and support the feasibility of stem cell-based therapy in the treatment of ALD.
In summary, the current number of trials for liver disease using stem cell-based therapy has provided fundamental data supporting the safety and potential therapeutic effects in various liver diseases. Unfortunately, due to the small number of trials, several obstacles need to be overcome to prove the effectiveness of the treatments, including (1) stem cell source and dose, (2) administration route, (3) time of intervention, and (4) clinical assessments during the follow-up period. Only by addressing these challenges we will be able to prove, facilitate and promote stem cell-based therapy as a mainstream treatment for liver diseases.
Arthritis is a general term describing cartilage conditions that cause pain and inflammation of the joints. Osteoarthritis (OA) is the most common form of arthritis caused by persistent degeneration and poor recovery of articular cartilage. 65 OA affects one or several diarthrodial joints, such as small joints at the hand and large joints at the knee and hips, leading to severe pain and subsequent reduction in the mobility of patients. There are two types of OA: (1) primary OA or idiopathic OA and secondary OA caused by causative factors such as trauma, surgery, and abnormal joint development at birth. 66 As conventional treatments for OA are not consistent in their effectiveness and might cause unbearable pain as well as long-term rehabilitation (in the case of joint replacement), there is a need for a more reliable, less painful, and curative therapy targeting the root of OA. 67 Thus, stem cell therapy has recently emerged as an alternative approach for OA and has drawn great attention in the regenerative field.
The administration of HSCs has been proven to reduce bone lesions, enhance bone regeneration and stimulate the vascularization process in degenerative cartilage. Attempts were made to evaluate the efficacy of peripheral blood stem cells in ten OA patients by three intraarticular injections. Post-administration analysis indicated a reduction in the WOMAC index with a significant reduction in all parameters. All patients completed 6-min walk tests with an increase of more than 54 meters. MRI analysis indicated an improvement in cartilage thickness, suggesting that cartilage degeneration was reduced post administration. To further enhance the therapeutic potential of HSCT, CD34 + stem cells were proposed to be used in combination with the rehabilitation algorithm, which included three stages: preoperative, hospitalization and outpatient periods. 68 Currently, a large wave of studies has been directed to MSC-based therapy for the treatment of OA due to their immunoregulatory functions and anti-inflammatory characteristics. MSCs have been used as the main cell source in several multiple and small-scale trials, proving their safety profile and potential effectiveness in alleviating pain, reducing cartilage degeneration, and enhancing the regeneration of cartilage structure and morphology in some cases. However, the best source of MSCs, whether from bone marrow, adipose tissue, or umbilical cord, for the management of OA is still a great question to be answered. A systematic review investigating over sixty-one of 3172 articles with approximately 2390 OA patients supported the positive effects of MSC-based therapy on OA patients, although the study also pointed out the fact that these therapeutic potentials were based on limited high-quality evidence and long-term follow-up. 69 Moreover, the study found no obvious evidence supporting the most effective source of MSCs for treating OA. Another systematic review covering 36 clinical trials, of which 14 studies were randomized trials, provides an interesting view in terms of the efficacy of autologous MSC-based therapy in the treatment of OA. 70 In terms of BM-MSCs, 14 clinical trials reported the clinical outcomes at the 1-year follow-up, in which 57% of trials reported clinical outcomes that were significantly better in comparison with the control group. However, strength analysis of the data set showed that outcomes from six trials were low, whereas the outcomes of the remaining eight trials were extremely low. Moreover, the positive evidence obtained from MRI analysis was low to very low strength of evidence after 1-year post administration. 70 Similar results were also found in the outcome analysis of autologous adipose tissue-derived MSCs (AT-MSCs). Thus, the review indicated low quality of evidence for the therapeutic potential of MSC therapy on clinical outcomes and MRI analysis. The low quality of clinical outcomes could be explained by the differences in interventions (including cell sources, cell doses, and administration routes), combination treatments (with hyaluronic acid, 71 peripheral blood plasma, 72 etc.), control treatments and clinical outcome measurements between randomized clinical trials. 73 In addition, the data of the systematic analysis could not prove the better source of MSCs for OA treatment. Taken together, although stem cell-based therapy has been shown to be safe and feasible in the management of OA, the authors support the notion that stem cell-based therapy could be considered an alternative treatment for OA when first-line treatments, such as education, exercise, and body weight management, have failed.
Cancer treatment
Stem cell therapy in the treatment of cancer is a sensitive term and needs to be used and discussed with caution. Clinicians and researchers should protect patients with cancer from expensive and potentially dangerous or ineffective stem cell-based therapy and patients without a cancer diagnosis from the risk of malignancy development. In general, unproven stem cell clinics employed three cell-based therapies for cancer management, including autologous HSCTs, stromal vascular fraction (SVF), and multipotent stem cells, such as MSCs. Allogeneic HSCTs confer the ability to generate donor lymphocytes that contribute to the suppression and regression of hematological malignancies and select solid tumors, a specific condition known as “graft-versus-tumor effects”. 74 However, stem cell clinics provide allogeneic cell-based therapy for the treatment of solid malignancies despite limited scientific evidence supporting the safety and efficacy of the treatment. High-quality evidence from the Cochrane library shows that marrow transplantation via autologous HSCTs in combination with high-dose chemotherapy does not improve the overall survival of women with metastatic breast cancer. In addition, a study including more than 41,000 breast cancer patients demonstrated no significant difference in survival benefits between patients who received HSCTs following high-dose chemotherapy and patients who underwent conventional treatment. 75 Thus, the use of autologous T-cell transplants as monotherapy and advertising stem cell-based therapies as if they are medically approved or preferred treatment of solid tumors is considered untrue statements and needs to be alerted to cancer patients. 76
Over the past decades, many preclinical studies have demonstrated the potential of MSC-based therapy in cancer treatment due to their unique properties. They confer the ability to migrate toward damaged sites via inherent tropism controlled by growth factors, chemokines, and cytokines. MSCs express specific C–X–C chemokine receptor type 4 (CXCR4) and other chemokine receptors (including CCR1, CCR2, CCR4, CCR7, etc.) that are essential to respond to the surrounding signals. 77 In addition, specific adherent proteins, including CD49d, CD44, CD54, CD102, and CD106, are also expressed on the MSC surface, allowing them to attach, rotate, migrate, and penetrate the blood vessel lumen to infiltrate the damaged tissue. 78 Similar to damaged tissues, tumors secrete a wide range of chemoattractant that also attract MSC migration via the CXCL12/CXCR4 axis. Previous studies also found that MSC migration toward the cancer site is tightly controlled by diffusible cytokines such as interleukin 8 (IL-8) and growth factors including transforming growth factor-beta 1 (TGF-β1), 79 platelet-derived growth factor (PDGF), 80 fibroblast growth factor 2 (FGF-2), 81 vascular endothelial growth factor (VEGF), 81 and extracellular matrix molecules such as matrix metalloproteinase-2 (MMP-2). 82 Once MSCs migrate successfully to cancerous tissue, accumulating evidence demonstrates the interaction between MSCs and cancer cells to exhibit their protumour and antitumour effects, which are the major concerns of MSC-based therapy. MSCs are well-known for their regenerative effects that regulate tissue repair and recovery. This unique ability is also attributed to the protumour functions of these cells. A previous study reported that breast cancer cells induce MSC secretion of chemokine (C–C motif) ligand 5 (CCL-5), which regulates the tumor invasion process. 83 , 84 Other studies also found that MSCs secrete a wide range of growth factors (VEGF, basic FGF, HGF, PDGF, etc.) that inhibits apoptosis of cancer cells. 85 Moreover, MSCs also respond to signals released from cancer cells, such as TGF-β, 86 to transform into cancer-associated fibroblasts, a specific cell type residing within the tumor microenvironment capable of promoting tumorigenesis. 87 Although MSCs have been proven to be involved in protumour activities, they also have potent tumor suppression abilities that have been used to develop cancer treatments. It has been suggested that MSCs exhibit their tumor inhibitory effects by inhibiting the Wnt and AKT signaling pathways, 88 reducing the angiogenesis process, 89 stimulating inflammatory cell infiltration, 90 and inducing tumor cell cycle arrest and apoptosis. 91 To date, the exact functions of MSCs in both protumour and antitumor activities are still a controversial issue across the stem cell field. Other approaches exploit gene editing and tissue engineering to convert MSCs into “a Trojan horse” that could exhibit antitumor functions. In addition, MSCs can also be modified to express specific anticancer miRNAs exhibiting tumor-suppressive behaviors. 92 However, genetically modified MSCs are still underdeveloped and require intensive investigation in the clinical setting.
To date, ~25 clinical trials have been registered on ClinicalTrials.gov aimed at using MSCs as a therapeutic treatment for cancer. 93 These trials are mostly phase 1 and 2 studies focusing on evaluating the safety and efficacy of the treatment. Studies exploiting MSC-based therapy have combined MSCs with an oncolytic virus approach. Oncolytic viruses are specific types of viruses that can be genetically engineered or naturally present, conferring the ability to selectively infect cancer cells and kill them without damaging the surrounding healthy cells. 94 A completed phase I/II study using BM-MSCs infected with the oncolytic adenovirus ICOVIR5 in the treatment of metastatic and refractory solid tumors in children and adult patients demonstrated the safety of the treatment and provided preliminary data supporting their therapeutic potential. 95 The same group also reported a complete disappearance of all signs of cancer in response to MSC-based therapy in one pediatric case three years post administration. 96 A reported study in 2019 claimed that adipose-derived MSCs infected with vaccinia virus have the potential to eradicate resistant tumor cells via the combination of potent virus amplification and senitization of the tumor cells to virus infection. 97 However, in a recently published review, a valid question was posed regarding the 2019 study that “do these reported data merit inclusion in the publication record when they were collected by such groups using a dubious therapeutic that was eventually confiscated by US Marshals?” 76
Taken together, cancer research and therapy have entered an innovative and fascinating era with advancements in traditional therapies such as chemotherapy, radiotherapy, and surgery on one hand and stem cell-based therapy on the other hand. Although stem cell-based therapy has been considered a novel and attractive therapeutic approach for cancer treatment, it has been hampered by contradictory results describing the protumour and antitumour effects in preclinical studies. Despite this contradictory reality, the use of stem cell-based therapy, especially MSCs, offers new hope to cancer patients by providing a new and more effective tool in personalized medicine. The authors support the use of MSC-based therapy as a Trojan horse to deliver specific anticancer functions toward cancer cells to suppress their proliferation, eradicate cancer cells, or limit the vascularization process of cancerous tissue to improve the clinical safety and efficacy of the treatment.
Human pluripotent stem cell-based therapy: a growing giant
The discovery of hPSCs, including human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs), has revolutionized stem cell research and cell-based therapy. 98 hESCs were first isolated from blastocyst-stage embryos in 1998, 99 followed by breakthrough reprogramming research that converted somatic cells into hiPSCs using just four genetic factors. 100 , 101 Methods have been developed to maintain these cells long-term in vitro and initiate their differentiation into a wide variety of cell types, opening a new era in regenerative medicine, particularly cell therapy to replace lost or damaged tissues.
History of hPSCs
hPSCs are defined as self-renewable cell types that confer the ability to differentiate into various cellular phenotypes of the human body, including three germ layers. 102 Historically, the first pluripotent cell lines to be generated were embryonic carcinoma (EC) cell lines established from human germ cell tumors 103 and murine undifferentiated compartments. 104 Although EC cells are a powerful tool in vitro, these cells are not suitable for clinical applications due to their cancer-derived origin and aneuploidy genotype. 105 The first murine ESCs were established in 1981 based on the culture techniques obtained from EC research. 106 Murine ESCs are derived from the inner cell mass (ICM) of the pre-implantation blastocyst, a unique biological structure that contains outer trophoblast layers that give rise to the placenta and ICM. 107 In vivo ESCs only exist for a short period during the embryo’s development, and they can be isolated and maintained indefinitely in vitro in an undifferentiated state. The discovery of murine ESCs has dramatically changed the field of biomedical research and regenerative medicine over the last 40 years. Since then, enormous investigations have been made to isolate and culture ESCs from other species, including hESCs, in 1998. 99 The success of Thomson et al. in 1998 triggered the great controversy in media and ethical research boards across the globe, with particularly strong objections being raised to the use of human embryos for research purposes. 108 Several studies using hESCs have been conducted demonstrating their therapeutic potential in the clinical setting. However, the use of hESCs is limited due to (1) the ethical barrier related to the destruction of human embryos and (2) the potential risk of immunological rejection, as hESCs are isolated from pre-implantation blastocysts, which are not autologous in origin. To overcome these two great obstacles, several research groups have been trying to develop technology to generate hESCs, including nuclear transfer technology, the well-known strategy that creates Dolly sheep, although the generation of human nuclear transfer ESCs remains technically challenging. 109 Taking a different approach, in 2006, Yamanaka and Takahashi generated artificial PSCs from adult and embryonic mouse somatic cells using four transcription factors ( Oct-3/4 , Sox2 , Klf4 , and c-Myc , called OSKM) reduced from 24 factors. 100 Thereafter, in 2007, Takahashi and colleagues successfully generated the first hiPSCs exhibiting molecular and biological features similar to those of hESCs using the same OSKM factors. 101 Since then, hiPSCs have been widely studied to expand our knowledge of the pathogenesis of numerous diseases and aid in developing new cell-based therapies as well as personalized medicine.
Clinical applications of hPSCs
Since its beginning 24 years ago, hPSC research has evolved momentously toward applications in regenerative medicine, disease modeling, drug screening and discovery, and stem cell-based therapy. In clinical trial settings, the uses of hESCs are restricted by ethical concerns and tight regulation, and the limited preclinical data support their therapeutic potential. However, it is important to acknowledge several successful outcomes of hESC-based therapies in treating human diseases. In 2012, Steven Schwartz and his team reported the first clinical evidence of using hESC-derived retinal pigment epithelium (RPE) in the treatment of Stargardt’s macular dystrophy, the most common pediatric macular degeneration, and an individual with dry age-related macular degeneration. 110 , 111 With a differentiation efficiency of RPE greater than 99%, 5 × 10 4 RPEs were injected into the subretinal space of one eye in each patient. As the hESC source of RPE differentiation was exposed to mouse embryonic stem cells, it was considered a xenotransplantation product and required a lower dose of immunosuppression treatment. This study showed that hESCs improved the vision of patients by differentiating into functional RPE without any severe adverse events. The trial was then expanded into two open-label, phase I/II studies with the published results in 2015 supporting the primary findings. 112 In these trials, patients were divided into three groups receiving three different doses of hESC-derived RPE, including 10 × 10 4 , 15 × 10 4 and 50 × 10 4 RPE cells per eye. After 22 months of follow-up, 19 patients showed improvement in eyesight, seven patients exhibited no improvement, and one patient experienced a further loss of eyesight. The technical challenge of hESC-derived RPE engraftment was an unbalanced proliferation of RPE post administration, which was observed in 72% of treated patients. A similar approach was also conducted in two South Korean patients diagnosed with age-induced macular degeneration and two patients with Stargardt macular dystrophy. 113 The results supported the safety of hESC-derived RPE cells and illustrated an improvement in visual acuity in three patients. Recently, clinical-graded hESC-derived RPE cells were also developed by Chinese researchers under xeno-free culture conditions to treat patients with wet age-related degeneration. 114 As hESC development is still associated with ethical concerns and immunological complications related to allogeneic administration, hiPSC-derived RPE cells have emerged as a potential cell source for macular degeneration. Although RPE differentiation protocols have been developed and optimized to improve the efficacy of hiPSC-derived RPE cells, they are still insufficient, time-consuming and labor intensive. 115 , 116 For clinical application, an efficient differentiation of “primed” to “naïve” state hiPSCs toward the RPE was developed using feeder-free culture conditions utilizing the transient inhibition of the FGF/MAPK signaling pathway. 117 Overexpression of specific transcription factors in hiPSCs throughout the differentiation process is also an interesting approach to generate a large number of RPE cells for clinical use. In a recent study, overexpression of three eye-field transcription factors, including OTX2, PAX6, and MITF , stimulated RPE differentiation in hiPSCs and generated functional RPE cells suitable for transplantation. 118 To date, although reported data from phase I/II clinical trials have been produced enough to support the safety of hESC-derived RPE cells, the treatment is still in its immature stage. Thus, future studies should focus on the development of the cellular manufacturing process of RPE and the subretinal administration route to further improve the outcomes of RPE fabrication and engraftment into the patient’s retina (recommended review 119 ).
Numerous studies have demonstrated that hESC-derived cardiomyocytes exhibit cardiac transcription factors and display a cardiomyocyte phenotype and immature electrical phenotype. In addition, using hPSC-derived cardiomyocytes could provide a large number of cells required for true remuscularization and transplantation. Thus, these cells can be a promising novel therapeutic approach for the treatment of human cardiovascular diseases. In a case report, hESC-derived cardiomyocytes showed potential therapeutic effects in patients with severe heart failure without any subsequent complications. 120 This study was a phase I trial (ESCORT [Transplantation of Human Embryonic Stem Cell-derived Progenitors in Severe Heart Failure] trial) to evaluate the safety of cardiomyocyte progenitor cells derived from hESCs seeded in fibrin gel scaffolds for 10 patients with severe heart failure (NCT02057900). The encouraging results from this study demonstrated the feasibility of producing hESC-derived cardiomyocyte progenitor cells toward clinical-grade standards and combining them with a tissue-engineered scaffold to treat severe heart disease (the first patient of this trial has already reached the 7-year follow-up in October 2021). 121 Currently, the two ongoing clinical trials using hPSC-derived cardiomyocytes have drawn great attention, as their results would pave the way to lift the bar for approving therapies for commercial use. The first trial was conducted by a team led by cardiac surgeon Yoshiki Sawa at Osaka University using hiPSC-derived cardiomyocytes embedded in a cell sheet for engraftment (jRCT2052190081). The trials started first with three patients followed by ten patients to assess the safety of the approach. Once safety is met, the treatment can be sold commercially under Japan’s fast-track system for regenerative medicine. 122 Another trial used a collagen-based construct called BioVAT-HF to contain hiPSC-derived cardiomyocytes. The trial was divided into two parts to evaluate the cell dose: (Part A) recruiting 18 patients and (Part B) recruiting 35 patients to test a broad range of engineered human myocardium (EHM) doses. The expected results from this study will provide the “proof-of-concept” for the use of EHM in the stimulation of heart remuscularization in humans. To date, no adverse events or severe adverse events have been reported from these trials, supporting the safety of the procedure. However, as the number of treated patients was relatively small, limitations in drawing conclusions regarding efficacy are not yet possible. 21 , 123
One of the first clinical trials using hPSC-based therapy was conducted by Geron Corporation in 2010 using hESC-derived oligodendrocyte progenitor cells (OPC1) to treat spinal cord injury (SCI). The results confirmed the safety one year post administration in five participants, and magnetic resonance imaging demonstrated improvement of spinal cord deterioration in four participants. 124 Asterias Biotherapeutic (AST) continued the Geron study by conducting the SCiStar Phase I/IIa study to evaluate the therapeutic effects of AST-OPC1 (NCT02302157). The trial’s results published in clinicaltrials.gov demonstrated significant improvement in running speed, forelimb stride length, forelimb longitudinal deviations, and rear stride frequency. Interestingly, the recently published data of a phase 1, multicentre, nonrandomized, single-group assignment, interventional trial illustrated no evidence of neurological decline, enlarging masses, further spinal cord damage, or syrinx formation in patients 10 years post administration of the OPC1 product. 125 This data set provides solid evidence supporting the safety of OPC1 with an event-free period of up to 10 years, which strengthens the safety profile of the SCiStar trial.
Analysis of the global trends in clinical trials using hPSC-based therapy showed that 77.1% of studies were observational (no cells were administered into patient), and only 22.9% of studies used hPSC-derived cells as interventional treatment. 126 The number of studies using hiPSCs was relatively higher than that using hESCs, which was 74.8% compared to 25.2%, respectively. The majority of observational studies were performed in developed countries, including the USA (41.6%) and France (16.8%), whereas interventional studies were conducted in Asian countries, including China (36.7%), Japan (13.3%), and South Korea (10%). The trends in therapeutic studies were also clear in terms of targeted diseases. The three most studied diseases were ophthalmological conditions, circulatory disorders, and nervous systems. 127 However, it is surprising that the clinical applications of hPSCs have achieved little progress since the first hESCs were discovered worldwide. The relatively low number of clinical trials focusing on using iPSCs as therapeutic agents to administer into patients could be ascribed to the unstable genome of hiPSCs, 128 immunological rejection, 129 and the potential for tumor formation. 130
Mesenchymal stem/stromal cell-based therapy: is it time to consider their origin toward targeted therapy?
Approximately 55 years ago, fibroblast-like, plastic-adherent cells, later named mesenchymal stem cells (MSCs) by Arnold L. Caplan, 18 were discovered for the first time in mouse bone marrow (BM) and were later demonstrated to be able to form colony-like structures, proliferate, and differentiate into bone/reticular tissue, cartilage, and fat. 131 Protocols were subsequently established to directly culture this subpopulation of stromal cells from BM in vitro and to stimulate their differentiation into adipocytes, chondroblasts, and osteoblasts. 132 Since then, MSCs have been found in and derived from different human tissue sources, including adipose tissue (AT), the umbilical cord (UC), UC blood, the placenta, dental pulp, amniotic fluid, etc. 133 To standardize and define MSCs, the International Society for Cell and Gene Therapy (ISCT) set minimal identification criteria for MSCs derived from multiple tissue sources. 134 Among them, MSCs derived from AT, BM, and UC are the most commonly studied MSCs in human clinical trials, 135 and they constitute the three major tissue sources of MSCs that will be discussed in this review.
The discovery of MSCs opened an era during which preclinical studies and clinical trials have been performed to assess the safety and efficacy of MSCs in the treatment of various diseases. The major conclusion of these studies and trials is that MSC-based therapy is safe, although the outcomes have usually been either neutral or at best marginally positive in terms of the clinically relevant endpoints regardless of MSC tissue origin, route of infusion, dose, administration duration, and preconditioning. 136 It is important to note that a solid background of knowledge has been generated from all these studies that has fueled the recent translational research in MSC-based therapy. As MSCs have been intensively studied over the last 55 years and have become the subject of multiple reviews, systematic reviews, and meta-analyses, the objective of this paper is not to duplicate these publications. Rather, we will discuss the questions that both clinicians and researchers are currently exploring with regard to MSC-based therapy, diligently seeking answers to the following:
“With a solid body of data supporting their safety profiles derived from both preclinical and clinical studies, does the tissue origin of MSCs also play a role in their downstream clinical applications in the treatment of different human diseases?”
“Do MSCs derived from AT, BM, and UC exhibit similar efficacy in the treatment of neurological diseases, metabolic/endocrine-related disorders, reproductive dysfunction, skin burns, lung fibrosis, pulmonary disease, and cardiovascular conditions?”
To answer these questions, we will first focus on the most recently published clinical data regarding these targeted conditions, including neurological disorders, pulmonary dysfunctions, metabolic/endocrine-related diseases, reproductive disorders, skin burns, and heart-related diseases, to analyze the potential efficacy of MSCs derived from AT, BM, and UC. Based on the level of clinical improvement observed in each trial, we analyzed the potential efficacy of MSCs derived from each source to visualize the correlation between patient improvement and MSC sources. We will then address recent trends in the exclusive use of MSC-based products, focusing on the efficacy of treatment with MSCs from each of the abovementioned sources, and we will analyze the relationship between the respective efficacies of MSCs from these sources in relation to the targeted disease conditions. Finally, we propose a hypothesis and mechanism to achieve the currently still unmet objective of evaluating the use of MSCs from AT, BM, and UC in regenerative medicine.
An overview of MSC tissue origins and therapeutic potential
In general, MSCs are reported to be isolated from numerous tissue types, but all of these types can be organized into two major sources: adult 137 and perinatal sources 138 (Fig. 2 ). Adult sources of MSCs are defined as tissues that can be harvested or obtained from an individual, such as dental pulp, 139 BM, peripheral blood, 140 AT, 141 lungs, 142 hair, 143 or the heart. 144 Adult MSCs usually reside in specialized structures called stem cell niches, which provide the microenvironment, growth factors, cell-to-cell contacts and external signals necessary for maintaining stemness and differentiation ability. 145 BM was the first adult source of MSCs discovered by Friedenstein 131 and has become one of the most documented and largely used MSC sources to date, followed by AT. BM-MSCs are isolated and cultured in vitro from BM aspirates using a Ficoll gradient-centrifugation method 146 or a red blood cell lysate buffer to collect BM mononuclear cell populations, whereas AT-MSCs are obtained from stromal vascular fractions of enzymatically digested AT obtained through liposuction, 141 lipoplasty, or lipectomy procedures. 147 These tissue collection procedures are invasive and painful for the patient and are accompanied by a risk of infection, although BM aspiration and adipose liposuction are considered safe procedures for BM and AT biopsies. The number of MSCs that can be isolated from these adult tissues varies significantly in a tissue-dependent manner. The percentage of MSCs in BM mononuclear cells ranges from 0.001 to 0.01% following gradient centrifugation. 132 The number of MSCs in AT is at least 500 times higher than that in BM, with approximately 5,000 MSCs per 1 g of AT. Perinatal sources of MSCs consist of UC-derived components, such as UC, Wharton’s jelly, and UC blood, and placental structures, such as the placental membrane, amnion, chorion membrane, and amniotic fluid. 138 The collection of perinatal MSCs, such as UC-MSCs, is noninvasive, as the placenta, UC, UC blood, and amnion are considered waste products that are usually discarded after birth (with no ethical barriers). 148 Although MSCs represent only 10 −7 % the cells found in UC, their higher proliferation rate and rapid population doubling time allow these cells to rapidly replicate and increase in number during in vitro culture. 149 Under standardized xeno-free and serum-free culture platforms, AT-MSCs show a faster proliferation rate and a higher number of colony-forming units than BM-MSCs. 149 UC-MSCs have the fastest population doubling time compared to AT-MSCs and BM-MSCs in both conventional culture conditions and xeno- and serum-free environments. 149 MSCs extracted from AT, BM and UC exhibit all minimal criteria listed by the ISCT, including morphology (plastic adherence and spindle shape), MSC surface markers (95% positive for CD73, CD90 and CD105; less than 2% negative for CD11, CD13, CD19, CD34, CD45, and HLR-DR) and differentiation ability into chondrocytes, osteocytes, and adipocytes. 150
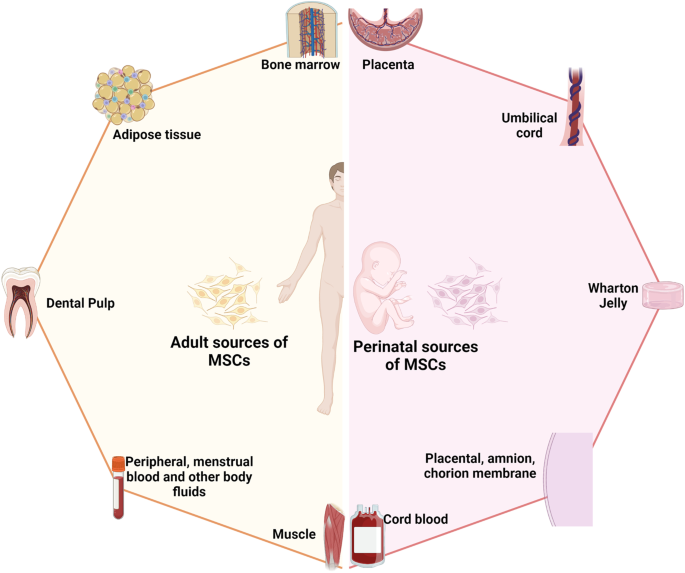
The two major sources of MSCs: adult and perinatal sources. The adult sources of MSCs are specific tissue in human body where MSCs could be isolated, including bone marrow, adipose tissue, dental pulp, peripheral blood, menstrual blood, muscle, etc. The perinatal sources of MSCs consist of umbilical cord-derived components, such as umbilical cord, Wharton’s jelly, umbilical cord blood, and placental structures, such as placental membrane, amnion, chorion membrane, amniotic fluid, etc. The figure was created with BioRender.com
In fact, although MSCs derived from either adult or perinatal sources exhibit similar morphology and the basic characteristics of MSCs, studies have demonstrated that these cells also differ from each other. Regarding immunophenotyping, AT-MSCs express high levels of CD49d and low levels of Stro-1. An analysis of the expression of CD49d and CD106 showed that the former is strongly expressed in AT-MSCs, in contrast to BM-MSCs, whereas CD106 is expressed in BM-MSCs but not in AT-MSCs. 151 Increased expression of CD133, which is associated with stem cell regeneration, differentiation, and metabolic functions, 152 was observed in BM-MSCs compared to MSCs from other sources. 153 A recent study showed that CD146 expression in UC-MSCs was higher than that in AT- and BM-MSCs, 153 supporting the observation that UC-MSCs have a stronger attachment and a higher proliferation rate than MSCs from other sources, as CD146 is a key cell adhesion protein in vascular and endothelial cell types. 154 In terms of differentiation ability, donor-matched BM-MSCs exhibit a higher ability to differentiate into chondrogenic and osteogenic cell types than AT-MSCs, whereas AT-MSCs show a stronger capacity toward the adipogenic lineage. 150 The findings from an in vitro differentiation study indicated that BM-MSCs are prone to osteogenic differentiation, whereas AT-MSCs possess stronger adipogenic differentiation ability, which can be explained by the fact that the epigenetic memory obtained from either BM or AT drives the favored MSC differentiation along an osteoblastic or adipocytic lineage. 155 Interestingly, although UC-MSCs have the ability to differentiate into adipocytes, osteocytes, or chondrocytes, their osteogenic differentiation ability has been proven to be stronger than that of BM-MSCs. 156 The most interesting characteristic of MSCs is their immunoregulatory functions, which are speculated to be related to either cell-to-cell contact or growth factor and cytokine secretion in response to environmental/microenvironmental stimuli. MSCs from different sources almost completely inhibit the proliferation of peripheral blood mononuclear cells (PBMCs) at PBMC:MSC ratios of 1:1 and 10:1. 149 At a higher ratio, BM-MSCs showed a significantly higher inhibitory effect than AT- or UC-MSCs. 153 Direct analysis of the immunosuppressive effects of BM- and UC-MSCs has revealed that these cells exert similar inhibitory effects in vitro with different mechanisms involved. 157 With these conflicting data, the mechanism of action related to the immune response of MSCs from different sources is still poorly understood, and long-term investigations both in preclinical studies and in clinical trial settings are needed to shed light on this complex immunomodulation function.
The great concern in MSC-based therapy is the fate of these cells post administration, especially through different delivery routes, including systemic administration via an intravenous (IV) route or tissue-specific administration, such as dorsal pancreatic administration. It is important to understand the distribution of these cells after injection to expand our understanding of the underlying mechanisms of action of treatments; in addition, this knowledge is required by authorized bodies (the Food and Drug Administration (FDA) in the United States or the regulation of advanced-therapy medicinal products in Europe, No. 1394/2007) prior to using these cells in clinical trials. The preclinical data using various labeling techniques provide important information demonstrating that MSCs do not have unwanted homing that could lead to the incorrect differentiation of the cells or inappropriate tumor formation. In a mouse model, human BM-MSCs and AT-MSCs delivered via an IV route are rapidly trapped in the lungs and then recirculate through the body after the first embolization process, with a small number of infused cells found mainly in the liver after the second embolization. 158 Using the technetium-99 m labeling method, intravenously infused human cells showed long-term persistence up to 13 months in the bone, BM compartment, spleen, muscle, and cartilage. 159 A similar result was reported in baboons, confirming the long-term homing of human MSCs in various tissues post administration. 160 Although the retainment of MSCs in the lungs might potentially reduce their systemic therapeutic effects, 161 it provides a strong advantage when these cells are used in the treatment of respiratory diseases. Local injection of MSCs also revealed their tissue-specific homing, as an injection of MSCs via the renal artery route resulted in the majority of the injected cells being found in the renal cortex. 162 Numerous studies have been conducted to track the migration of administered MSCs in human subjects. Henriksson and his team used MSCs labeled with iron sucrose in the treatment of intervertebral disc degeneration. 163 Their study showed that chondrocytes differentiated from infused MSCs could be detected at the injured intervertebral discs at 8 months but not at 28 months. A study conducted in a patient with hemophilia A using In-oxine-labeled MSCs showed that the majority of the cells were trapped in the lungs and liver 1 h post administration, followed by a reduction in the lungs and an increase in the number of cells in the liver after 6 days. 164 Interestingly, a small proportion of infused MSCs were found in the hemarthrosis site at the right ankle after 24 h, suggesting that MSCs are attracted and migrate to the injured site. The distribution of MSCs was also reported in the treatment of 21 patients diagnosed with type 2 diabetes using 18-FDG-tagged MSCs and visualized using positron emission tomography (PET). 165 The results illustrated that local delivery of MSCs via an intraarterial route is more effective than delivery via an IV route, as MSCs home to the pancreatic head (pancreaticoduodenal artery) or body (splenic artery). Therefore, although the available data related to the biodistribution of infused MSCs are still limited, the results obtained from both preclinical and clinical studies illustrate a comparable set of data supporting results on homing, migration to the injured site, and the major organs where infused MSCs are located. The following comprehensive and interesting reviews are highly recommended. 166 , 167 , 168
To date, 1426 registered clinical trials spanning different trial phases have used MSCs for therapeutic purposes, which is four times the number reported in 2013. 169 , 170 As supported by a large body of preclinical studies and advancements in conducting clinical trials, MSCs have been proven to be effective in the treatment of numerous diseases, including nervous system and brain disorders, pulmonary diseases, 171 cardiovascular conditions, 172 wound healing, etc. The outcomes of MSC-based therapy have been the subject of many intensive reviews and systematic analyses with the solid conclusion that these cells exhibit strong safety profiles and positive outcomes in most tested conditions. 173 , 174 , 175 In addition, the available data have revealed several potential mechanisms that could explain the beneficial effects of MSCs, including their homing efficiency, differentiation potential, production of trophic factors (including cytokines, chemokines, and growth factors), and immunomodulatory abilities. However, it is still not known which MSC types should be used for which diseases, as it seems to be that MSCs exhibit beneficial effects regardless of their sources. 169
Acquired brain and spinal cord injury treatment: BM-MSCs have emerged as key players
The theory that brain cells can never regenerate has been challenged by the discovery of newly formed neurons in the human adult hippocampus or the migration of stem cells in the brain in animal models. 176 These observations have triggered hope for regeneration in the context of neuronal diseases by using exogenous stem cell sources to replenish or boost the stem cell population in the brain. Moreover, the limited regenerative capacity of the brain and spinal cord is an obstacle for traditional treatments of neurodegenerative diseases, such as autism, cerebral palsy, stroke, and spinal cord injury (SCI). As current treatments cannot halt the progression of these diseases, studies throughout the world have sought to exploit cell-based therapies to treat neurodegenerative diseases on the basis of advances in the understanding and development of stem cell technology, including the use of MSCs. Successful stem cell therapy for treating brain disease requires therapeutic cells to reach the injured sites, where they can repair, replace, or at least prevent the deteriorative effects of neuronal damage. 177 Hence, the gold standard of cell-based therapy is to deliver the cells to the target site, stimulate the tissue repair machinery, and regulate immunological responses via either cell-to-cell contact or paracrine effects. 178 Among 315 registered clinical trials using stem cells for the treatment of brain diseases, MSCs and hematopoietic stem cells (HSCs; CD34+ cells isolated from either BM aspirate or UC blood) are the two main cell types investigated, whereas approximately 102 clinical trials used MSCs and 62 trials used HSCs for the treatment of brain disease (main search data from clinicaltrial.gov). MSCs are widely used in almost all clinical trials targeting different neuronal diseases, including multiple sclerosis, 179 stroke, 180 SCI, 181 cerebral palsy, 182 hypoxic-ischemic encephalopathy, 183 autism, 184 Parkinson’s disease, 185 Alzheimer’s disease 185 and ataxia. Among these trials in which MSCs were the major cells used, nearly two-thirds were for stroke, SCI, or multiple sclerosis. MSCs have been widely used in 29 registered clinical trials for stroke, with BM-MSCs being used in 16 of these trials. With 26 registered clinical trials, SCI is the second most common indication for using MSCs, with 16 of these trials using mainly expanded BM-MSCs. For multiple sclerosis, 15 trials employed BM-MSCs among a total of 23 trials conducted for the treatment of this disease. Hence, it is important to note that in neuronal diseases and disorders, BM-MSCs have emerged as the most commonly used therapeutic cells among other MSCs, such as AT-MSCs and UC-MSCs.
The outcomes of the use of BM-MSCs in the treatment of neuronal diseases have been widely reported in various clinical trial types. A review by Zheng et al. indicated that although the treatments appeared to be safe in patients diagnosed with stroke, there is a need for well-designed phase II multicentre studies to confirm the outcomes. 173 One of the earliest trials using autologous BM-MSCs was conducted by Bang et al. in five patients diagnosed with stroke in 2005. The results supported the safety and showed an improved Barthel index (BI) in MSC-treated patients. 186 In a 2-year follow-up clinical trial, 16 patients with stroke received BM-MSC infusions, and the results showed that the treatment was safe and improved clinical outcomes, such as motor impairment scale scores. 187 A study conducted in 12 patients with ischemic stroke showed that autologous BM-MSCs expanded in vitro using autologous serum improved the patient’s modified Rankin Scale (mRS), with a mean lesion volume reduced by 20% at 1 week post cell infusion. 188 In 2011, a modest increase in the Fugl Meyer and modified BI scores was observed after autologous administration of BM-MSCs in patients with chronic stroke. 189 More recently, a prospective, open-label, randomized controlled trial with blinded outcome evaluation was conducted, with 39 patients and 15 patients in the BM-MSC administration and control groups, respectively. The results of this study indicated that autologous BM-MSCs with autologous serum administration were safe, but the treatment led to no improvements at 3 months in modified Rankin Scale (mRS) scores, although leg motor improvement was observed. 180 Researchers explored whether the efficacy of BM-MSC administration was maintained over time in a 5-year follow-up clinical trial. Patients (85) were randomly assigned to either the MSC group or the control group, and follow-ups on safety and efficacy were performed for 5 years, with 52 patients being examined at the end of the study. The MSC group exhibited a significant improvement in terms of decreased mRS scores, whereas the number of patients with an mRS score increase of 0–3 was statistically significant. 187 Although autologous BM-MSCs did not improve the Basel index, mRS, or National Institutes of Health Stroke Scale (NIHSS) score 2 years post infusion, patients who received BM-MSC therapy showed improvement in their motor function score. 190 In addition, a prospective, open-label, randomized controlled trial by Lee et al. showed that autologous BM-MSCs primed with autologous “ischemic” serum significantly improved motor functions in the MSC-treated group. Neuroimaging analysis also illustrated a significant increase in interhemispheric connectivity and ipsilesional connectivity in the MSC group. 191 Recently, a single intravenous infection of allogeneic BM-MSCs has been proven to be safe and feasible in patients with chronic stroke with a significant improvement in BI score and NIHSS score. 192
In two systematic reviews using MSCs for the treatment of SCI, BM-MSCs ( n = 16) and UC-MSCs ( n = 5) were reported to be safe and well-tolerated. 193 , 194 The results indicated significant improvements in the stem cell administration groups compared with the control groups in terms of a composite of the American Spinal Injury Association (ASIA) impairment scale (AIS) grade, AIS grade A, and ASIA sensory scores and bladder function (Table 1 ). However, larger experimental groups with a randomized and multicentre design are needed for further confirmation of these findings. For multiple sclerosis, several early-phase (phase I/II) registered clinical studies have used BM-MSCs. A study compared the potential efficacy of BM-MSC and BM mononuclear cell (BMMNC) transplantation in 105 patients with spastic cerebral palsy. 195 The results showed that the GMFM (gross motor function measure) and the FMFM (fine motor function measure) scores of the BM-MSC transplant group were higher than those of the BMNNC transplant group at 3, 6, and 12 months of assessment. In terms of autism spectrum disorder, a review of 254 children after BMMNC transplantation found that over 90% of patients’ ISAA (Indian Scale for Assessment of Autism) and CARS (Childhood Autism Rating Scale) scores improved. Young patients and those in whom autism spectrum disorder was detected early generally showed better improvement. 196
One of the biggest limitations when using BM-MSCs is the bone marrow aspiration process, as it is an invasive procedure that can introduce a risk of complications, especially in pediatric and elderly patients. 197 Therefore, UC-MSCs have been suggested as an alternative to BM-MSCs and are being studied in clinical trials for the treatment of neurological diseases in approximately 1550 patients throughout the world; however, only three studies have been completed, with data published recently. 198 A recent study showed that UC-MSC administration improved both gross motor function and cognitive skills, assessed using the Activities of Daily Living (ADL), Comprehensive Function Assessment (CFA), and GMFM, in patients diagnosed with cerebral palsy. The improvements peaked 6 months post administration and lasted for 12 months after the first transplantation. 199 In a single-targeted phase I/II clinical trial using UC-MSCs for the treatment of autism, Riordan et al. reported decreases in Autism Treatment Evaluation Checklist (ATEC) and CARS scores for eight patients, but the paper has been retracted due to a violation of the journal’s guidelines. 200 In an open-label, phase I study, UC-MSCs were used as the main cells to treat 12 patients with autism spectrum disorder via IV infusions. It is important to note that five participants developed new class I anti-human leukocyte antigen in response to the specific lot of manufactured UC-MSCs, although these responses did not exhibit any immunological response or clinical manifestations. Only 50% of participants showed improvements in at least two autism-specific measurements. 201 Although not as widely used as BM-MSCs, these trials have demonstrated the efficacy of using UC-MSCs in the treatment of SCIs. In a pilot clinical study, Yang et al. showed that the use of UC-MSCs has the potential to improve disease status through an increase in total ASIA and SCI Functional Rating Scale of the International Association of Neurorestoratology (IANR-SCIFRS) scores, as well as an improvement in pinprick, light touch, motor and sphincter scores. 202 A study of 22 patients with SCIs showed a potential therapeutic effect in 13 patients post UC-MSC infusion. 203 AT-MSCs were also used to treat SCI, with a single case report indicating an improvement in neurological and motor functions in a domestic ferret patient. 204 However, a result obtained from another phase I trial using AT-MSCs showed mild improvements in neurological function in a small number of patients. 205 A phase II, randomized, double-blind, placebo-controlled, single-center, pilot clinical trial using AT-MSCs in the treatment of acute ischemic stroke published a data set that supports the safety of the therapy, although patients who received AT-MSCs showed a nonsignificant improvement after 24 months of follow-up. 206 In all of the above studies, the safety of using either AT-MSCs or UC-MSCs was evaluated, and no significant reactions were reported after infusion.
Therefore, based on the number of recovered patients post-transplantation and the number of recruited patients in large-scale trials using BM-MSCs, it seems that BM-MSCs are the prominent cells in regard to treating neurodegenerative disease with potentially good outcomes (Table 1 ). It is important to note that we do not negate the fact that AT- and UC-MSCs also show positive outcomes in the treatment of neuronal diseases, with numerous ongoing large-scale, multicentre, randomized, and placebo-control trials, 207 , 208 but we suggest alternative and thoughtful decisions regarding which sources of MSCs are best for the treatment of neuronal diseases and degenerative disorders.
Respiratory disease and lung fibrosis: clinical data support UC as a good source of MSCs
In the last decade, significant increases in respiratory disease incidence due to air pollution, smoking behavior, population aging, and recently, respiratory virus infections such as coronavirus disease 2019 (COVID-19) 209 have been observed, leading to substantial burdens on public health and healthcare systems worldwide. Respiratory inflammatory diseases, including bronchopulmonary dysplasia (BPD), chronic obstructive pulmonary disease (COPD), and acute respiratory distress syndrome (ARDS), have recently emerged as three prevalent pulmonary diseases in children and adults. These conditions are usually associated with inflammatory cell infiltration, a disruption of alveolar structural integrity, a reduction in alveolar fluid clearance ability, cytokine release and associated cytokine storms, airway remodeling, and the development of pulmonary fibrosis. Traditional treatments are focused on relieving symptoms and preventing disease progression using surfactants, artificial respiratory support, mechanical ventilation, and antibiotic/anti-inflammatory drugs, with limited effects on the damaged airway, alveolar fluid clearance, and other detrimental effects caused by the inflammatory response. MSCs are known for their immunomodulatory abilities, showing potential in injury reduction and aiding lung recovery after injury. According to ClinicalTrials.gov, from 2017 to date, there have been 159 studies testing the application of MSCs in the treatment of pulmonary diseases, including but not limited to BPD, COPD, and ARDS, suggesting a trend in the use of MSCs as an alternative approach for the treatment of respiratory diseases, especially MSCs from UC as an “off-the-shelf” and allogeneic source.
Extremely premature infants are born with arrested lung development at the canalicular-saccular phases prior to alveolarization and before pulmonary maturation occurs, which results in the development of BPD. 210 These infants require intensive care during the first three months of life using postnatal interventions, including positive pressure mechanical ventilation, external oxygen support, and surfactant infusions, and the newborns have recurrent infections that further compromise normal lung development. 211 To date, 13 clinical trials have been proposed to use UC-MSCs in the treatment of BPD, recruiting ~566 premature infants throughout the world, including Vietnam, Korea, the United States, Spain, Australia, and China. The majority of these trials use UC-derived stem cells for phases I and II, focusing on evaluating the safety and efficacy of stem cell-based therapy. 212 Human UC tissue and its derivative components are considered the most attractive cell sources for MSCs in the treatment of BPD due to the ease of obtaining them, being readily available, with no ethical concerns, low antigenicity, a high cell proliferation rate, and superior regenerative potential. Chang et al. used MSCs derived from UC blood in a phase I dose-escalation clinical trial to treat 9 preterm infants via intratracheal administration to prevent the development of BPD. 213 All 9 preterm infants survived, and only three developed BPD; these infants had significantly decreased BPD severity compared with the historically matched control group. A follow-up study of the same patients after 24 months indicated that only one infant had an E. cloacae infection after discharge at 4 months, with subsequent disseminated intravascular coagulation, which was later proven to be unrelated to the intervention. The remaining eight patients survived with normal pulmonary development and function, suggesting that the therapy was safe. MSCs from UC blood were also used for the treatment of 12 extremely low birthweight preterm patients using the same administration route, which further confirmed the safety of the therapy in the treatment of BPD, although ten of 12 infants still developed severe BPD at 36 weeks. 214 Our group also reported the safety and potential efficacy of using UC-MSCs in the treatment of four preterm infants, and the results supported the safety of UC-MSCs and demonstrated that patients could be weaned from oxygen supply and develop normal lung structure and function. 215 A phase II clinical trial of 66 infants born at 23–28 weeks with a birthweight of 500–1250 g who were recruited and randomized into an MSC-administration group and a control group was conducted. Although the results supported the safety of MSC administration in preterm infants, the efficacy of the treatment was not supported by statistical analysis, potentially due to the small sample size. Subgroup analysis showed that patients with severe BPD born at 23–24 weeks showed a significant improvement in BPD severity, but those born at 25–28 weeks did not. 216 Hence, it is important to conduct controlled phase II clinical trials with larger cohort sizes to further substantiate the efficacy of UC blood-derived MSCs in the treatment of infants with BPD.
With more than 65 million patients worldwide, COPD was the third-leading cause of death in 2020, according to World Health Organization records. COPD is classified as a chronic inflammatory and destructive pulmonary disease characterized by a progressive reduction in lung function. Averyanov et al. performed a randomized, placebo-controlled phase I/IIa study in 20 patients with mild to moderate idiopathic pulmonary fibrosis (IPF). Treatment group patients received two IV doses of allogeneic MSCs (2 × 10 8 cells) every 3 months, and the second group received a placebo. 217 Evaluation tests were performed at weeks 13, 26, 39, and 52. The 6-min walking test distance (6MWTD) results showed that patient fitness improved from week 13 onwards and was maintained until up to the 52nd week. Pulmonary function indicators improved markedly before and after treatment in the treated group but did not change significantly in the placebo group. The goal of MSC therapy in the treatment of COPD is to promote the regeneration of parenchymal cells and alveolar structure and the restoration of lung function. Based on the results of a phase I trial of a commercial BM-MSC product, Prochymal TM , which led to improvements in pulmonary function in treated patients, a multicentre, double-blind, placebo-controlled phase II trial was conducted in 62 patients diagnosed with COPD to determine the safety and potential efficacy of the product. Although the results supported the safety of BM-MSCs, their effectiveness in the treatment of COPD was not assured. No statistically significant differences in FEV 1 or FEV 1% , total lung capacity, or carbon monoxide diffusing capacity were detected after 2 years of follow-up between the two treatment groups. To date, there have been five clinical trials using BM-MSCs as the main stem cells for the treatment of COPD, but the overall clinical outcomes did not demonstrate the potential therapeutic effects of the treatment. 218 , 219 , 220 , 221 , 222 In clinical trial NCT001110252, the results showed that there was an overall reduction in the process of COPD pathological development 3 years after the administration of BM-MSCs, although the trial had a phase I design, with no control group, and evaluated only a small cohort (four patients). 219 To alleviate local inflammatory progression in COPD, Oliveira et al. studied the combination treatment of one-way endobronchial valve (EBV) and BM-MSC intubation. 223 Ten GOLD (Global Initiative for Obstructive Lung Disease) stage C or D patients were equally divided into 2 groups: one group received a dose of 10 8 cells before valve insertion, and the other group received a normal saline infusion. The follow-up time was 90 days. Inflammation was significantly improved as assessed by the CRP (C-reactive protein) index at 30 and 90 days after infusion. In addition, improvements in St. George’s Respiratory Questionnaire (SGRQ) scores indicated improved patient quality of life. Furthermore, an investigation into the homing ability of MSCs in vivo was performed on 9 GOLD patients, from stage A to stage D. Each patient received two 2 × 10 6 BM-MSC/kg IV infusions 1-week apart. 224 The marking of MSCs with indium-111 showed that MSCs were retained in the pulmonary vasculature longer in patients with mild COPD and that the levels of inflammatory mediators improved after 7 days of treatment. The results of the evaluation survey conducted after 1 year showed that the number of COPD exacerbations decreased to six times/year compared to 11 times/year before treatment. In addition, AT-MSCs present in the stromal vascular fraction were used to treat patients with COPD, and no adverse events were observed after 12 months of follow-up, but the clinical improvements post administration were not clear. 225 The results from a phase I clinical trial using AT-MSCs in eight patients with COPD also reported no significant change in pulmonary function test parameters. 226 A study evaluating the use of AT-MSCs as adjunctive therapy for COPD in 12 patients was performed. 227 AT was obtained using standard liposuction, MSCs were isolated, and 150–300 million cells were intravenously infused. The patients showed improvements in quality of life, with improved SGRQ scores after 3 and 6 months of treatment. Recently, UC-MSCs have emerged as potential allogeneic stem cell candidates for the treatment of COPD. 228 In a pilot clinical study, it was demonstrated that allogeneic administration of UC-MSCs in the treatment of COPD was safe and potentially effective. 229 In one study, 20 patients, including 9 at stage C and 11 at stage D per the GOLD classification, with histories of smoking were recruited and received cell-based therapy. The patients who received UC-MSC treatment showed significant reductions in Modified Medical Research Council scores, COPD assessment test scores, and the number of pulmonary exacerbations 6 months post administration. The results of the second trial using UC-MSCs showed that the mean FEV 1 /FVC ratios were increased along with improvements in SGRQ scores and 6MWTDs at three months post administration. 230 Although thorough assessments of the effectiveness of UC-MSCs are still in the early stages, the number of trials using UC-MSCs for the treatment of COPD is increasing steadily, with larger sample sizes and stronger designs (randomized or matched case–control studies), providing a data set strongly supporting the future applications of UC-MSCs. 231
The ongoing pandemic of the 21st century, the COVID-19 pandemic, emerged as a major pulmonary health problem worldwide, with a relatively high mortality rate. Numerous studies, reviews, and systematic analyses have been conducted to discuss and expand our knowledge of the virus and propose different mechanisms by which the virus could alter the immune system. 232 One of the most critical mechanisms is the generation of cytokine storms, which result from the initiation of hyperreactions of the adaptive immune response to viral infection. 233 These cytokine storms are formed by the establishment of waves of hypercytokinaemia generated from overreactive immune cells, which enhance their expression of TNF-α, IL-6, and IL-10, preventing T-lymphocyte recruitment and proliferation and culminating in T-lymphocyte apoptosis and T-cell exhaustion. In COVID-19, once a cytokine storm is formed, it spreads from an initial focal area through the body via circulation, which has been discussed in a comprehensive review by Jamilloux et al. 234 At the time of writing this review, there were 74 clinical trials using MSCs from UC (29 trials; including WJ-derived MSCs (WJ-MSCs) and placenta-derived MSCs (PL-MSCs)), AT (15 trials), and BM (11 trials) (comprehensive review 171 , 235 ). Hence, UC-MSCs have emerged as the most common MSCs for the treatment of COVID-19, with a total of 1047 patients participating in these trials. Among these trials, 15 completed trials using UC-MSCs (including WJ- and PL-MSCs) have been reported, with clinical data from approximately 600 recruited patients. 232 Eight of these 15 studies used allogenic UC-MSC transplantation to treat critically ill patients. 236 A list of case reports using UC-MSCs showed that the treatments were safe and well-tolerated in 14 patients with COVID-19, with the primary outcomes including increased percentages and numbers of T cells, 237 , 238 improved respiratory and renal functions, 239 reductions in inflammatory biomarker levels, 240 and positive outcomes in the PaO 2 /FiO 2 ratio. 240 In a pilot study conducted in ten patients with severe COVID-19, a single dose of UC-MSCs was safe and improved clinical outcomes, although the study did not investigate whether multiple doses of UC-MSCs could further improve the outcomes. 241 Two trials without a control group were conducted in 47 patients, and the results indicated that UC-MSCs were safe and feasible for the treatment of patients with COVID-19. 235 , 242 A single-center, open-label, individually randomized, standard treatment-controlled trial was performed in 41 patients (12 patients assigned to the UC-MSC group), and the results showed that significant improvements in C-reactive protein levels, IL-6 levels, oxygen indices, and lymphocyte numbers were found in the MSC groups. Chest computed tomography (CT) illustrated significant reductions in lung inflammatory responses as reflected by CT findings, the number of lobes involved, and pulmonary consolidation. 238 In a phase I trial conducted in 18 hospitalized patients with COVID-19, UC-MSCs were administered via an IV route in nine patients (five patients with moderate COVID-19 and 4 patients with severe COVID-19) at days 0, 3, and 6, with no treatment-related adverse events or severe adverse events. 243 Only one patient in the UC-MSC group required mechanical ventilation, compared to four patients in the control group. However, the clinical outcomes, such as COVID-19 symptoms, laboratory test results, CT findings of lung damage, and pulmonary function test parameters, were improved in both groups. Interestingly, a 1-year follow-up of the same sample revealed that the patients who received UC-MSC administration improved in terms of whole-lung lesion volume compared to the control group. 244 Moreover, chest CT at 12 months showed significant regeneration of lung tissue in the MSC-administered groups, whereas lung fibrosis was found in all patients in the control group. This finding is of interest because it indicates that a long time is needed to detect the regenerative functions of MSC-based therapy, as the biological process to enhance lung tissue regeneration occurs relatively slowly and requires multiple steps. The effects of UC-MSCs in the attenuation and prevention of the development of cytokine storms were illustrated in an interventional, prospective, three-parallel arm study with two control arms conducted in 30 patients in moderate and critical clinical conditions. 245 The results indicated a significant decrease in proinflammatory cytokines (IFNγ, IL-6, IL-17A, IL-2, and IL-12) and an increase in anti-inflammatory cytokines (IL-10, IL-13, and IL-1ra), suggesting that UC-MSCs might participate in the prevention of cytokine storm development. Lanzoni et al. performed a double-blind, randomized, controlled trial and found that UC-MSC infusions significantly decreased cytokine levels at day 6 and improved survival in patients with COVID-19 with ARDS. In this trial, 24 patients were randomized and assigned 1:1 to receive either MSCs or placebo. 246 MSC treatment was associated with a significant improvement in the survival rate without serious adverse events. To date, other trials conducted using UC-MSCs as the main MSCs provide a solid data set on their safety and efficacy in preventing the development of cytokine storms, reducing the inflammatory response, improving pulmonary function, reducing intensive care unit (ICU) stay duration, enhancing lung tissue regeneration, and reducing lung fibrosis progression. 240 , 247 , 248 , 249 In two large cohort studies (phase I with 210 patients and phase II with 100 patients), the volume of lung lesions and solid component injuries of patients’ lungs were reduced significantly after the administration of UC-MSCs, 250 and clinical symptoms and inflammatory levels were improved. 251 Of the 26 reported clinical trials for the treatment of COVID-19 with MSCs, 1 study used AT-MSCs as the main MSCs. 236 Thirteen COVID-19 adult patients under invasive mechanical ventilation who had received previous antiviral and/or anti-inflammatory treatments (including steroids, lopinavir/ritonavir, hydroxychloroquine, and/or tocilizumab, among others) were treated with allogeneic AT-MSCs. With a mean follow-up time of 16 days after infusion, 9/13 patients’ clinical symptoms improved, and 7/13 patients were intubated. A decrease in inflammatory cytokines and an increase in immunoregulatory cells were also observed in patients, especially in the group of patients with overall clinical improvement. Although there is a lack of clinical efficacy data supporting the use of AT-MSCs in the treatment of patients with COVID-19, AT-MSCs are still potential candidates for inhibiting COVID-19 due to their high secretory activity, strong immune-modulatory effects, and homing ability. 252 , 253 , 254
For ARDS, in a phase IIa trial, 60 patients with moderate to severe disease were randomized into 2 groups. A group of 40 patients received a single infusion of BM-MSCs at a dose of 1 × 10 6 cells/kg body weight, and another 20 patients received a placebo. 255 After 6 and 24 h of infusion, the decrease in plasma inflammatory cytokine levels in the MSC group was significantly greater than that in the placebo group. For severe pulmonary hypertension (PH) associated with BPD (BPD-PH), in a small trial, two preterm infants born at 26–27 weeks of age were intravenously administered heterologous BM-MSCs at a dose of 5 × 10 6 cells per kg of body weight; the treatment reduced oxygen requirements and supported respiration in the infants. 256 The administration of allogeneic AT-MSCs in the treatment of ARDS appeared to be safe and well-tolerated in 12 adult patients, but clinical outcomes were not observed. 257 The results of two patients who received BM-MSCs showed that both patients had improved respiratory function and hemodynamic function and a reduction in multiorgan failure. 258 Although the safety of BM-MSCs was confirmed in a multicentre, open-label, dose-escalation, phase I clinical trial (The Stem cells for ARDS treatment—START trial), 259 no significant improvements were found in a phase II trial, including in respiratory function and ARDS conditions. 260 The safety profile of UC-MSCs is also supported by the findings of a previous phase I clinical trial conducted in 9 patients, which showed that a single IV administration of UC-MSCs was safe and led to positive outcomes in terms of respiratory function and a reduction in the inflammatory response. 261 The findings of this study were also supported by those of the REALIST (Repair of Acute Respiratory Distress with Stromal Cell Administration) trial, which further confirmed the maximum tolerated dose of allogeneic UC-MSCs in patients with moderate to severe ARDS. 262
Although AT- and BM-MSCs have demonstrated therapeutic potential with similar mechanisms of action, UC-MSCs have emerged as potential candidates in the treatment of pulmonary diseases due to their ease of production as “off-the-shelf” products, rapid proliferation, noninvasive isolation methods, and supreme immunological regulation as well as anti-inflammatory effects. 263 However, it is important to note that there is a need to conduct phase III clinical trials with larger cohorts and trials with at least two sources of MSCs in the treatment of pulmonary conditions to further confirm this speculation. 264 Table 2 summarizes several clinical trials with published results discussed in this review.
Endocrine disorders, infertility/reproductive function recovery, and skin burns: should we consider AT-MSCs as the main MSCs based on their origin?
Endocrine disorders.
The human body maintains function and homeostatic regulation via a complex network of endocrine glands that synthesize and release a wide range of hormones. The endocrine system regulates body functions, including heartbeat, bone regeneration, sexual function, and metabolic activity. Endocrine system dysregulation plays a vital role in the development of diabetes, thyroid disease, growth disorder, sexual dysfunction, reproductive malfunction, and other metabolic disorders. The central dogma of regenerative medicine is the use of adult stem cells as a footprint for tissue regeneration and organ renewal. The functions of these stem cells are tightly regulated by microenvironmental stimuli from the nervous system (rapid response) and endocrine signals via hormones, growth factors, and cytokines. This harmonized and orchestrated system creates a symphony of signals that directly regulate tissue homeostasis and repair after injury. The disruption of these complex networks results in an imbalance of tissue homeostasis and regeneration that can lead to the development of endocrine disorders in humans, such as diabetes, sexual hormone deficiency, premature ovarian failure (POF), and Asherman syndrome.
In recent years, obesity and diabetes (type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM)) have been the two biggest challenges in endocrinology research, and the application of MSCs has emerged as a novel approach for therapeutic consideration. T1DM is characterized by the autoimmune destruction of pancreatic β-cells, whereas T2DM is defined as a combination of insulin resistance and pancreatic insulin-producing cell dysfunction. Regenerative medicine seeks to provide an exogenous cell source for replacing damaged or lost β-cells to achieve the goal of stabilizing patients’ blood glucose levels. To date, there are 28 clinical trials using MSCs in the treatment of T1DM ( http://www.clinicaltrials.gov , searched in October 2021), among which three trials were completed using autologous BM-MSCs (NCT01068951), allogeneic BM-MSCs (NCT00690066), and allogeneic AT-MSCs (NCT03920397). Interestingly, UC-MSCs were the most favored MSCs for the remaining trials. All published studies confirmed the safety of MSC therapy in the treatment of T1DM with no adverse events. The first study using autologous BM-MSCs showed that patients who were randomized into the MSC-administration group showed an increase in C-peptide levels in response to a mixed-meal tolerance test (MMTT) in comparison to the control group. 265 Unfortunately, there was no significant improvement in C-peptide levels, HbA1 C or insulin requirements. The use of autologous AT-MSCs in combination with vitamin D was safe and improved HbA1 C levels 6 months post administration. 266 WJ-MSCs were used as the main MSCs for the treatment of new-onset T1DM, which showed a significant improvement in both HbA1 C and C-peptide levels when compared to those of the control group at three and six months post administration. 267 , 268 The combination of allogeneic WJ-MSCs with autologous BM-derived mononuclear cells improved insulin secretion and reduced insulin requirements in patients with T1DM. 269 In terms of T2DM, 23 studies were registered on clinicaltrials.gov (searched in October 2021), with six completed studies (three studies used BM-MSCs and three studies used allogeneic UC-MSCs). Although the number of studies using MSCs for the treatment of T2DM is small, their findings support the safety of MSCs, with no severe adverse events observed during the course of these studies. 270 It was confirmed that MSC therapy potentially reduced fasting blood glucose and HbA1 C levels and increased C-peptide levels. However, these effects were short-term, and multiple doses were required to maintain the MSC effects. Interestingly, the autologous MSC approach in the treatment of patients with diabetes in general is hampered, as both BM-MSCs and AT-MSCs isolated from patients with diabetes showed reduced stemness and functional characteristics. 271 , 272 In addition, the durations of diabetes and obesity are strongly associated with autologous BM-MSC metabolic function, especially mitochondrial respiration, and the accumulation of mitochondrial DNA, which directly interfere with the functions of BM-MSCs and reduce the effectiveness of the therapy. 271 Therefore, the allogeneic approach using MSCs from healthy donors provides an alternative approach for stem cell therapy in the treatment of patients with diabetes.
Infertility and reproductive function recovery
Modern society is increasingly facing the problem of infertility, which is defined as the inability to become pregnant after more than 1 year of unprotected intercourse. 273 This problem has emerged as an important worldwide health issue and social burden. Assisted reproductive techniques and in vitro fertilization technology have recently become the most effective methods for the treatment of infertility in humans, but the use of these approaches is limited, as they cannot be applied in patients with no sperm or those who are unable to support implantation during pregnancy, they are associated with complications, they are time-consuming and expensive, and they are associated with ethical issues in certain territories. 274 Numerous conditions are related to infertility, including POF, nonobstructive azoospermia, endometrial dysfunction, and Asherman syndrome. Recent progress has been illustrated in preclinical studies for the potential applications of stem cell-based therapy for reproductive function recovery, especially recent studies in the field of MSCs, which provide new hope for patients with infertility and reproductive disorders. 275
POF is characterized by a loss of ovarian activity during middle age (before 40 years old) and affects 1–2% of women of reproductive age. 276 Patients diagnosed with POF exhibit oligo-/amenorrhea for at least 4 months, with increased levels of follicle-stimulating hormone (FSH) (>25 IU/L) on two occasions more than 1 month apart. 277 Diverse factors, such as genetic backgrounds, autoimmune disorders, environmental conditions, and iatrogenic and idiopathic situations, have been reported to be the cause of POF. 278 POF can be treated with limited effectiveness via psychosocial support, hormone replacement intervention, and fertility management. 279 MSCs from AT, BM, and UC have been used in the treatment of POF, with improvements in ovarian function in preclinical studies using chemotherapy-induced POF animal models. The early published POF study using BM-MSCs as the main cell source is a single case report in which a perimenopausal woman showed an improvement in follicular regeneration, and increased AMH levels resulted in a successful pregnancy followed by delivery of a healthy infant. 280 A report using autologous BM-MSCs in two women with POF illustrated an increase in baseline estrogen levels and the volume of the treated ovaries along with amelioration of menopausal symptoms. 281 The clinical procedures used in this early trial were invasive, as patients underwent two operations: (1) BM aspiration and (2) laparoscopy. A similar approach was used in two trials conducted in 10 women with POF (age range from 26–33 years old) and 30 patients (age from 18 to 40 years old). 282 A later study investigated two different routes of cell delivery, including laparoscopy and the ovarian artery, but the results have not been reported at this time. 282 Based on the positive outcomes of the mouse model, an autologous stem cell ovarian transplantation (ASCOT) trial was deployed using BM-derived stem cells with encouraging observations of improved ovarian function, as determined by elevated levels of AMH and AFC in 81.3% of participants, six pregnancies, and the successful delivery of three healthy babies. 283 A randomized trial (NCT03535480) was conducted in 20 patients with POF aged less than 39 years to further elaborate on the results of the ASCOT trial. 284 To date, there are no completed trials using AT-MSCs or UC-MSCs in the treatment of patients with POF, limiting the evaluation of these MSCs in the treatment of POF. The speculated reason is that POF is a rare disease, affecting 1% of women younger than 40 years, and with improvements in assisted productive technology, patients have several alternative options to enhance the recovery of reproductive function. 285
Wound healing and skin burns
Burns are the fourth most common injury worldwide, affecting ~11 million people, and are a major cause of death (180,000 patients annually). The severity of burns is defined based on the percentage of surface area burned, burn depth, burn location and patient age, and burns are usually classified into first-, second-, third-, and fourth-degree burns on the basis of their severity. 286 Postburn recovery depends on the severity of the burn and the effectiveness of treatment. Rapid healing may occur over weeks, while alternatively, healing can take months, with the ultimate result being scar formation and disability in patients with severe burns. Different from mechanical injury, burn injury is an invasive progression of damage to tissue at the burn site, including both mechanical damage to the skin surface and biological damage caused by natural apoptosis that prolongs excessive inflammation, oxidative stress, and impaired tissue perfusion. 287 To date, completely reversing the devastating damage of severe burns remains unachievable in medicine, and stem cell therapy provides an alternative option for patients with burn injury. The first case report of the use of BM-MSCs to treat a 45-year-old patient with burns on 40% of their body demonstrated the safety of the therapy and showed partial improvements in vascularization at the wound site and reduced coarse cicatrices. 288 , 289 Later, patients with second- and third-degree burns as well as deep burns were treated using either autologous BM-MSCs or allogeneic BM-MSCs by spraying the MSCs onto the burn sites or adding MSCs over a dermal matrix sheet to cover the wound. The results in these case reports revealed the potential efficacy of MSC-based therapy, which not only enhanced the speed of wound recovery but also reduced pain and improved blood supply without introducing infection. 288 , 290 , 291 In 2017, a study conducted in 60 patients with 10–25% of their total body surface areas burned treated with either autologous BM-MSCs or UC-MSCs showed that both MSC types improved the rate of healing and reduced the hospitalization period. 292 The drawback of BM-MSCs in the treatment of burns is the invasive harvesting method, which causes pain and possible complications in patients. Hence, treatment with allogeneic MSCs obtained from healthy donors is the method of choice, and AT- and UC-MSCs are two suitable candidates for this option. To date, a limited number of clinical trials have been conducted using MSC therapy. These trials have several limitations in trial design, such as a lack of a negative control group and blinding, small sample sizes, and the use of standardized measurement tools for burn injury and wound healing. Currently, AT-MSCs are being used in seven ongoing phase I and II trials in the treatment of burns. Hence, it is important to note that among the most widely studied MSCs, AT-MSCs have advantages over BM-MSCs when obtained from an allogeneic source, while their abilities in burn treatment remain to be determined. The main MSCs that should be used in the regeneration of burn tissue remain undefined (Table 3 ), and we observed the trend that AT-MSCs are more suitable candidates due to their biological nature, which contributes to the generation of keratinocytes and secretion profiles that strongly enhance the skin regeneration process. 293 , 294 , 295 , 296
MSC applications in cardiovascular disease: a promising but still controversial field
In the last two decades, great advancements have been achieved in the development of novel regenerative medicine and cardiovascular research, especially stem cell technology. 297 The discovery of human embryonic stem cells and human induced pluripotent stem cells (hiPSCs) opened a new door for basic research and therapeutic investigation of the use of these cells to treat different diseases. 298 However, the clinical path of hiPSCs and hiPSC-derived cardiomyocytes in the treatment of cardiovascular diseases is limited due to the potential for teratoma formation with hiPSCs and the immaturity of hiPSC-derived cardiomyocytes, which might pose a risk of cancer formation, 299 arrhythmia, and cardiac arrest to patients. 300 A recently emerged stem cell type is adult stem cells/progenitor cells, including MSCs, which can stimulate myocardial repair post administration due to their paracrine effects. Promising results of MSC-based therapy obtained from preclinical studies of cardiac diseases enhance the knowledge and strengthen the clinical research to investigate the safety and efficacy in a clinical trial setting. There are papers that discuss the importance of MSC therapy in the treatment of cardiovascular diseases, with the following references being highly recommended. 301 , 302 , 303 , 304 , 305 , 306 To date, 36 trials have evaluated the therapeutic potential of MSCs in different pathological conditions, with the most prevalent types being BM-MSCs (25 trials), followed by UC-MSCs (7 trials) and AT-MSCs (4 trials). 303 However, the reported results are contradictory and create controversy about the efficacy of the treatments.
One of the first trials using MSCs in the treatment of chronic heart failure was the Cardiopoietic Stem Cell Therapy in Heart Failure (C-CURE) trial, a multicentre, randomized clinical trial that recruited 47 patients. The trial findings supported the safety of BM-MSC therapy and provided a data set that demonstrated improvements in cardiovascular scores along with New York Heart Association functional class, quality of life, and general physical health. 307 Despite these encouraging results in the phase I trial, the treatment failed to achieve the primary outcomes in the phase II/III trial (CHART-1 trial), including no significant improvements in cardiac structure or function or patient quality of life. 308 A positive outcome was also found in a phase I/II, randomized pilot study called the POSEIDON trial, which was the first trial to demonstrate the superior effectiveness of the administration of allogeneic BM-MSCs compared to allogeneic MSCs from other sources. 309 , 310 Published results from the MSC-HF study, with 4 years of follow-up results, 311 , 312 and the TRIDENT study 313 illustrated the positive outcomes of BM-MSCs in the treatment of heart failure. However, a contradictory result from the recently published CONCERT-HF trial demonstrated that the administration of autologous BM-MSCs to patients diagnosed with chronic ischemic heart failure did not improve left ventricular function or reduce scar size at 12 months post administration, but the patient’s quality of life was improved. 314 This observation is similar to that of the TAC-HFT trial 315 but completely different from the reported results of the MSC-HF trial. A comprehensive investigation is still needed to determine the reasons behind these contradictory results. The largest clinical trial to date using BM-MSCs is the DREAM-HF study, which was a randomized, double-blind, placebo-controlled, phase III trial that was conducted at 55 sites across North America and recruited a total of 565 patients with ischemic and nonischaemic heart failure. 172 Although recent reports from the sponsor confirmed that the trial missed its primary endpoint (a reduction in recurrent heart failure-related hospitalization), other prespecified endpoints were met, such as a reduction in overall major adverse cardiac events (including death, myocardial infarction, and stroke). 306 Thus, a complete report from the DREAM-HF trial will provide pivotal data supporting the therapeutic potential of BM-MSCs in the treatment of heart failure and open a new path for the FDA to approve cell-based therapy for cardiovascular diseases.
The early trial using AT-derived cells was the PRECISE trial, which was a phase I, randomized, placebo-controlled, double-blind study that examined the safety and efficacy of adipose-derived regenerative cells (ADRCs) in the treatment of chronic ischemic cardiomyopathy. 316 ADRCs are a homogenous population of cells obtained from the vascular stromal fraction of AT, which contains a small proportion of AT-MSCs. 317 Although the study supported the safety of ADRC administration and illustrated a preserved functional capacity (peak VO 2 ) in the treated group and improvements in heart wall motion, neither poor left ventricle (LV) volume nor poor left ventricular ejection fraction (LVEF) was ameliorated. The follow-up trial of the PRECISE trial, called the ATHENA trial, was conducted in 31 patients, although the study was terminated prematurely because two cerebrovascular events occurred, which were not related to the cell product itself. 318 The results of the study illustrated increases in functional capacity, hospitalization rate, and MLHFQ scores, but the LV volume and LVEF were not significantly different between the two groups. Kastrup and colleagues conducted the first in vitro expanded AT-MSC trial in ten patients with ischemic heart disease and ischemic heart failure in 2017. The results confirmed that ready-to-use AT-MSCs were well-tolerated and potentially effective in the treatment of ischemic heart disease and heart failure. 319 Comparable results of AT-MSCs were also reported from the MyStromalCell Trial, which was a randomized placebo-controlled study. In this trial, 61 patients were randomized at a 2:1 ratio into two groups, with the results showing no significant difference in the primary endpoint, which was a change in the maximal bicycle exercise tolerance test (ETT) score from baseline to 6 months post administration. 320 A 3-year follow-up report from the MyStromalCell Trial confirmed that patients who received AT-MSC administration maintained their preserved exercise capacity and their cardiac symptoms improved, whereas the control group experienced a significant reduction in exercise performance and a worsened cardiovascular condition. 321
UC-MSCs are potential allogeneic cells for the treatment of cardiovascular disease, as they are “ready to use” and easy to isolate, they rapidly proliferate, and they secrete hepatocyte growth factors, 322 which are involved in cardioprotection and cardiovascular regeneration. 323 The pilot study using UC-MSCs in 30 patients with heart failure, called the RIMECARD trial, was the first reported trial for which the results supported the effectiveness of UC-MSCs, as seen in the improved ejection fraction, left ventricular function, functional status, and quality of life in patients administered UC-MSCs. 324 Encouraging results reported from a phase I/II HUC-HEART trial 325 showed improvements in LVEF and reductions in the size of the injured area of the myocardium. However, the opposite observations were also reported from a recently published phase I randomized trial using a combination of UC-MSCs and a collagen scaffold in patients with ischemic heart conditions, in which the size of fibrotic scar tissue was not significantly reduced. 326
Although MSCs from AT, BM, and UC have proven to be safe and feasible in the treatment of cardiovascular diseases, the correlation between the MSC types and their therapeutic potentials is still uncertain because different results have been reported from different clinical trials (Table 4 ). The mechanisms by which MSCs participate in recovery and enhance myocardial regeneration have been discussed comprehensively in a recently published review; 305 , 327 therefore, they will not be discussed in this review. In fact, the challenges of MSC-based therapy in cardiovascular diseases have been clearly described previously, 328 including (1) the lack of an in vitro evaluation of the transdifferentiation potential of MSCs to functional cardiac and endothelial cells, 329 (2) the uncontrollable differentiation of MSCs to undesirable cell types post administration, 330 and (3) the undistinguishable nature of MSCs derived from different sources with various levels of differentiation potential. 331 Therefore, the applications of MSC-based therapy in cardiovascular disease are still in their immature stage, with potential benefits to patients. Thus, there is a need to conduct large-scale, well-designed randomized clinical trials not only to confirm the therapeutic potential of MSCs from various sources but also to enhance our knowledge of cardiovascular regeneration post administration.
Proposed mechanism of BM-MSCs in the treatment of acquired brain and spinal injury
Bones are complex structures constituting a part of the vertebrate skeleton, and they play a vital role in the production of blood cells from HSCs. Similar to the functions of most vertebrate organs, bone function is tightly regulated by its constituents and by long-range signaling from AT and the adrenal glands, parathyroid glands, and nervous system. 332 The central nervous system (CNS) orchestrates the voluntary and involuntary input transmitted by a network of peripheral nerves, which act as the bridge between the nervous system and target organs. The CNS controls involuntary responses via the autonomic nervous system (ANS), consisting of the sympathetic nervous system and the parasympathetic nervous system, and voluntary responses via the somatic nervous system. The ANS penetrates deep into the BM cavity, reaching the regions of hematopoietic activity to deliver neurotransmitters that tightly regulate BM stem cell niches. 333 The BM microenvironment consists of various cell types that participate in the maintenance of HSC niches, which are composed of specialized cells, including BM-MSCs (Fig. 3a ). The release of a specific neurotransmitter, circadian norepinephrine, from the sympathetic nervous system at nerve terminals leads to a reduction in the circadian expression of C–X-C chemokine ligand 12 (CXCL12, which is also known as stromal cell-derived factor-1 (SDF-1)) by Nestin + /NG2 2+ BM-MSCs, resulting in the secretion of HSCs into the peripheral bloodstream. 334 , 335 In fact, BM-MSCs play a significant role in the regulation of HSC quiescence and are closely associated with arterioles and sympathetic nervous system nerve fibers. Nestin-expressing BM-MSCs have been shown to express high levels of SDF-1, stem cell factor (SCF), angiopoietin-1 (Ang-1), interleukin-7, vascular cell adhesion molecule 1 (VCAM-1), and osteopontin (OPN), which are directly involved in the regulation and maintenance of HSC quiescence. 336 The depletion of BM-MSCs in BM leads to the mobilization of HSCs into the peripheral bloodstream and spleen. The findings from a previous study demonstrated that reduced SDF-1 expression in norepinephrine-treated BM-MSCs resulted in the mobilization of CXCR4 + HSCs into circulation. 337 The ability of BM-MSCs to produce SDF-1 is tightly related to their neuronal protective functions. 338 SDF-1 is a member of a chemokine subfamily that orchestrates an enormous diversity of pathways and functions in the CNS, such as neuronal survival and proliferation. The chemokine has two receptors, CXCR4 and CXCR7, that are involved in the pathogenic development of neurodegenerative and neuroinflammatory diseases. 339 In the damaged brain, SDF-1 functions as a stem cell homing signal, and in acquired immune deficiency syndrome (AIDS), SDF-1 has been reported to be involved in the protection of damaged neurons by preventing apoptosis. In a traumatic brain injury model, SDF-1 was found to function as an inhibitor of the caspase-3 pathway by upregulating the Bcl-2/Bax ratio, which in turn protects neurons from apoptosis. 340 Moreover, the release of SDF-1 also facilitates cell recruitment, cell migration, and the homing of neuronal precursor cells in the adult CNS by activating the CXCR4 receptor. 341 , 342 Existing data support that SDF-1 acts as the guiding signal for the regeneration of axon growth in damaged neurons and enhances spinal nerve regeneration. 343 , 344 Hence, the ability of BM-MSCs to express SDF-1 in response to the neuronal environment provides a unique neuronal protective effect that could explain the potential therapeutic efficacy of BM-MSCs in the treatment of neurodegenerative diseases (Fig. 3b ).
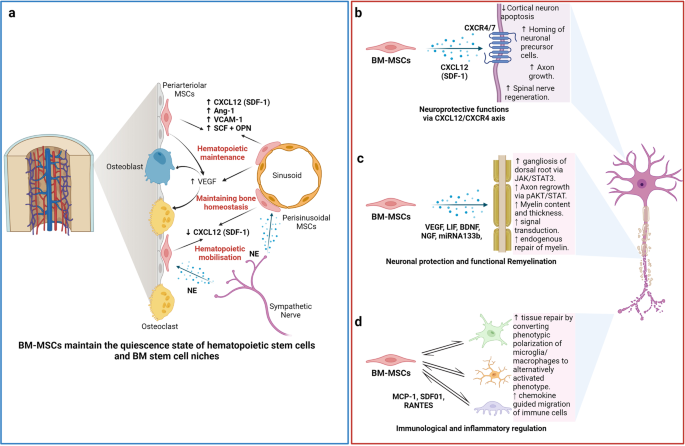
The nature of the “stem niche” of bone marrow-derived mesenchymal stem cells (BM-MSCs) supports their therapeutic potential in neuron-related diseases. a Bone marrow is a complex stem cell niche regulated directly by the central nervous system to maintain bone marrow homeostasis and haematopoietic stem cell (HSC) functions. MSCs in bone marrow respond to the environmental changes through the release of norepinephrine (NE) from the sympathetic nerves that regulate the synthesis of SDF-1 and the migration of HSCs through the sinusoids. The secretion of stem cell factors (SCFs), VCAM-1 and angiotensin-1 from MSCs also plays a significant role in the maintenance of HSCs. b BM-MSCs have the ability to produce and release SDF-1, which directly contributes to neuroprotective functions at the damaged site through interaction with its receptors CXCR4/7, located on the neuronal membrane. c Neuronal protection and the functional remyelination induced by BM-MSCs are also modulated by the release of a wide range of growth factors, including VEGF, BDNF, and NGF, by the BM-MSCs. d BM-MSCs also have the ability to regulate neuronal immune responses by direct interaction or paracrine communication with microglia. Figure was created with BioRender.com
The migration of exogenous MSCs after systemic administration to the brain is limited by the physical blood–brain barrier (BBB), which is a selective barrier formed by CNS endothelial cells to restrict the passage of molecules and cells. The mechanism of molecular movement across the BBB is well established, but how stem cells can bypass the BBB and home to the brain remains unclear. Recent studies have reported that MSCs are able to migrate through endothelial cell sheets by paracellular or transcellular transport followed by migration to the injured or inflammatory site of the brain. 345 , 346 During certain injuries or ischemic events, such as brain injury, stroke, or cerebral palsy, the integrity and efficiency of BBB protection is compromised, which allows MSC migration across the BBB via paracellular transport through the transient formation of interendothelial gaps. 347 CD24 expression has been detected in human BM-MSCs, which are regulated by TGF-β3, 348 allowing them to interact with activated endothelial cells via P-selectin and initiate the tethering and rolling steps of MSCs. 349 Additionally, BM-MSCs express high levels of CXCR4 or CXCR7, 350 , 351 which bind to integrin receptors, such as VLA-4, to activate the integrin-binding process and allow the cells to anchor to endothelial cells, followed by the migration of MSCs through the endothelial cell layer and basement membrane in a process called transmigration. 352 This process is facilitated by the secretion of matrix metalloproteinases (MMPs), which degrade the endothelial basement membrane, allowing BM-MSCs to enter the brain environment. 353 , 354 BM-MSCs can also regulate the integrity of the BBB via the secretion of tissue inhibitor of matrix metalloproteinase-3 (TIMP3), which has been shown to ameliorate the effects of a compromised BBB in traumatic brain injury. 355 The secretion of TIMP3 from MSCs directly blocked vascular endothelial growth factor a (VEGF-a)-induced breakdown of endothelial cell adherent junctions, demonstrating the potential mechanism of BM-MSCs in the regulation of BBB integrity.
The therapeutic applications of BM-MSCs in neurodegenerative conditions have been significantly increased by the demonstration of BM-MSC involvement in axonal and functional remyelination processes. Remyelination is a spontaneous regenerative process occurring in the human CNS to protect oligodendrocytes, neurons, and myelin sheaths from neuronal degenerative diseases. 356 Remyelination is considered a neuroprotective process that limits axonal degeneration by demyelination and neuronal damage. The first mechanism of action of BM-MSCs related to remyelination is the activation of the JAK/STAT3 pathway to regulate dorsal root ganglia development. 357 It was reported that BM-MSCs secrete vascular endothelial growth factor-A (VEGF-A), 358 brain-derived neurotrophic factor (BDNF), interleukin-6, and leukemia inhibitor factor (LIF), which directly function in neurogenesis and neurite growth. 357 VEGF-A is a key regulator of hemangiogenesis during development and bone homeostasis. Postnatally, osteoblast- and MSC-derived VEGF plays a critical role in maintaining and regulating bone homeostasis by stimulating MSC differentiation into osteoblasts and suppressing their adipogenic differentiation. 359 , 360 , 361 To balance osteoblast and adipogenic differentiation, VEGF forms a functional link with the nuclear envelope protein laminin A, which in turn directly regulates the osteoblast and adipocyte transcription factors Runx2 and PPARγ, respectively. 361 , 362 In the brain, VEGF is a potent growth factor mediating angiogenesis, neural migration, and neuroprotection. VEGF-A, secreted from BM-MSCs under in vitro xeno- and serum-free culture conditions, is the most studied member of the VEGF family and is suggested to play a protective role against cognitive impairment, such as in the context of Alzheimer’s disease pathology or stroke. 363 , 364 , 365 Recently, it was reported that the neurotrophic and neuroprotective function of VEGF is mediated through VEGFR2/Flk-1 receptors, which are expressed in the neuroproliferative zones and extend to astroglia and endothelial cells. 366 In animal models of intracerebral hemorrhage and cerebral ischemia, the transfusion of Flk-1-positive BM-MSCs promotes behavioral recovery and anti-inflammatory and angiogenic effects. 367 , 368 Moreover, supplementation with VEGF-A in neuronal disorders enhances intraneural angiogenesis, improves nerve regeneration, and promotes neurotrophic capacities, which in turn increase myelin thickness via the activation of the prosurvival transcription factor nuclear factor-kappa B (NF-kB). This activation, together with the downregulation of Mdm2 and increased expression of the pro-apoptotic transcription factor p53, is considered to be the neuroprotective process associated with an increased VEGF-A level. 369 , 370 , 371 An analysis of microRNA (miRNA) in extracellular vesicles (EVs) secreted from BM-MSCs revealed that BM-MSCs release substantial amounts of miRNA133b, which suppresses the expression of connective tissue growth factor (CTGF) and protects hippocampal neurons from apoptosis and inflammatory injury 372 , 373 , 374 (Fig. 3c ).
In terms of immunoregulatory functions, the administration of human BM-MSCs into immunocompetent mice subjected to SCI or brain ischemia showed that BM-MSCs exhibited a short-term neuronal protective function against neurological damage (Fig. 3d ). Further investigation demonstrated the ability of BM-MSCs to directly communicate with host microglia/macrophages and convert them from phenotypic polarization into alternative activated microglia/macrophages (AAMs), which are key players in axonal extension and the reconstruction of neuronal networks. 375 Other studies have also illustrated that the administration of AAMs directly to the injured spinal cord induced axonal regrowth and functional improvement. 376 The mechanism by which BM-MSCs activate the conversion of microglia/macrophages occurs through two representative macrophage-related chemokine axes, CCL2/CCR2 and CCL-5/CCR5, both of which exhibit acute or chronic elevation following brain injury or SCI. 377 The CCL2/CCR2 axis contributed to the enhancement of inflammatory function, and BM-MSC-mediated induction of CCL2 did not alter the total granulocyte number (Fig. 3d ). Although the chemokine-mediated mechanism of BM-MSCs in the activation of AAMs and enhanced axonal regeneration at the damage sites is evident, the direct mechanism by which the communication between BM-MSCs and the target cells results in these phenomena remains unclear, and further investigation is needed.
BM-MSCs also confer the ability to regulate the inflammatory regulation of the immune cells present in the brain by (1) promoting the polarization of macrophages toward the M2 type, (2) suppressing T-lymphocyte activities, (3) stimulating the proliferation and differentiation of regulatory T cells (Tregs), and (4) inhibiting the activation of natural killer (NK) cells. BM-MSCs secrete glial cell line-derived neurotrophic factor (GDNF), a specific growth factor that contributes directly to the transition of the microglial destructive M1 phenotype into the regenerative M2 phenotype during the neuroinflammatory process. 378 A similar result was also found in AT- 379 and UC-MSCs 380 under neuroinflammation-associated conditions, suggesting that AT-, BM-, and UC-MSCs share the same mechanism in promoting macrophage polarization. In terms of T-lymphocyte suppression, compared to MSCs from AT and BM, UC-MSCs show the strongest potential to inhibit the proliferation of T-lymphocytes by promoting cell cycle arrest (G0/G1 phase) and apoptosis. 381 In addition, UC-MSCs have been proven to be more effective in promoting the proliferation of Tregs 382 and inhibiting NK activation. 383 Although MSCs are well-known for their inflammatory regulatory ability, the mechanism is not exclusive to BM-MSCs, especially in neurological disorders. 384
Proposed mechanism of UC-MSCs in the treatment of pulmonary diseases and lung fibrosis
In contrast to AT-MSCs and BM-MSCs, UC-MSCs have lower expression of major histocompatibility complex I (MHC I) and no expression of MHC II, which prevents the complications of immune rejection. 385 Moreover, as UC is considered a waste product after birth, with the option of noninvasive collection, UC-MSCs are easier to obtain and culture than AD- and BM-MSCs. 386 These advantages of UC-MSCs have contributed to their use in the treatment of pulmonary diseases, especially during the rampant COVID-19 pandemic, as “off-the-shelf” products. Numerous pulmonary diseases have been the subject of applications of UC-MSCs, including BPD, COPD, ARDS, and COVID-19-induced ARDS. In BPD, premature infants are born before the alveolarization process, resulting in arrested lung development and alveolar maturation. Upon administration via an IV route, the majority of exogenous UC-MSCs reach the immature lung and directly interact with immune cells to exert their immunomodulatory properties via cell-to-cell interaction mechanisms (Fig. 4a ). UC-MSCs interact with T cells via the PD-L1 ligand, which binds to the PD-1 inhibitory molecule on T cells, resulting in the suppression of CD3+ T-cell proliferation and effector T-cell responses. 387 In addition, UC-MSCs also express CD54 (ICAM-1), which plays a crucial role in the immunomodulatory functions of T cells. 388 Direct contact between UC-MSCs and macrophages via CD54 expression on UC-MSCs promotes the immune regulation of UC-MSCs via the regulation of phagocytosis by monocytes. 389 Moreover, the contact of UC-MSCs with macrophages during proinflammatory responses increases the secretion of TSG-6 by UC-MSCs, which in turn promotes the inhibitory regulation of CD3+ T cells, macrophages, and monocytes by MSCs. 390 Recently, upregulation of SDF-1 was described in neonatal lung injury, especially in layers of the respiratory epithelium. 391 SDF-1 has been shown to participate in the migration and initiation of the homing process of MSCs via the CXCR4 receptors on their surface. 392 It was reported that UC-MSCs express low levels of CXCR4, allowing them to induce SDF-1-associated migration processes via the Akt, ERK, and p38 signal transduction pathways. 393 Hence, in BPD, the upregulation of SDF-1 together with the homing ability of UC-MSCs strongly supports the therapeutic effects of UC-MSCs in the treatment of BPD. Furthermore, UC-MSCs have the ability to communicate with immune cells via cell-to-cell contact to reduce proinflammatory responses and the production of proinflammatory cytokines (such as TGF-β, INF-γ, macrophage MIF, and TNF-α). The modulation of the human innate immune system by UC-MSCs is mediated by cell–cell interactions via CD54-LFA-1 that switch macrophage polarization processes, promoting the proliferation of M2 macrophages, which in turn reduce inflammatory responses in the immature lung. 394 Moreover, UC-MSCs also have the ability to produce VEGF and hepatocyte growth factors (HGFs), promoting angiogenesis and enhancing lung maturation. 395
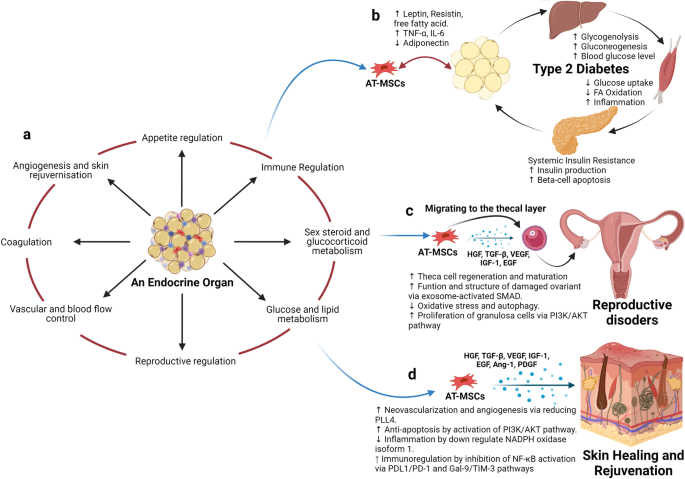
Adipose tissue-derived mesenchymal stem cells (AT-MSCs) and the nature of their tissue of origin support their use in therapeutic applications. a Adipose tissue is considered an endocrine organ, supporting and regulating various functions, including appetite regulation, immune regulation, sex hormone and glucocorticoid metabolism, energy production, the orchestration of reproduction, the control of vascularization, and blood flow, the regulation of coagulation, and angiogenesis and skin regeneration. b In terms of metabolic disorders, such as type 2 diabetes mellitus (T2DM), as adipose tissue is directly involved in the metabolism of glucose and lipids and the regulation of appetite, the detrimental effects of T2DM also alter the functions of AT-MSCs, which in turn, hampers their therapeutic effects. Hence, the use of autologous AT-MSCs is not recommended for the treatment of metabolic disorders, including T2DM, suggesting that allogeneic AT-MSCs from healthy donors could be a better alternative approach. c AT-MSCs are suitable for the treatment of reproductive disorders due to their unique ability to mobilize and home to the thecal layer of the injured ovary, enhance the regeneration and maturation of thecal cells, increase the structure and function of damaged ovaries via exosome-activated SMAD, decrease oxidative stress and autophagy, and increase the proliferation of granulosa cells via PI3K/AKT pathways. These functions are regulated specifically by growth hormones produced by AT-MSCs in response to the surrounding environment, including HGF, TGF-β, IGF-1, and EGF. d AT-MSCs are also good candidates for skin healing and regeneration as their growth factors strongly support neovascularization and angiogenesis by reducing PLL4, increase anti-apoptosis via the activation of PI3K/AKT pathways, regulate inflammation by downregulating NADPH oxidase isoform 1, and increase immunoregulation through the inhibition of NF-κB activation. The figure was created with BioRender.com
COPD is characterized by an increase in hyperinflammatory reactions in the lung, compromising lung function and increasing the development of lung fibrosis. The mechanism by which UC-MSCs contribute to the response to COPD is inflammatory regulation (Fig. 4b ). The administration of UC-MSCs prevented the infiltration of inflammatory cells in peribronchiolar, perivascular, and alveolar septa and switched macrophage polarization to M2. 396 A significant reduction in proinflammatory cytokines, including IL-1β, TNF-α, and IL-8, was also observed following UC-MSC administration. 224 MSCs, including UC-MSCs, have been reported to trigger the production of secretory leukocyte protease inhibitors in epithelial cells through the secretion of HGF and epidermal growth factor (EGF), which is believed to have beneficial effects on COPD. 397 , 398 In addition to their inflammatory regulation ability, UC-MSCs exhibit antimicrobial effects through the inhibition of bacterial growth and the alleviation of antibiotic resistance during Pseudomonas aeruginosa infection. 399 The combination of the regulation of the host immune response and the antimicrobial effects of UC-MSCs may be relevant for the prevention and treatment of COPD exacerbations, as inflammation and bacterial infections are important risk factors that significantly contribute to the morbidity and mortality of patients with COPD. In terms of regenerative functions, UC-MSCs were reported to be able to differentiate into type 2 alveolar epithelial cells in vitro and alleviate the development of pulmonary fibrosis via β-catenin-regulated cell apoptosis. 400 Furthermore, UC-MSCs enhanced alveolar epithelial cell migration and proliferation by increasing matrix metalloproteinase-2 levels and reduced their endogenous inhibitors, tissue inhibitors of matrix metalloproteinases, providing a potential mechanism underlying their anti-pulmonary-fibrosis effects. 401 , 402
In ARDS, especially that associated with COVID-19, the proinflammatory state is initiated by increases in plasma concentrations of proinflammatory cytokines, such as IL-1 beta, IL-7, IL-8, IL-9, IL-10, bFGF, granulocyte colony-stimulating factor (G-CSF), GM-CSF, IFN-γ, and TNF-α. The significant increases in the concentrations of these cytokines in patient plasma suggest the development of a cytokine storm, which is a leading cause of COVID-induced mortality. In addition to the immunomodulatory functions regulated via cell-to-cell interactions between UC-MSCs and immune cells, such as macrophages, monocytes, and T cells, UC-MSCs exert their functions via paracrine effects through the secretion of growth factors, cytokines, and exosomes (Fig. 4c ). The most relevant immunomodulatory function of UC-MSCs is considered to be their inhibition of effector T cells via the induction of T-cell apoptosis and cell cycle arrest by the production of indoleamine 2,3- dioxygenase (IDO), prostaglandin E2 (PGE-2), and TGF-β. Elevated levels of PGE-2 in patients with COVID-19 are reported to be a crucial factor in the initiation of inflammatory regulation by UC-MSCs post administration and prevent the development of cytokine storms by direct inhibition of T- and B lymphocytes. 403 UC-MSCs exert these inhibitory activities through a PGE-2-dependent mechanism. 404 It was reported that UC-MSCs confer the ability to secrete tolerogenic mediators, including TGF-β1, PGE-2, nitric oxide (NO), and TNF-α, which are directly involved in their immunoregulatory mechanism. The secretion of NO from UC-MSCs is reported to be associated with the desensitization of T cells via the IFN-inducible nitric oxide synthase (iNOS) pathways and to stimulate the migration of T cells in close proximity to MSCs that subsequently suppress T-cell sensitivities via NO. 405 Lung infection with viruses usually leads to impairments in alveolar fluid clearance and protein permeability. The administration of UC-MSCs enhances alveolar protection and restores fluid clearance in patients with COVID-19. UC-MSCs secrete growth factors associated with angiogenesis and the regeneration of pulmonary blood vessels and micronetworks, including angiotensin-1, VEGF, and HGF, which also reduce oxidative stress and prevent fibrosis formation in the lungs. These trophic factors have been identified as key players in the modulation of the microenvironment and promote pulmonary repair. Additionally, UC-MSCs are more effective than BM-MSCs in the restoration of impaired alveolar fluid clearance and the permeability of airways in vitro, supporting the use of UC-MSCs in the treatment of patients with pulmonary pneumonia. 406 In the context of pulmonary regeneration, UC-MSCs were shown to inhibit apoptosis and fibrosis in pulmonary tissue by activating the PI3K/AKT/mTOR pathways via the secretion of HGF, which also acts as an inhibitory stimulus that blocks alveolar epithelial-to-mesenchymal transition. 407 , 408 Moreover, UC-MSCs can reverse the process of fibrosis via enhanced expression of macrophage matrix-metallopeptidase-9 for collagen degradation and facilitate alveolar regeneration via Toll-like receptor-4 signaling pathways. 409 UC-MSCs were shown to communicate with CD4+ T cells through HGF induction not only to inhibit their differentiation into Th17 cells, reducing the secretion of IL-17 and IL-22 but also to switch their differentiation into regulatory T cells. 410 , 411 In addition, UC-MSCs conferred the ability to facilitate the number of M2 macrophages and reduce M1 cells via the control of the macrophage polarization process. 412
There are several potential mechanisms of UC-MSCs in the treatment of patients with pulmonary diseases and pneumonia, including the regulation of immune cell function, immunomodulation, the enhancement of alveolar fluid clearance and protein permeability, the modulation of endoplasmic reticulum stress, and the attenuation of pulmonary fibrosis. Hence, based on these discussions, UC-MSCs are recommended as suitable candidates for the treatment of pulmonary disease both in pediatric and adult patients.
Proposed mechanism of AT-MSCs in the treatment of endocrinological diseases, reproductive disorders, and skin burns
Human AT was first viewed as a passive reservoir for energy storage and later as a major site for sex hormone metabolism, the production of endocrine factors (such as adipsin and leptin), and a secretion source of bioactive peptides known as adipokines. 413 It is now clear that AT functions as a complex and highly active metabolic and endocrine organ, orchestrating numerous different biological features 414 (Fig. 5a ). In addition to adipocytes, AT contains hematopoietic-derived progenitor cells, connective tissue, nerve tissue, stromal cells, endothelial cells, MSCs, and pericytes. AT-MSCs and pericytes mobilize from their perivascular locations to aid in healing and tissue regeneration throughout the body. As AT is involved directly in energy storage and metabolism, AT-MSCs are also mediated and regulated by growth factors related to these pathways. In particular, interleukin-6 (IL-6), IL-33, and leptin regulate the maintenance of metabolic activities by increasing insulin sensitivity and preserving homeostasis related to AT. Nevertheless, in the development of obesity and diabetes, omental and subcutaneous AT maintains a low-grade state of inflammation, resulting in the impairment of glucose metabolism and potentially contributing to the development of insulin resistance. 415 In normal AT, direct regulation of Pre-B-cell leukemia homeobox (Pbx)-regulating protein-1 (PREP1) by leptin and thyroid growth factor-beta 1 (TGF-β1) in AT-MSCs and mature adipocytes is involved in the protective function and maintenance of AT homeostasis. However, under diabetic conditions, the balance between the expression of leptin and the secretion of TGF-β1 is compromised, resulting in the malfunction of AT-MSC metabolic activity and the proliferation, differentiation, and maturation of adipocytes. Therefore, the use of autologous AT-MSCs in the treatment of diabetic conditions is not a suitable option, as the functions of AT-MSCs are directly altered by diabetic conditions, which reduces their effectiveness in cell-based therapy (Fig. 5b ).
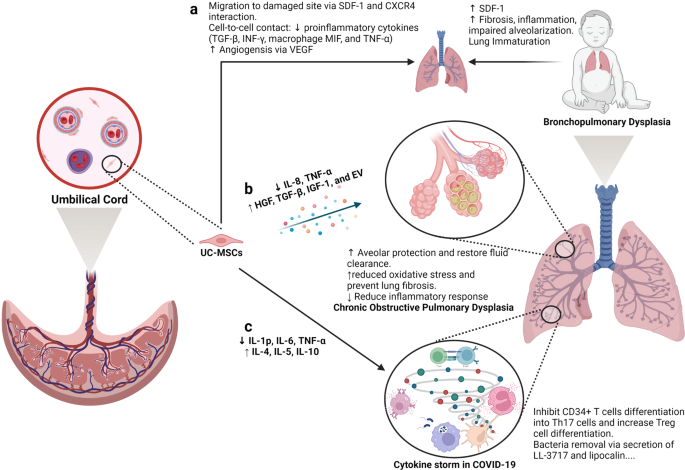
Umbilical cord-derived mesenchymal stem cells (UC-MSCs) are good candidates for the treatment of pulmonary diseases. a Lung immaturity and fibrosis are the major problems of patients with bronchopulmonary dysplasia and lead to increased levels of SDF-1, the development of fibrosis, the induction of the inflammatory response, and the impairment of alveolarization. UC-MSCs are attracted to the damaged lung via the chemoattractant SDF-1, which is constantly released from the immature lung via SDF-1 and CXCR4 communication. Moreover, UC-MSCs reduce the level of proinflammatory cytokines (TGF-β, INF-γ, macrophage MIF, and TNF-α) via a cell-to-cell contact mechanism. The ability of UC-MSCs to produce and secrete VEGF also involves in the regeneration of the immature lung through enhanced angiogenesis. b Upon an exacerbation of chronic obstructive pulmonary disease (COPD), UC-MSCs respond to the surrounding stimuli by reducing IL-8 and TNF-α levels, resulting in the inhibition of the inflammatory response but an increase in the secretion of growth factors participating in the protection of alveoli, fluid clearance and reduced oxidative stress and lung fibrosis, including HGF, TGF-β, IGF-1, and exosomes. c In a similar manner, UC-MSCs prevent the formation of cytokine storms in coronavirus disease 2019 (COVID-19) by inhibiting CD34+ T-cell differentiation into Th17 cells and enhancing the number of regulatory T cells. Moreover, UC-MSCs also have antibacterial activity by secreting LL-3717 and lipocalin. Figure was created with BioRender.com
Preclinical studies and clinical trials have revealed the therapeutic effects of MSCs, in general, and AT-MSCs, in particular, in the management of POF, with relatively high efficacy and enhanced regeneration of the ovaries. Understanding the molecular and cellular mechanisms underlying these effects is the first step in the development of suitable MSC-based therapies for POF. One of the mechanisms by which MSCs exert their therapeutic effects is their ability to migrate to sites of injury, a process known as “homing”. Studies have shown that MSCs from different sources have the ability to migrate to different compartments of the injured ovary. For example, BM-MSCs administered through IV routes migrated mostly to the ovarian hilum and medulla, 416 whereas a significant number of UC-MSCs were found in the medulla. 417 Interestingly, AT-MSCs were found to be engrafted in the theca layers of the ovary but not in the follicles, where they acted as supportive cells to promote follicular growth and the regeneration of thecal layers. 418 The structure and function of the thecal layer have a great impact on fertility, which has been reviewed elsewhere. 419 In brief, the thecal layer consists of two distinct parts, the theca interna, which contains endocrine cells, and the theca externa, which is an outer fibrous layer. The thecal layer contains not only endocrine-derived cells but also vascular- and immune-derived cells, whose functions are to maintain the structural integrity of the follicles, transport nutrients to the inner compartment of the ovary and produce key reproductive hormones such as androgens (testosterone and dihydrotestosterone) and growth factors (morphogenic proteins, e.g., BMPs and TGF-β). 420 As AT-MSCs originate from an endocrine organ, their ability to sense signals and migrate to the thecal layer is anticipated. Additionally, secretome analysis of AT-MSCs showed a wide range of growth factors, including HGF, TBG-β, VEGF, insulin-like growth factor-1 (IGF-1), and EGF, 421 that are directly involved in the restoration of the structure and function of damaged ovaries by stimulating cell proliferation and reducing the aging process of oocytes via the activation of the SIRT1/FOXO1 pathway, a key regulator of vascular endothelial homeostasis. 422 , 423 In POF pathology, autophagy and its correlated oxidative stress contribute to the development of POF throughout a patient’s life. Recently, AT-MSCs were shown to be able to improve the structure and function of mouse ovaries by reducing oxidative stress and inflammation, providing essential data supporting the mechanism of AT-MSCs in the treatment of POF. 424 Several studies have illustrated that AT-MSCs secrete biologically active EVs that regulate the proliferation of ovarian granulosa cells via the PI3K/AKT pathway, resulting in the enhancement of ovarian function. 425 Direct regulation of ovarian cell proliferation modulates the state of these cells, which in turn restores the ovarian reserve. 426 Other mechanisms supporting the effectiveness of MSCs have been carefully reviewed, confirming the therapeutic potential of MSCs derived from different sources 426 (Fig. 5c ).
In the last decade, the number of clinical trials using AT-MSCs in the treatment of chronic skin wounds and skin regeneration has exponentially increased, with data supporting the enhancement of the skin healing processes, the reduction of scar formation, and improvements in skin structure and quality. Several mechanisms are directly linked to the origin of AT-MSCs, including differentiation ability, neovascularization, anti-apoptosis, and immunological regulation. AT is a connective and supportive tissue positioned just beneath the skin layers. AT-MSCs have a strong ability to differentiate into adipocytes, endothelial cells, 427 epithelial cells 428 and muscle cells. 429 The adipogenic differentiation of AT-MSCs is one of the three mesoderm lineages that defines MSC features, and AT-MSCs are likely to be the best MSC type harboring this ability compared to BM- and UC-MSCs. Recent reports detailed that AT-MSCs accelerated diabetic wound tissue closure through the recruitment and differentiation of endothelial cell progenitor cells into endothelial cells mediated by the VEGF-PLCγ-ERK1/ERK2 pathway. 430 Upon injury, the skin must be healed as quickly as possible to prevent inflammation and excessive blood loss. The reparation process occurs through distinct overlapping phases and involves various cell types and processes, including endothelial cells, keratinocyte proliferation, stem cell differentiation, and the restoration of skin homeostasis. 431 Hence, the differentiation ability of AT-MSCs plays a critical role in their therapeutic effect on skin wound regeneration and healing processes. AT-MSCs accelerate wound healing via the production of exosomes that serve as paracrine factors. It was reported that AT-MSCs responded to skin wound injury stimuli by increasing their expression of the lncRNA H19 exosome, which upregulated SOX9 expression via miR-19b, resulting in the acceleration of human skin fibroblast proliferation, migration, and invasion. 432 In addition, the engraftment of AT-MSCs supported wound bed blood flow and epithelialization processes. 433 Anti-apoptosis plays a critical role in AT-MSC-based therapy, as without a microvascular supply network established within 4 days post injury, adipocytes undergo apoptosis and degenerate. Exogenous sources of AT-MSCs mediate anti-apoptosis via IGF-1 and exosome secretion by triggering the activation of PI3K signaling pathways. 434 Another mechanism supporting the therapeutic potential of AT-MSCs is their anti-inflammatory function, which results in the reduction of proinflammatory factors, such as tumor necrosis factor (TNF) and interferon-γ (IFN-γ), and increases the production of the anti-inflammatory factors IL-10 and IL-4. Exosomes from AT-MSCs in response to a wound environment were found to contain high levels of Nrf2, which downregulated wound NADPH oxidase isoform 1 (NOX1), NADPH oxidase isoform 4 (NOX4), IL-1β, IL-6, and TNF-α expression. The anti-inflammatory functions of AT-MSCs are also regulated by their immunomodulatory ability, partially through the inhibition of NF-κB activation in T cells via the PD-L1/PD-1 and Gal-9/TIM-3 pathways, providing a novel target for the acceleration of wound healing 435 (Fig. 5d ).
Therefore, as an endocrine organ in the human body, AT and its derivative stem cells, including AT-MSCs, have shown great potential in the treatment of reproductive disorders and skin diseases. Their potential is supported by mechanisms that are directly related to the nature of AT-MSCs in the maintenance of tissue homeostasis, angiogenesis, anti-apoptosis, and the regulation of inflammatory responses.
The current challenges for MSC-based therapies
Over the past decades, MSC-based research and therapy have made tremendous advancements due to their advantages, including immune evasion, diverse tissue sources for harvesting, ease of isolation, rapid expansion, and cryopreservation as “off-the-shelf” products. However, several important challenges have to be addressed to further enhance the safety profile and efficacy of MSC-based therapy. In our opinion, the most important challenge of MSC-based therapy is the fate of these cells post administration, especially the long-term survival of allogeneic cells in the treatment of certain diseases. Although reported data confirm that the majority of MSCs are trapped in the lung and rapidly removed from the circulation, caution has been raised related to the occurrence of embolism events post infusion, which was proven to be related to MSC-induced innate immune attack (called instant blood-mediated inflammatory reaction). 436 Another related challenge is the homing ability of infused cells, as successful homing at targeted tissue might result in long-term benefits to patients. Other concerns related to MSC-based therapy are the number of dead cells infused into the patients. An interesting study reported that dead MSCs alone still exerted the same immunomodulatory property as live MSCs by releasing phosphatidylserine. 437 This is an interesting observation, as there is always a certain number of dead cells present in the cell-based product, and concerns are always raised related to their effects on the patient’s health. Finally, the hypothesis presented in this review is also a great challenge of the field, which has been proposed for future studies to answer the question: “What is the impact of MSC sources on their downstream application?”. Tables 5 and 6 illustrate the comparative studies that were conducted in preclinical and clinical settings to address the MSC source challenge. Other challenges of MSC-based therapies have been discussed in several reviews and systematic studies, 135 , 185 , 438 , 439 which are highly recommended.
Limitations of the current hypothesis
The proposed hypothesis presented in this review was made based on (1) the calculated number of recovered patients from published clinical trials; (2) the empirical experience of the authors in the treatment of brain-related diseases, 440 pulmonary disorders, 215 and endocrinological conditions; 271 , 441 and (3) the proposed mechanisms by which each type of MSC exhibits its best potential for downstream applications. The authors understand that the approach that we used has a certain level of research bias, as a comprehensive meta-analysis is needed to first confirm the correlation between the origins of MSCs and their downstream clinical outcomes before a complete hypothesis can be made. However, to date, a limited number of clinical trials have been conducted to directly compare the efficacy of MSCs from different sources in treating the same disease, which in turn dampened our analysis to prove this hypothesis. In addition, MSC-based therapy is still in its early stages, as controversy and arguments are still present in the field, including (1) the name of MSCs (medicinal signaling cells vs. MSCs or mesenchymal stromal cells), 442 , 443 (2) the existence of “magic cells” (one cell type for the treatment of all diseases), 444 , 445 (3) the conflicting results from large-scale clinical trials, 135 and (4) the dangerous issues of unauthorized, unproven stem cell therapies and clinics. 446 , 447 Therefore, our hypothesis is proposed at this time to encourage active researchers and clinicians to either prove or disprove it so that future research can strengthen the uses of MSC-based therapies with solid mechanistic study results and clarify results for “one cell type for the treatment of all diseases”.
Another limitation is the knowledge coverage in the field of MSC-based regenerative medicine, as discussed in this study. First, the abovementioned diseases were narrowed to four major disease categories for which MSC-based therapy is widely applied, including neuronal, pulmonary, cardiovascular, and endocrinological conditions. In fact, other diseases also receive great benefits from MSC therapy, including liver cirrhosis, 448 bone regeneration, 360 plastic surgery, 449 autoimmune disease, 450 etc., which are not fully discussed in this review and included in our hypothesis. Recently, the secretome profile of MSCs and its potential application in clinical settings have emerged as a new player in the field, with a recently published comprehensive review including MSC-derived exosomes. 451 , 452 To date, the therapeutic potential of MSCs is believed to be strongly influenced by their secretomes, including growth factors, cytokines, chemokines, and exosomes. 453 However, this body of knowledge is also not fully included in our discussion, as this review focuses on the function and potency of MSCs as a whole with considerations derived from published clinical data. Therefore, the authors believe in and support the future applications of the secreted components derived from MSCs, including exosomes, in the treatment of human diseases. In fact, this potential approach could elevate the uses of MSCs to the next level, where the sources of MSCs could be neglected with advancements in the development of protocols that allow strict control of the secretome profiles of MSCs under specific conditions. 454 , 455 , 456 Finally, strategies that could potentially enhance the therapeutic outcomes of MSC-based therapy, such as the “priming” process, are not discussed in this review. The idea of “priming” MSCs is based on the nature of MSCs, which is similar to the immune cells, 457 that MSCs have proven to be able to “remember” the stimulus from the surrounding environment. 458 , 459 Thus, activating or priming MSCs using certain conditions, such as hypoxia, matrix mechanics, 3D environment, hormones, or inflammatory cytokines, could trigger the memory mechanism of the MSCs in vitro so that these cells are ready to function towards specific therapeutic activities without the need for in vivo activation. 3 , 460
From a cellular and molecular perspective and from our own experience in a clinical trial setting, AD-, BM- and UC-MSCs exhibit different functional activities and treatment effectiveness across a wide range of human diseases. In this paper, we have provided up-to-date data from the most recently published clinical trials conducted in neuronal diseases, endocrine and reproductive disorders, skin regeneration, pulmonary dysplasia, and cardiovascular diseases. The implications of the results and discussions presented in this review and in a very large body of comprehensive and excellent reviews as well as systematic analyses in the literature provide a different aspect and perspective on the use of MSCs from different sources in the treatment of human diseases. We strongly believe that the field of regenerative medicine and MSC-based therapy will benefit from active discussion, which in turn will significantly advance our knowledge of MSCs. Based on the proposed mechanisms presented in this review, we suggest several key mechanistic issues and questions that need to be addressed in the future:
The confirmation and demonstration of the mechanism of action prove that tissue origin plays a significant role in the downstream applications of the originated MSCs.
Is it required that MSCs derived from particular cell sources need to have certain functionalities that are unique to or superior in the original tissue sources?
As mechanisms may rely on the secretion of factors from MSCs, it is important to identify the specific stimuli from the wound environments to understand how MSCs from different sources can exhibit similar functions in the same disease and whether or not MSCs derived from a particular source have stronger effects than their counterparts derived from other tissue sources.
Should we create “universal” MSCs that could be functionally equal in the treatment of all diseases regardless of their origin by modeling their genetic materials?
Can new sources of MSCs from either perinatal or adult tissues better stimulate the innate mechanisms of specific cell types in our body, providing a better tool for MSC-based treatment?
A potential ‘priming’ protocol that allows priming, activating, and switching the potency of MSCs from one source to another with a more appropriate clinical phenotype to treat certain diseases. This idea is potentially relevant to our suggestion that each MSC type could be more beneficial in downstream applications, and the development of such a “priming” protocol would allow us to expand the bioavailability of specific MSC types.
From our clinical perspective, the underlying proposal in our review is to no longer use MSCs for applications while disregarding their sources but rather to match the MSC tissue source to the application, shifting from one cell type for the treatment of all diseases to cell source-specific disease treatments. Whether the application of MSCs from different sources still shows their effectiveness to a certain extent in the treatment of diseases or not, the transplantation of MSCs derived from different sources for each particular disease needs to be further investigated, and protocols need to be established via multicentre, randomized, placebo-controlled phase II and III clinical trials (Fig. 6 ).
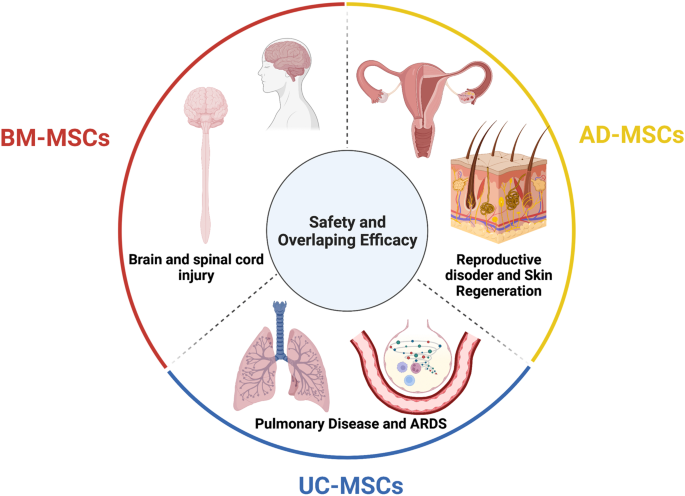
The tissue sources of mesenchymal stem cells (MSCs) contribute greatly to their therapeutic potential, as all MSC types share safety profiles and overlapping efficacy. Although a large body of data and their review and systematic analysis indicated the shared safety and potential efficacy of MSCs derived from different tissue sources, targeted therapies considering MSC origin as an important factor are imperative to enhance the downstream therapeutic effects of MSCs. We suggest that bone marrow-derived MSCs (BM-MSCs) are good candidates for the treatment of brain and spinal cord injury, adipose tissue-derived MSCs (AT-MSCs) are suitable for the treatment of reproductive disorders and skin regeneration, and umbilical cord-derived MSCs (UC-MSCs) could be alternatives for the treatment of pulmonary diseases and acute respiratory distress syndrome (ARDS). Figure was created with BioRender.com
Data availability
All data generated or analyzed in this study are included in this published article.
Mellman, I., Coukos, G. & Dranoff, G. Cancer immunotherapy comes of age. Nature 480 , 480–489 (2011).
Article PubMed PubMed Central CAS Google Scholar
Ancans, J. Cell therapy medicinal product regulatory framework in Europe and its application for MSC-based therapy development. Front. Immunol. 3 , 253 (2012).
Article PubMed PubMed Central Google Scholar
Yin, J. Q., Zhu, J. & Ankrum, J. A. Manufacturing of primed mesenchymal stromal cells for therapy. Nat. Biomed. Eng. 3 , 90–104 (2019).
Article PubMed CAS Google Scholar
O’Brien, T. & Barry, F. P. Stem cell therapy and regenerative medicine. Mayo Clin. Proc. 84 , 859–861 (2009).
Mousaei Ghasroldasht, M., Seok, J., Park, H. S., Liakath Ali, F. B. & Al-Hendy, A. Stem cell therapy: from idea to clinical practice. Int. J. Mol. Sci . 23 , 2850 (2022).
Kuriyan, A. E. et al. Vision loss after intravitreal injection of autologous “stem cells” for AMD. N. Engl. J. Med. 376 , 1047–1053 (2017).
Biehl, J. K. & Russell, B. Introduction to stem cell therapy. J. Cardiovasc. Nurs. 24 , 98–103 (2009). quiz 104-105.
Srijaya, T. C., Ramasamy, T. S. & Kasim, N. H. Advancing stem cell therapy from bench to bedside: lessons from drug therapies. J. Transl. Med. 12 , 243 (2014).
Ramalho-Santos, M. & Willenbring, H. On the origin of the term “stem cell”. Cell Stem Cell 1 , 35–38 (2007).
Konstantinov, I. E. In search of Alexander A. Maximow: the man behind the unitarian theory of hematopoiesis. Perspect. Biol. Med. 43 , 269–276 (2000).
Droscher, A. Images of cell trees, cell lines, and cell fates: the legacy of Ernst Haeckel and August Weismann in stem cell research. Hist. Philos. Life Sci. 36 , 157–186 (2014).
Article PubMed Google Scholar
Jansen, J. The first successful allogeneic bone-marrow transplant: Georges Mathe. Transfus. Med. Rev. 19 , 246–248 (2005).
Blume, K. G. & Weissman, I. L. E. Donnall Thomas (1920-2012). Proc. Natl Acad. Sci. USA 109 , 20777–20778 (2012).
Cheng, M. Hartmann Stahelin (1925-2011) and the contested history of cyclosporin A. Clin. Transpl. 27 , 326–329 (2013).
Article CAS Google Scholar
Thomas, E. D. et al. Aplastic anaemia treated by marrow transplantation. Lancet 1 , 284–289 (1972).
Friedenstein, A. J., Chailakhyan, R. K. & Gerasimov, U. V. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 20 , 263–272 (1987).
PubMed CAS Google Scholar
Friedenstein, A. J., Chailakhjan, R. K. & Lalykina, K. S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 3 , 393–403 (1970).
Caplan, A. I. Mesenchymal stem cells. J. Orthop. Res. 9 , 641–650 (1991).
Bolli, R., Tang, X. L., Guo, Y. & Li, Q. After the storm: an objective appraisal of the efficacy of c-kit+ cardiac progenitor cells in preclinical models of heart disease. Can. J. Physiol. Pharm. 99 , 129–139 (2021).
Liu, C., Han, D., Liang, P., Li, Y. & Cao, F. The current dilemma and breakthrough of stem cell therapy in ischemic heart disease. Front. Cell Dev. Biol. 9 , 636136 (2021).
Zhang, J. et al. Basic and translational research in cardiac repair and regeneration: JACC state-of-the-art review. J. Am. Coll. Cardiol. 78 , 2092–2105 (2021).
Gyongyosi, M., Wojakowski, W., Navarese, E. P., Moye, L. A. & Investigators, A. Meta-analyses of human cell-based cardiac regeneration therapies: controversies in meta-analyses results on cardiac cell-based regenerative studies. Circ. Res. 118 , 1254–1263 (2016).
Okamoto, R., Matsumoto, T. & Watanabe, M. Regeneration of the intestinal epithelia: regulation of bone marrow-derived epithelial cell differentiation towards secretory lineage cells. Hum. Cell 19 , 71–75 (2006).
Gehart, H. & Clevers, H. Tales from the crypt: new insights into intestinal stem cells. Nat. Rev. Gastroenterol. Hepatol. 16 , 19–34 (2019).
Santos, A. J. M., Lo, Y. H., Mah, A. T. & Kuo, C. J. The intestinal stem cell niche: homeostasis and adaptations. Trends Cell Biol. 28 , 1062–1078 (2018).
Roda, G. et al. Crohn’s disease. Nat. Rev. Dis. Prim. 6 , 22 (2020).
Kobayashi, T. et al. Ulcerative colitis. Nat. Rev. Dis. Prim. 6 , 74 (2020).
Gratwohl, A. et al. Autologous hematopoietic stem cell transplantation for autoimmune diseases. Bone Marrow Transpl. 35 , 869–879 (2005).
Kashyap, A. & Forman, S. J. Autologous bone marrow transplantation for non-Hodgkin’s lymphoma resulting in long-term remission of coincidental Crohn’s disease. Br. J. Haematol. 103 , 651–652 (1998).
Hurley, J. M., Lee, S. G., Andrews, R. E. Jr., Klowden, M. J. & Bulla, L. A. Jr. Separation of the cytolytic and mosquitocidal proteins of Bacillus thuringiensis subsp. israelensis. Biochem Biophys. Res. Commun. 126 , 961–965 (1985).
Oyama, Y. et al. Autologous hematopoietic stem cell transplantation in patients with refractory Crohn’s disease. Gastroenterology 128 , 552–563 (2005).
Burt, R. K. et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in patients with severe anti-TNF refractory Crohn disease: long-term follow-up. Blood 116 , 6123–6132 (2010).
Hasselblatt, P. et al. Remission of refractory Crohn’s disease by high-dose cyclophosphamide and autologous peripheral blood stem cell transplantation. Aliment Pharm. Ther. 36 , 725–735 (2012).
Hawkey, C. J. et al. Autologous hematopoetic stem cell transplantation for refractory Crohn disease: a randomized clinical trial. J. Am. Med. Assoc. 314 , 2524–2534 (2015).
Lindsay, J. O. et al. Autologous stem-cell transplantation in treatment-refractory Crohn’s disease: an analysis of pooled data from the ASTIC trial. Lancet Gastroenterol. Hepatol. 2 , 399–406 (2017).
Wang, R. et al. Stem cell therapy for Crohn’s disease: systematic review and meta-analysis of preclinical and clinical studies. Stem Cell Res Ther. 12 , 463 (2021).
Hawkey, C. J. Hematopoietic stem cell transplantation in Crohn’s disease: state-of-the-art treatment. Dig. Dis. 35 , 107–114 (2017).
Si-Tayeb, K., Lemaigre, F. P. & Duncan, S. A. Organogenesis and development of the liver. Dev. Cell 18 , 175–189 (2010).
Xue, R. et al. Clinical performance of stem cell therapy in patients with acute-on-chronic liver failure: a systematic review and meta-analysis. J. Transl. Med. 16 , 126 (2018).
Shi, M. et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl. Med. 1 , 725–731 (2012).
Liu, Y., Dong, Y., Wu, X., Xu, X. & Niu, J. The assessment of mesenchymal stem cells therapy in acute on chronic liver failure and chronic liver disease: a systematic review and meta-analysis of randomized controlled clinical trials. Stem Cell Res. Ther. 13 , 204 (2022).
Lin, B. L. et al. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: a randomized controlled trial. Hepatology 66 , 209–219 (2017).
Gordon, M. Y. et al. Characterization and clinical application of human CD34+ stem/progenitor cell populations mobilized into the blood by granulocyte colony-stimulating factor. Stem Cells 24 , 1822–1830 (2006).
Arroyo, V. et al. Acute-on-chronic liver failure in cirrhosis. Nat. Rev. Dis. Prim. 2 , 16041 (2016).
Zhang, Z. et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J. Gastroenterol. Hepatol. 27 (Suppl 2), 112–120 (2012).
El-Ansary, M. et al. Phase II trial: undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Rev. Rep. 8 , 972–981 (2012).
Xu, L. et al. Randomized trial of autologous bone marrow mesenchymal stem cells transplantation for hepatitis B virus cirrhosis: regulation of Treg/Th17 cells. J. Gastroenterol. Hepatol. 29 , 1620–1628 (2014).
Suk, K. T. et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology 64 , 2185–2197 (2016).
Fang, X. et al. A study about immunomodulatory effect and efficacy and prognosis of human umbilical cord mesenchymal stem cells in patients with chronic hepatitis B-induced decompensated liver cirrhosis. J. Gastroenterol. Hepatol. 33 , 774–780 (2018).
Mohamadnejad, M. et al. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. 33 , 1490–1496 (2013).
Nguyen, T. L. et al. Autologous bone marrow mononuclear cell infusion for liver cirrhosis after the Kasai operation in children with biliary atresia. Stem Cell Res. Ther. 13 , 108 (2022).
Bai, Y. Q. et al. Outcomes of autologous bone marrow mononuclear cell transplantation in decompensated liver cirrhosis. World J. Gastroenterol. 20 , 8660–8666 (2014).
Guo, C. et al. Long-term outcomes of autologous peripheral blood stem cell transplantation in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 17 , 1175–1182 e1172 (2019).
Newsome, P. N. et al. Granulocyte colony-stimulating factor and autologous CD133-positive stem-cell therapy in liver cirrhosis (REALISTIC): an open-label, randomised, controlled phase 2 trial. Lancet Gastroenterol. Hepatol. 3 , 25–36 (2018).
Spahr, L. et al. Autologous bone marrow mononuclear cell transplantation in patients with decompensated alcoholic liver disease: a randomized controlled trial. PLoS ONE 8 , e53719 (2013).
Maurice, J. & Manousou, P. Non-alcoholic fatty liver disease. Clin. Med. 18 , 245–250 (2018).
Article Google Scholar
Huang, T. D., Behary, J. & Zekry, A. Non-alcoholic fatty liver disease: a review of epidemiology, risk factors, diagnosis and management. Intern. Med. J. 50 , 1038–1047 (2020).
Sakai, Y. et al. Clinical trial of autologous adipose tissue-derived regenerative (stem) cells therapy for exploration of its safety and efficacy. Regen. Ther. 18 , 97–101 (2021).
Mieli-Vergani, G. et al. Autoimmune hepatitis. Nat. Rev. Dis. Primers 4 , 18018 (2018).
Calore, E. et al. Haploidentical stem cell transplantation cures autoimmune hepatitis and cerebrovascular disease in a patient with sickle cell disease. Bone Marrow Transpl. 53 , 644–646 (2018).
Vento, S., Cainelli, F., Renzini, C., Ghironzi, G. & Concia, E. Resolution of autoimmune hepatitis after bone-marrow transplantation. Lancet 348 , 544–545 (1996).
Terziroli Beretta-Piccoli, B., Mieli-Vergani, G. & Vergani, D. Autoimmmune hepatitis. Cell Mol. Immunol. 19 , 158–176 (2022).
Wang, L. et al. Pilot study of umbilical cord-derived mesenchymal stem cell transfusion in patients with primary biliary cirrhosis. J. Gastroenterol. Hepatol. 28 (Suppl 1), 85–92 (2013).
Wang, L. et al. Allogeneic bone marrow mesenchymal stem cell transplantation in patients with UDCA-resistant primary biliary cirrhosis. Stem Cells Dev. 23 , 2482–2489 (2014).
Martel-Pelletier, J. et al. Osteoarthritis. Nat. Rev. Dis. Prim. 2 , 16072 (2016).
Olsson, S., Akbarian, E., Lind, A., Razavian, A. S. & Gordon, M. Automating classification of osteoarthritis according to Kellgren-Lawrence in the knee using deep learning in an unfiltered adult population. BMC Musculoskelet. Disord. 22 , 844 (2021).
Mahmoudian, A., Lohmander, L. S., Mobasheri, A., Englund, M. & Luyten, F. P. Early-stage symptomatic osteoarthritis of the knee—time for action. Nat. Rev. Rheumatol. 17 , 621–632 (2021).
Kubsik-Gidlewska, A. et al. CD34+ stem cell treatment for knee osteoarthritis: a treatment and rehabilitation algorithm. J. Rehabil. Med Clin. Commun. 3 , 1000012 (2018).
Jevotovsky, D. S., Alfonso, A. R., Einhorn, T. A. & Chiu, E. S. Osteoarthritis and stem cell therapy in humans: a systematic review. Osteoarthr. Cartil. 26 , 711–729 (2018).
Wiggers, T. G., Winters, M., Van den Boom, N. A., Haisma, H. J. & Moen, M. H. Autologous stem cell therapy in knee osteoarthritis: a systematic review of randomised controlled trials. Br. J. Sports Med 55 , 1161–1169 (2021).
Han, S. B., Seo, I. W. & Shin, Y. S. Intra-articular injections of hyaluronic acid or steroids associated with better outcomes than platelet-rich plasma, adipose mesenchymal stromal cells, or placebo in knee osteoarthritis: a network meta-analysis. Arthroscopy 37 , 292–306 (2021).
Bastos, R. et al. Intra-articular injections of expanded mesenchymal stem cells with and without addition of platelet-rich plasma are safe and effective for knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 26 , 3342–3350 (2018).
Molnar, V. et al. Mesenchymal stem cell mechanisms of action and clinical effects in osteoarthritis: a narrative review. Genes 13 , 949 (2022).
Barisic, S. & Childs, R. W. Graft-versus-solid-tumor effect: from hematopoietic stem cell transplantation to adoptive cell therapies. Stem Cells 40 , 556–563 (2022).
Mello, M. M. & Brennan, T. A. The controversy over high-dose chemotherapy with autologous bone marrow transplant for breast cancer. Health Aff. (Millwood) 20 , 101–117 (2001).
Sissung, T. M. & Figg, W. D. Stem cell clinics: risk of proliferation. Lancet Oncol. 21 , 205–206 (2020).
Fu, X. et al. Mesenchymal stem cell migration and tissue repair. Cells 8 , 784 (2019).
Zachar, L., Bacenkova, D. & Rosocha, J. Activation, homing, and role of the mesenchymal stem cells in the inflammatory environment. J. Inflamm. Res. 9 , 231–240 (2016).
de Araujo Farias, V., Carrillo-Galvez, A. B., Martin, F. & Anderson, P. TGF-beta and mesenchymal stromal cells in regenerative medicine, autoimmunity and cancer. Cytokine Growth Factor Rev. 43 , 25–37 (2018).
Ding, W. et al. Platelet-derived growth factor (PDGF)-PDGF receptor interaction activates bone marrow-derived mesenchymal stromal cells derived from chronic lymphocytic leukemia: implications for an angiogenic switch. Blood 116 , 2984–2993 (2010).
Ritter, E. et al. Breast cancer cell-derived fibroblast growth factor 2 and vascular endothelial growth factor are chemoattractants for bone marrow stromal stem cells. Ann. Surg. 247 , 310–314 (2008).
Cronwright, G. et al. Cancer/testis antigen expression in human mesenchymal stem cells: down-regulation of SSX impairs cell migration and matrix metalloproteinase 2 expression. Cancer Res. 65 , 2207–2215 (2005).
Aldinucci, D., Borghese, C. & Casagrande, N. The CCL5/CCR5 axis in cancer progression. Cancers 12 , 1765 (2020).
Karnoub, A. E. et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449 , 557–563 (2007).
Kucerova, L., Matuskova, M., Hlubinova, K., Altanerova, V. & Altaner, C. Tumor cell behaviour modulation by mesenchymal stromal cells. Mol. Cancer 9 , 129 (2010).
Schmohl, K. A., Muller, A. M., Nelson, P. J. & Spitzweg, C. Thyroid hormone effects on mesenchymal stem cell biology in the tumour microenvironment. Exp. Clin. Endocrinol. Diabetes 128 , 462–468 (2020).
Mishra, P. J. et al. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 68 , 4331–4339 (2008).
Liu, J., Han, G., Liu, H. & Qin, C. Suppression of cholangiocarcinoma cell growth by human umbilical cord mesenchymal stem cells: a possible role of Wnt and Akt signaling. PLoS ONE 8 , e62844 (2013).
Ho, I. A. et al. Human bone marrow-derived mesenchymal stem cells suppress human glioma growth through inhibition of angiogenesis. Stem Cells 31 , 146–155 (2013).
Sun, Z., Wang, S. & Zhao, R. C. The roles of mesenchymal stem cells in tumor inflammatory microenvironment. J. Hematol. Oncol. 7 , 14 (2014).
Rhee, K. J., Lee, J. I. & Eom, Y. W. Mesenchymal stem cell-mediated effects of tumor support or suppression. Int. J. Mol. Sci. 16 , 30015–30033 (2015).
Liang, W. et al. Mesenchymal stem cells as a double-edged sword in tumor growth: focusing on MSC-derived cytokines. Cell Mol. Biol. Lett. 26 , 3 (2021).
Hmadcha, A., Martin-Montalvo, A., Gauthier, B. R., Soria, B. & Capilla-Gonzalez, V. Therapeutic potential of mesenchymal stem cells for cancer therapy. Front. Bioeng. Biotechnol. 8 , 43 (2020).
Cao, G. D. et al. The oncolytic virus in cancer diagnosis and treatment. Front. Oncol. 10 , 1786 (2020).
Melen, G. J. et al. Influence of carrier cells on the clinical outcome of children with neuroblastoma treated with high dose of oncolytic adenovirus delivered in mesenchymal stem cells. Cancer Lett. 371 , 161–170 (2016).
Garcia-Castro, J. et al. Treatment of metastatic neuroblastoma with systemic oncolytic virotherapy delivered by autologous mesenchymal stem cells: an exploratory study. Cancer Gene Ther. 17 , 476–483 (2010).
Draganov, D. D. et al. Delivery of oncolytic vaccinia virus by matched allogeneic stem cells overcomes critical innate and adaptive immune barriers. J. Transl. Med. 17 , 100 (2019).
Cyranoski, D. How human embryonic stem cells sparked a revolution. Nature 555 , 428–430 (2018).
Thomson, J. A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282 , 1145–1147 (1998).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126 , 663–676 (2006).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131 , 861–872 (2007).
Gepstein, L. Derivation and potential applications of human embryonic stem cells. Circ. Res. 91 , 866–876 (2002).
Andrews, P. W. From teratocarcinomas to embryonic stem cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 357 , 405–417 (2002).
Finch, B. W. & Ephrussi, B. Retention of multiple developmental potentialities by cells of a mouse testicular teratocarcinoma during prolonged culture in vitro and their extinction upon hybridization with cells of permanent lines. Proc. Natl Acad. Sci. USA 57 , 615–621 (1967).
Ried, T. et al. The consequences of chromosomal aneuploidy on the transcriptome of cancer cells. Biochim Biophys. Acta 1819 , 784–793 (2012).
Evans, M. J. & Kaufman, M. H. Establishment in culture of pluripotential cells from mouse embryos. Nature 292 , 154–156 (1981).
Martin, G. R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl Acad. Sci. USA 78 , 7634–7638 (1981).
Lo, B. & Parham, L. Ethical issues in stem cell research. Endocr. Rev. 30 , 204–213 (2009).
Wilmut, I., Schnieke, A. E., McWhir, J., Kind, A. J. & Campbell, K. H. Viable offspring derived from fetal and adult mammalian cells. Nature 385 , 810–813 (1997).
Schwartz, S. D. et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet 379 , 713–720 (2012).
Atala, A. Human embryonic stem cells: early hints on safety and efficacy. Lancet 379 , 689–690 (2012).
Schwartz, S. D. et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet 385 , 509–516 (2015).
Song, W. K. et al. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: preliminary results in Asian patients. Stem Cell Rep. 4 , 860–872 (2015).
Liu, Y. et al. Human embryonic stem cell-derived retinal pigment epithelium transplants as a potential treatment for wet age-related macular degeneration. Cell Discov. 4 , 50 (2018).
Limnios, I. J., Chau, Y. Q., Skabo, S. J., Surrao, D. C. & O’Neill, H. C. Efficient differentiation of human embryonic stem cells to retinal pigment epithelium under defined conditions. Stem Cell Res. Ther. 12 , 248 (2021).
Foltz, L. P. & Clegg, D. O. Rapid, directed differentiation of retinal pigment epithelial cells from human embryonic or induced pluripotent stem cells. J. Vis. Exp. 128 , e56274 (2017).
Kuroda, T., Ando, S., Takeno, Y., Kishino, A. & Kimura, T. Robust induction of retinal pigment epithelium cells from human induced pluripotent stem cells by inhibiting FGF/MAPK signaling. Stem Cell Res 39 , 101514 (2019).
Dewell, T. E. et al. Transcription factor overexpression drives reliable differentiation of retinal pigment epithelium from human induced pluripotent stem cells. Stem Cell Res. 53 , 102368 (2021).
Dehghan, S., Mirshahi, R., Shoae-Hassani, A. & Naseripour, M. Human-induced pluripotent stem cells-derived retinal pigmented epithelium, a new horizon for cells-based therapies for age-related macular degeneration. Stem Cell Res. Ther. 13 , 217 (2022).
Menasche, P. et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur. Heart J. 36 , 2011–2017 (2015).
Menasche, P. et al. Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J. Am. Coll. Cardiol. 71 , 429–438 (2018).
Cyranoski, D. ‘Reprogrammed’ stem cells approved to mend human hearts for the first time. Nature 557 , 619–620 (2018).
Povsic, T. J. & Gersh, B. J. Stem cells in cardiovascular diseases: 30,000-foot view. Cells 10 , 600 (2021).
Romito, A. & Cobellis, G. Pluripotent stem cells: current understanding and future directions. Stem Cells Int. 2016 , 9451492 (2016).
McKenna, S. L. et al. Ten-year safety of pluripotent stem cell transplantation in acute thoracic spinal cord injury. J. Neurosurg. Spine 1 , 1–10 (2022).
Deinsberger, J., Reisinger, D. & Weber, B. Global trends in clinical trials involving pluripotent stem cells: a systematic multi-database analysis. NPJ Regen. Med. 5 , 15 (2020).
Kim, J. Y., Nam, Y., Rim, Y. A. & Ju, J. H. Review of the current trends in clinical trials involving induced pluripotent stem cells. Stem Cell Rev. Rep. 18 , 142–154 (2022).
Ji, P., Manupipatpong, S., Xie, N. & Li, Y. Induced pluripotent stem cells: generation strategy and epigenetic mystery behind reprogramming. Stem Cells Int. 2016 , 8415010 (2016).
Fu, X. The immunogenicity of cells derived from induced pluripotent stem cells. Cell Mol. Immunol. 11 , 14–16 (2014).
Lee, A. S., Tang, C., Rao, M. S., Weissman, I. L. & Wu, J. C. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat. Med. 19 , 998–1004 (2013).
Friedenstein, A. J., Piatetzky, S. II & Petrakova, K. V. Osteogenesis in transplants of bone marrow cells. J. Embryol. Exp. Morphol. 16 , 381–390 (1966).
Pittenger, M. F. et al. Multilineage potential of adult human mesenchymal stem cells. Science 284 , 143–147 (1999).
Nombela-Arrieta, C., Ritz, J. & Silberstein, L. E. The elusive nature and function of mesenchymal stem cells. Nat. Rev. Mol. Cell Biol. 12 , 126–131 (2011).
Dominici, M. et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8 , 315–317 (2006).
Zhou, T. et al. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 14 , 24 (2021).
Ankrum, J. & Karp, J. M. Mesenchymal stem cell therapy: two steps forward, one step back. Trends Mol. Med. 16 , 203–209 (2010).
Tuan, R. S., Boland, G. & Tuli, R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res. Ther. 5 , 32–45 (2003).
Witkowska-Zimny, M. & Wrobel, E. Perinatal sources of mesenchymal stem cells: Wharton’s jelly, amnion and chorion. Cell Mol. Biol. Lett. 16 , 493–514 (2011).
Alkhalil, M., Smajilagic, A. & Redzic, A. Human dental pulp mesenchymal stem cells isolation and osteoblast differentiation. Med. Glas. (Zenica) 12 , 27–32 (2015).
Google Scholar
Ouryazdanpanah, N., Dabiri, S., Derakhshani, A., Vahidi, R. & Farsinejad, A. Peripheral blood-derived mesenchymal stem cells: growth factor-free isolation, molecular characterization and differentiation. Iran. J. Pathol. 13 , 461–466 (2018).
PubMed PubMed Central Google Scholar
Francis, M. P., Sachs, P. C., Elmore, L. W. & Holt, S. E. Isolating adipose-derived mesenchymal stem cells from lipoaspirate blood and saline fraction. Organogenesis 6 , 11–14 (2010).
Gong, X. et al. Isolation and characterization of lung resident mesenchymal stem cells capable of differentiating into alveolar epithelial type II cells. Cell Biol. Int. 38 , 405–411 (2014).
Wang, B. et al. Human hair follicle-derived mesenchymal stem cells: Isolation, expansion, and differentiation. World J. Stem Cells 12 , 462–470 (2020).
Pilato, C. A. et al. Isolation and characterization of cardiac mesenchymal stromal cells from endomyocardial bioptic samples of arrhythmogenic cardiomyopathy patients. J. Vis. Exp . 132 , e57263 (2018).
Mannino, G. et al. Adult stem cell niches for tissue homeostasis. J. Cell Physiol. 237 , 239–257 (2022).
Pavlushina, S. V., Orlova, T. G. & Tabagari, D. Z. [Isolation of mononuclear cells from the bone marrow of patients with hemoblastoses using one-step ficoll-verographin density gradient separation]. Eksp. Onkol. 6 , 68–70 (1984).
Schneider, S., Unger, M., van Griensven, M. & Balmayor, E. R. Adipose-derived mesenchymal stem cells from liposuction and resected fat are feasible sources for regenerative medicine. Eur. J. Med Res. 22 , 17 (2017).
Torre, P. & Flores, A. I. Current status and future prospects of perinatal stem cells. Genes 12 , 6 (2020).
Hoang, V. T. et al. Standardized xeno- and serum-free culture platform enables large-scale expansion of high-quality mesenchymal stem/stromal cells from perinatal and adult tissue sources. Cytotherapy 23 , 88–99 (2020).
Mohamed-Ahmed, S. et al. Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison. Stem Cell Res. Ther. 9 , 168 (2018).
Zuk, P. A. et al. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 13 , 4279–4295 (2002).
Li, Z. CD133: a stem cell biomarker and beyond. Exp. Hematol. Oncol. 2 , 17 (2013).
Petrenko, Y. et al. A comparative analysis of multipotent mesenchymal stromal cells derived from different sources, with a focus on neuroregenerative potential. Sci. Rep. 10 , 4290 (2020).
Wang, Z. & Yan, X. CD146, a multi-functional molecule beyond adhesion. Cancer Lett. 330 , 150–162 (2013).
Xu, L. et al. Tissue source determines the differentiation potentials of mesenchymal stem cells: a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res Ther. 8 , 275 (2017).
Han, I., Kwon, B. S., Park, H. K. & Kim, K. S. Differentiation potential of mesenchymal stem cells is related to their intrinsic mechanical properties. Int. Neurourol. J. 21 , S24–S31 (2017).
Song, Y. et al. Human mesenchymal stem cells derived from umbilical cord and bone marrow exert immunomodulatory effects in different mechanisms. World J. Stem Cells 12 , 1032–1049 (2020).
Lee, R. H. et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 5 , 54–63 (2009).
Allers, C. et al. Dynamic of distribution of human bone marrow-derived mesenchymal stem cells after transplantation into adult unconditioned mice. Transplantation 78 , 503–508 (2004).
Devine, S. M., Cobbs, C., Jennings, M., Bartholomew, A. & Hoffman, R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood 101 , 2999–3001 (2003).
Fischer, U. M. et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 18 , 683–692 (2009).
Sierra-Parraga, J. M. et al. Mesenchymal stromal cells are retained in the porcine renal cortex independently of their metabolic state after renal intra-arterial infusion. Stem Cells Dev. 28 , 1224–1235 (2019).
Henriksson, H. B. et al. The traceability of mesenchymal stromal cells after injection into degenerated discs in patients with low back pain. Stem Cells Dev. 28 , 1203–1211 (2019).
Sokal, E. M. et al. Biodistribution of liver-derived mesenchymal stem cells after peripheral injection in a hemophilia A patient. Transplantation 101 , 1845–1851 (2017).
Sood, V. et al. Biodistribution of 18F-FDG-labeled autologous bone marrow-derived stem cells in patients with type 2 diabetes mellitus: exploring targeted and intravenous routes of delivery. Clin. Nucl. Med. 40 , 697–700 (2015).
Sanchez-Diaz, M. et al. Biodistribution of mesenchymal stromal cells after administration in animal models and humans: a systematic review. J. Clin. Med. 10 , 2925 (2021).
Sensebe, L. & Fleury-Cappellesso, S. Biodistribution of mesenchymal stem/stromal cells in a preclinical setting. Stem Cells Int. 2013 , 678063 (2013).
Zhuang, W. Z. et al. Mesenchymal stem/stromal cell-based therapy: mechanism, systemic safety and biodistribution for precision clinical applications. J. Biomed. Sci. 28 , 28 (2021).
Wei, X. et al. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharm. Sin. 34 , 747–754 (2013).
Kouchakian, M. R. et al. The clinical trials of mesenchymal stromal cells therapy. Stem Cells Int. 2021 , 1634782 (2021).
Chen, L. et al. Mesenchymal stem cell-based treatments for COVID-19: status and future perspectives for clinical applications. Cell Mol. Life Sci. 79 , 142 (2022).
Borow, K. M., Yaroshinsky, A., Greenberg, B. & Perin, E. C. Phase 3 DREAM-HF trial of mesenchymal precursor cells in chronic heart failure. Circ. Res. 125 , 265–281 (2019).
Zheng, H. et al. Mesenchymal stem cell therapy in stroke: a systematic review of literature in pre-clinical and clinical research. Cell Transpl. 27 , 1723–1730 (2018).
Rodriguez-Fuentes, D. E. et al. Mesenchymal stem cells current clinical applications: a systematic review. Arch. Med. Res. 52 , 93–101 (2021).
Shi, L. et al. Mesenchymal stem cell therapy for severe COVID-19. Signal Transduct. Target Ther. 6 , 339 (2021).
Carney, B. J. & Shah, K. Migration and fate of therapeutic stem cells in different brain disease models. Neuroscience 197 , 37–47 (2011).
Yao, P., Zhou, L., Zhu, L., Zhou, B. & Yu, Q. Mesenchymal stem cells: a potential therapeutic strategy for neurodegenerative diseases. Eur. Neurol. 83 , 235–241 (2020).
Bonaventura, G. et al. Stem cells: innovative therapeutic options for neurodegenerative diseases? Cells 10 , 1992 (2021).
Mansoor, S. R., Zabihi, E. & Ghasemi-Kasman, M. The potential use of mesenchymal stem cells for the treatment of multiple sclerosis. Life Sci. 235 , 116830 (2019).
Chung, J. W. et al. Efficacy and safety of intravenous mesenchymal stem cells for ischemic stroke. Neurology 96 , e1012–e1023 (2021).
Yamazaki, K., Kawabori, M., Seki, T. & Houkin, K. Clinical trials of stem cell treatment for spinal cord injury. Int. J. Mol. Sci . 21 , 3994 (2020).
Xie, B., Chen, M., Hu, R., Han, W. & Ding, S. Therapeutic evidence of human mesenchymal stem cell transplantation for cerebral palsy: a meta-analysis of randomized controlled trials. Stem Cells Int. 2020 , 5701920 (2020).
McDonald, C. A. et al. Intranasal delivery of mesenchymal stromal cells protects against neonatal hypoxic(-)ischemic brain injury. Int. J. Mol. Sci . 20 , 2449 (2019).
Liu, Q. et al. Rational use of mesenchymal stem cells in the treatment of autism spectrum disorders. World J. Stem Cells 11 , 55–72 (2019).
Fricova, D., Korchak, J. A. & Zubair, A. C. Challenges and translational considerations of mesenchymal stem/stromal cell therapy for Parkinson’s disease. npj Regen. Med. 5 , 20 (2020).
Bang, O. Y., Lee, J. S., Lee, P. H. & Lee, G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 57 , 874–882 (2005).
Lee, J. S. et al. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 28 , 1099–1106 (2010).
Honmou, O. et al. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain 134 , 1790–1807 (2011).
Bhasin, A. et al. Autologous mesenchymal stem cells in chronic stroke. Cerebrovasc. Dis. Extra 1 , 93–104 (2011).
Jaillard, A. et al. Autologous mesenchymal stem cells improve motor recovery in subacute ischemic stroke: a randomized clinical trial. Transl. Stroke Res. 11 , 910–923 (2020).
Lee, J. et al. Efficacy of intravenous mesenchymal stem cells for motor recovery after ischemic stroke: a neuroimaging study. Stroke 53 , 20–28 (2022).
Levy, M. L. et al. Phase I/II study of safety and preliminary efficacy of intravenous allogeneic mesenchymal stem cells in chronic stroke. Stroke 50 , 2835–2841 (2019).
Xu, P. & Yang, X. The efficacy and safety of mesenchymal stem cell transplantation for spinal cord injury patients: a meta-analysis and systematic review. Cell Transpl. 28 , 36–46 (2019).
Liau, L. L. et al. Treatment of spinal cord injury with mesenchymal stem cells. Cell Biosci. 10 , 112 (2020).
Liu, X. et al. Comparative analysis of curative effect of bone marrow mesenchymal stem cell and bone marrow mononuclear cell transplantation for spastic cerebral palsy. J. Transl. Med. 15 , 1–9 (2017).
Sharma, A. K. et al. Cell transplantation as a novel therapeutic strategy for autism spectrum disorders: a clinical study. Am J. Stem Cells 9 , 89 (2020).
Ballen, K. & Kurtzberg, J. Exploring new therapies for children with autism: “Do no harm” does not mean do not try. Stem Cells Transl. Med. 10 , 823–825 (2021).
Reyhani, S., Abbaspanah, B. & Mousavi, S. H. Umbilical cord-derived mesenchymal stem cells in neurodegenerative disorders: from literature to clinical practice. Regen. Med. 15 , 1561–1578 (2020).
Gu, J. et al. Therapeutic evidence of umbilical cord-derived mesenchymal stem cell transplantation for cerebral palsy: a randomized, controlled trial. Stem Cell Res Ther. 11 , 43 (2020).
Retraction. Stem Cells Transl. Med. 10 , 1717 (2021).
Sun, J. M. et al. Infusion of human umbilical cord tissue mesenchymal stromal cells in children with autism spectrum disorder. Stem Cells Transl. Med. 9 , 1137–1146 (2020).
Yang, Y. et al. Repeated subarachnoid administrations of allogeneic human umbilical cord mesenchymal stem cells for spinal cord injury: a phase 1/2 pilot study. Cytotherapy 23 , 57–64 (2021).
Liu, J. et al. Clinical analysis of the treatment of spinal cord injury with umbilical cord mesenchymal stem cells. Cytotherapy 15 , 185–191 (2013).
Przekora, A. & Juszkiewicz, L. The effect of autologous adipose tissue-derived mesenchymal stem cells’ therapy in the treatment of chronic posttraumatic spinal cord injury in a domestic ferret patient. Cell Transpl. 29 , 963689720928982 (2020).
Hur, J. W. et al. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: a human trial. J. Spinal Cord. Med. 39 , 655–664 (2016).
de Celis-Ruiz, E. et al. Final results of allogeneic adipose tissue-derived mesenchymal stem cells in acute ischemic stroke (AMASCIS): a phase II, randomized, double-blind, placebo-controlled, single-center, pilot clinical trial. Cell Transpl. 31 , 9636897221083863 (2022).
Yang, Y. et al. Human umbilical cord mesenchymal stem cells to treat spinal cord injury in the early chronic phase: study protocol for a prospective, multicenter, randomized, placebo-controlled, single-blinded clinical trial. Neural Regen. Res. 15 , 1532–1538 (2020).
de Celis-Ruiz, E. et al. Allogeneic adipose tissue-derived mesenchymal stem cells in ischaemic stroke (AMASCIS-02): a phase IIb, multicentre, double-blind, placebo-controlled clinical trial protocol. BMJ Open 11 , e051790 (2021).
Murray, C. J. L. COVID-19 will continue but the end of the pandemic is near. Lancet 399 , 417–419 (2022).
Thebaud, B. et al. Bronchopulmonary dysplasia. Nat. Rev. Dis. Prim. 5 , 78 (2019).
Mohamed, T., Abdul-Hafez, A., Gewolb, I. H. & Uhal, B. D. Oxygen injury in neonates: which is worse? hyperoxia, hypoxia, or alternating hyperoxia/hypoxia. J. Lung Pulm. Respir. Res. 7 , 4–13 (2020).
Omar, S. A. et al. Stem-cell therapy for bronchopulmonary dysplasia (BPD) in newborns. Cells 11 , 1275 (2022).
Chang, Y. S. et al. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J. Pediatr. 164 , 966–972 e966 (2014).
Powell, S. B. & Silvestri, J. M. Safety of intratracheal administration of human umbilical cord blood derived mesenchymal stromal cells in extremely low birth weight preterm infants. J. Pediatr. 210 , 209–213 e202 (2019).
Nguyen, L. T. et al. Allogeneic administration of human umbilical cord-derived mesenchymal stem/stromal cells for bronchopulmonary dysplasia: preliminary outcomes in four Vietnamese infants. J. Transl. Med. 18 , 398 (2020).
Ahn, S. Y. et al. Stem cells for bronchopulmonary dysplasia in preterm infants: a randomized controlled phase II trial. Stem Cells Transl. Med. 10 , 1129–1137 (2021).
Averyanov, A. et al. First-in-human high-cumulative-dose stem cell therapy in idiopathic pulmonary fibrosis with rapid lung function decline. Stem Cells Transl. Med. 9 , 6–16 (2020).
Ribeiro-Paes, J. T. et al. Unicentric study of cell therapy in chronic obstructive pulmonary disease/pulmonary emphysema. Int. J. Chron. Obstruct Pulmon Dis. 6 , 63–71 (2011).
Stessuk, T. et al. Phase I clinical trial of cell therapy in patients with advanced chronic obstructive pulmonary disease: follow-up of up to 3 years. Rev. Bras. Hematol. Hemoter. 35 , 352–357 (2013).
Weiss, D. J., Casaburi, R., Flannery, R., LeRoux-Williams, M. & Tashkin, D. P. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest 143 , 1590–1598 (2013).
de Oliveira, H. G. et al. Combined bone marrow-derived mesenchymal stromal cell therapy and one-way endobronchial valve placement in patients with pulmonary emphysema: a phase I clinical trial. Stem Cells Transl. Med. 6 , 962–969 (2017).
Stolk, J. et al. A phase I study for intravenous autologous mesenchymal stromal cell administration to patients with severe emphysema. QJM 109 , 331–336 (2016).
Armitage, J. et al. Mesenchymal stromal cell infusion modulates systemic immunological responses in stable COPD patients: a phase I pilot study. Eur. Respir. J. 51 , 1702369 (2018).
Comella, K. et al. Autologous stromal vascular fraction in the intravenous treatment of end-stage chronic obstructive pulmonary disease: a phase I trial of safety and tolerability. J. Clin. Med. Res. 9 , 701–708 (2017).
Tzilas, V. et al . Prospective phase 1 open clinical trial to study the safety of adipose derived mesenchymal stem cells (ADMSCs) in COPD and combined pulmonary fibrosis and emphysema (CPFE). Eur. Respir. J. 46 , (2015).
Glassberg, M. K., Csete, I., Simonet, E. & Elliot, S. J. Stem cell therapy for COPD: hope and exploitation. Chest 160 , 1271–1281 (2021).
Le Thi Bich, P. et al. Allogeneic umbilical cord-derived mesenchymal stem cell transplantation for treating chronic obstructive pulmonary disease: a pilot clinical study. Stem Cell Res. Ther. 60 , 11 (2020).
Karaoz, E., Kalemci, S. & Ece, F. Improving effects of mesenchymal stem cells on symptoms of chronic obstructive pulmonary disease. Bratisl. Lek. Listy. 121 , 188–191 (2020).
Hoang, D. M., Nguyen, K. T., Nguyen, A. H., Nguyen, B. N. & Nguyen, L. T. Allogeneic human umbilical cord-derived mesenchymal stem/stromal cells for chronic obstructive pulmonary disease (COPD): study protocol for a matched case-control, phase I/II trial. BMJ Open 11 , e045788 (2021).
Xu, R., Feng, Z. & Wang, F. S. Mesenchymal stem cell treatment for COVID-19. EBioMedicine 77 , 103920 (2022).
Khoury, M. et al. Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. Eur. Respir. J. 55 , 2000858 (2020).
Jamilloux, Y. et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun. Rev. 19 , 102567 (2020).
Feng, Y. et al. Safety and feasibility of umbilical cord mesenchymal stem cells in patients with COVID-19 pneumonia: a pilot study. Cell Prolif. 53 , e12947 (2020).
Primorac, D. et al. Mesenchymal stromal cells: potential option for COVID-19 treatment. Pharmaceutic 13 , 1481 (2021).
Zhang, Y. et al. Intravenous infusion of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells as a potential treatment for patients with COVID-19 pneumonia. Stem Cell Res. Ther. 11 , 207 (2020).
Shu, L. et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 11 , 361 (2020).
Tao, J. et al. Umbilical cord blood-derived mesenchymal stem cells in treating a critically ill COVID-19 patient. J. Infect. Dev. Ctries 14 , 1138–1145 (2020).
Saleh, M. et al. Cell therapy in patients with COVID-19 using Wharton’s jelly mesenchymal stem cells: a phase 1 clinical trial. Stem Cell Res. Ther. 12 , 410 (2021).
Leng, Z. et al. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 11 , 216–228 (2020).
Guo, Z. et al. Administration of umbilical cord mesenchymal stem cells in patients with severe COVID-19 pneumonia. Crit. Care 24 , 420 (2020).
Meng, F. et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduct. Target Ther. 5 , 172 (2020).
Shi, L. et al. Human mesenchymal stem cells treatment for severe COVID-19: 1-year follow-up results of a randomized, double-blind, placebo-controlled trial. EBioMedicine 75 , 103789 (2021).
Adas, G. et al. The systematic effect of mesenchymal stem cell therapy in critical COVID-19 patients: a prospective double controlled trial. Cell Transpl. 30 , 9636897211024942 (2021).
Shi, L. et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct. Targeted Ther. 6 , 58 (2021).
Lanzoni, G. et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl. Med. 10 , 660–673 (2021).
Hashemian, S. R. et al. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series. Stem Cell Res Ther. 12 , 91 (2021).
Zhu, R. et al. Mesenchymal stem cell treatment improves outcome of COVID-19 patients via multiple immunomodulatory mechanisms. Cell Res. 31 , 1244–1262 (2021).
Shi, L. et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct. Target Ther. 6 , 58 (2021).
N, O. E., Pekkoc-Uyanik, K. C., Alpaydin, N., Gulay, G. R. & Simsek, M. Clinical experience on umbilical cord mesenchymal stem cell treatment in 210 severe and critical COVID-19 cases in Turkey. Stem Cell Rev. Rep. 17 , 1917–1925 (2021).
Gentile, P., Sterodimas, A., Pizzicannella, J., Calabrese, C. & Garcovich, S. Research progress on mesenchymal stem cells (MSCs), adipose-derived mesenchymal stem cells (AD-MSCs), drugs, and vaccines in inhibiting COVID-19 disease. Aging Dis. 11 , 1191–1201 (2020).
Copcu, H. E. Potential using of fat-derived stromal cells in the treatment of active disease, and also, in both pre- and post-periods in COVID-19. Aging Dis. 11 , 730–736 (2020).
Gentile, P. & Sterodimas, A. Adipose-derived stromal stem cells (ASCs) as a new regenerative immediate therapy combating coronavirus (COVID-19)-induced pneumonia. Expert Opin. Biol. Ther. 20 , 711–716 (2020).
Matthay, M. A. et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir. Med. 7 , 154–162 (2019).
Álvarez-Fuente, M. et al. Off-label mesenchymal stromal cell treatment in two infants with severe bronchopulmonary dysplasia: clinical course and biomarkers profile. Cytotherapy 20 , 1337–1344 (2018).
Zheng, G. et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir. Res. 15 , 39 (2014).
Simonson, O. E. et al. In vivo effects of mesenchymal stromal cells in two patients with severe acute respiratory distress syndrome. Stem Cells Transl. Med. 4 , 1199–1213 (2015).
Wilson, J. G. et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir. Med. 3 , 24–32 (2015).
Yip, H. K. et al. Human umbilical cord-derived mesenchymal stem cells for acute respiratory distress syndrome. Crit. Care Med 48 , e391–e399 (2020).
Gorman, E. et al. Repair of acute respiratory distress syndrome by stromal cell administration (REALIST) trial: a phase 1 trial. EClinicalMedicine 41 , 101167 (2021).
Le Thi Bich, P. et al. Allogeneic umbilical cord-derived mesenchymal stem cell transplantation for treating chronic obstructive pulmonary disease: a pilot clinical study. Stem Cell Res. Ther. 11 , 60 (2020).
Wang, M. Y. et al. Current therapeutic strategies for respiratory diseases using mesenchymal stem cells. MedComm 2 , 351–380 (2021).
Carlsson, P. O., Schwarcz, E., Korsgren, O. & Le Blanc, K. Preserved beta-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes 64 , 587–592 (2015).
Dantas, J. R. et al. Adipose tissue-derived stromal/stem cells + cholecalciferol: a pilot study in recent-onset type 1 diabetes patients. Arch. Endocrinol. Metab. 65 , 342–351 (2021).
PubMed Google Scholar
Joseph, U. A. & Jhingran, S. G. Technetium-99m labeled RBC imaging in gastrointestinal bleeding from gastric leiomyoma. Clin. Nucl. Med. 13 , 23–25 (1988).
Hu, J. et al. Long term effects of the implantation of Wharton’s jelly-derived mesenchymal stem cells from the umbilical cord for newly-onset type 1 diabetes mellitus. Endocr. J. 60 , 347–357 (2013).
Cai, J. et al. Umbilical cord mesenchymal stromal cell with autologous bone marrow cell transplantation in established type 1 diabetes: a pilot randomized controlled open-label clinical study to assess safety and impact on insulin secretion. Diabetes Care 39 , 149–157 (2016).
Huang, Q., Huang, Y. & Liu, J. Mesenchymal stem cells: an excellent candidate for the treatment of diabetes mellitus. Int. J. Endocrinol. 2021 , 9938658 (2021).
Nguyen, L. T. et al. Type 2 diabetes mellitus duration and obesity alter the efficacy of autologously transplanted bone marrow-derived mesenchymal stem/stromal cells. Stem Cells Transl. Med. 10 , 1266–1278 (2021).
Alicka, M., Major, P., Wysocki, M. & Marycz, K. Adipose-derived mesenchymal stem cells isolated from patients with type 2 diabetes show reduced “stemness” through an altered secretome profile, impaired anti-oxidative protection, and mitochondrial dynamics deterioration. J. Clin. Med. 8 , 765 (2019).
Agarwal, A. et al. Male infertility. Lancet 397 , 319–333 (2021).
Farquhar, C. & Marjoribanks, J. Assisted reproductive technology: an overview of Cochrane reviews. Cochrane Database Syst. Rev. 8 , CD010537 (2018).
Chang, Z. et al. Mesenchymal stem cells in preclinical infertility cytotherapy: a retrospective review. Stem Cells Int. 2021 , 8882368 (2021).
Fenton, A. J. Premature ovarian insufficiency: pathogenesis and management. J. Midlife Health 6 , 147–153 (2015).
Coulam, C. B. Premature gonadal failure. Fertil. Steril. 38 , 645–655 (1982).
Huhtaniemi, I. et al. Advances in the molecular pathophysiology, genetics, and treatment of primary ovarian insufficiency. Trends Endocrinol. Metab. 29 , 400–419 (2018).
Torrealday, S., Kodaman, P. & Pal, L. Premature ovarian Insufficiency—an update on recent advances in understanding and management. F1000Res 6 , 2069 (2017).
Gupta, S., Lodha, P., Karthick, M. S. & Tandulwadkar, S. R. Role of autologous bone marrow-derived stem cell therapy for follicular recruitment in premature ovarian insufficiency: review of literature and a case report of world’s first baby with ovarian autologous stem cell therapy in a perimenopausal woman of age 45 year. J. Hum. Reprod. Sci. 11 , 125–130 (2018).
Igboeli, P. et al. Intraovarian injection of autologous human mesenchymal stem cells increases estrogen production and reduces menopausal symptoms in women with premature ovarian failure: two case reports and a review of the literature. J. Med. Case Rep. 14 , 108 (2020).
Ulin, M. et al. Human mesenchymal stem cell therapy and other novel treatment approaches for premature ovarian insufficiency. Reprod. Sci. 28 , 1688–1696 (2021).
Herraiz, S. et al. Autologous stem cell ovarian transplantation to increase reproductive potential in patients who are poor responders. Fertil. Steril. 110 , 496–505 e491 (2018).
Ding, L. et al. Transplantation of UC-MSCs on collagen scaffold activates follicles in dormant ovaries of POF patients with long history of infertility. Sci. China Life Sci. 61 , 1554–1565 (2018).
Wang, M. Y., Wang, Y. X., Li-Ling, J. & Xie, H. Q. Adult stem cell therapy for premature ovarian failure: from bench to bedside. Tissue Eng. Part B Rev. 28 , 63–78 (2022).
Kaddoura, I., Abu-Sittah, G., Ibrahim, A., Karamanoukian, R. & Papazian, N. Burn injury: review of pathophysiology and therapeutic modalities in major burns. Ann. Burns Fire Disasters 30 , 95–102 (2017).
PubMed PubMed Central CAS Google Scholar
Jeschke, M. G. et al. Burn injury. Nat. Rev. Dis. Prim. 6 , 11 (2020).
Rasulov, M. F. et al. First experience of the use bone marrow mesenchymal stem cells for the treatment of a patient with deep skin burns. Bull. Exp. Biol. Med. 139 , 141–144 (2005).
Mansilla, E. et al. Cadaveric bone marrow mesenchymal stem cells: first experience treating a patient with large severe burns. Burns Trauma 3 , 17 (2015).
Xu, Y., Huang, S. & Fu, X. Autologous transplantation of bone marrow-derived mesenchymal stem cells: a promising therapeutic strategy for prevention of skin-graft contraction. Clin. Exp. Dermatol. 37 , 497–500 (2012).
Yoshikawa, T. et al. Wound therapy by marrow mesenchymal cell transplantation. Plast. Reconstr. Surg. 121 , 860–877 (2008).
Abo-Elkheir, W. et al. Role of cord blood and bone marrow mesenchymal stem cells in recent deep burn: a case-control prospective study. Am. J. Stem Cells 6 , 23–35 (2017).
Li, L. et al. Conditioned medium from human adipose-derived mesenchymal stem cell culture prevents UVB-induced skin aging in human keratinocytes and dermal fibroblasts. Int. J. Mol. Sci. 21 , 49 (2019).
Lotfi, M. et al. Adipose tissue-derived mesenchymal stem cells and keratinocytes co-culture on gelatin/chitosan/beta-glycerol phosphate nanoscaffold in skin regeneration. Cell Biol. Int. 43 , 1365–1378 (2019).
Yang, J. A., Chung, H. M., Won, C. H. & Sung, J. H. Potential application of adipose-derived stem cells and their secretory factors to skin: discussion from both clinical and industrial viewpoints. Expert Opin. Biol. Ther. 10 , 495–503 (2010).
Zhou, Y. et al. Combined topical and systemic administration with human adipose-derived mesenchymal stem cells (hADSC) and hADSC-derived exosomes markedly promoted cutaneous wound healing and regeneration. Stem Cell Res. Ther. 12 , 257 (2021).
Arjmand, B. et al. Regenerative medicine for the treatment of ischemic heart disease; status and future perspectives. Front. Cell Dev. Biol. 9 , 704903 (2021).
Denning, C. et al. Cardiomyocytes from human pluripotent stem cells: from laboratory curiosity to industrial biomedical platform. Biochim Biophys. Acta 1863 , 1728–1748 (2016).
Wu, R., Hu, X. & Wang, J. Concise review: optimized strategies for stem cell-based therapy in myocardial repair: clinical translatability and potential limitation. Stem Cells 36 , 482–500 (2018).
Chong, J. J. et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510 , 273–277 (2014).
Bagno, L., Hatzistergos, K. E., Balkan, W. & Hare, J. M. Mesenchymal stem cell-based therapy for cardiovascular disease: progress and challenges. Mol. Ther. 26 , 1610–1623 (2018).
Demurtas, J. et al. Stem cells for treatment of cardiovascular diseases: an umbrella review of randomized controlled trials. Ageing Res. Rev. 67 , 101257 (2021).
Gubert, F. et al. Mesenchymal stem cells therapies on fibrotic heart diseases. Int. J. Mol. Sci. 22 , 7447 (2021).
da Silva, J. S. et al. Mesenchymal stem cell therapy in diabetic cardiomyopathy. Cells 11 , 240 (2022).
He, X. et al. Signaling cascades in the failing heart and emerging therapeutic strategies. Signal Transduct. Target Ther. 7 , 134 (2022).
Bolli, R., Solankhi, M., Tang, X. L. & Kahlon, A. Cell therapy in patients with heart failure: a comprehensive review and emerging concepts. Cardiovasc Res. 118 , 951–976 (2022).
Bartunek, J. et al. Cardiopoietic stem cell therapy in heart failure: the C-CURE (cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J. Am. Coll. Cardiol. 61 , 2329–2338 (2013).
Bartunek, J. et al. Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur. Heart J. 38 , 648–660 (2017).
Hare, J. M. et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. J. Am. Med. Assoc. 308 , 2369–2379 (2012).
Hare, J. M. et al. Randomized comparison of allogeneic versus autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDON-DCM trial. J. Am. Coll. Cardiol. 69 , 526–537 (2017).
Mathiasen, A. B. et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial). Eur. Heart J. 36 , 1744–1753 (2015).
Mathiasen, A. B. et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with ischaemic heart failure: final 4-year follow-up of the MSC-HF trial. Eur. J. Heart Fail 22 , 884–892 (2020).
Florea, V. et al. Dose comparison study of allogeneic mesenchymal stem cells in patients with ischemic cardiomyopathy (The TRIDENT Study). Circ. Res. 121 , 1279–1290 (2017).
Bolli, R. et al. A Phase II study of autologous mesenchymal stromal cells and c-kit positive cardiac cells, alone or in combination, in patients with ischaemic heart failure: the CCTRN CONCERT-HF trial. Eur. J. Heart Fail 23 , 661–674 (2021).
Heldman, A. W. et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. J. Am. Med. Assoc. 311 , 62–73 (2014).
Perin, E. C. et al. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: the PRECISE trial. Am. Heart J. 168 , 88–95 e82 (2014).
Han, S., Sun, H. M., Hwang, K. C. & Kim, S. W. Adipose-derived stromal vascular fraction cells: update on clinical utility and efficacy. Crit. Rev. Eukaryot. Gene Expr. 25 , 145–152 (2015).
Henry, T. D. et al. The Athena trials: autologous adipose-derived regenerative cells for refractory chronic myocardial ischemia with left ventricular dysfunction. Catheter Cardiovasc Inter. 89 , 169–177 (2017).
Kastrup, J. et al. Cryopreserved off-the-shelf allogeneic adipose-derived stromal cells for therapy in patients with ischemic heart disease and heart failure—a safety study. Stem Cells Transl. Med. 6 , 1963–1971 (2017).
Qayyum, A. A. et al. Adipose-derived stromal cells for treatment of patients with chronic ischemic heart disease (MyStromalCell Trial): a randomized placebo-controlled study. Stem Cells Int. 2017 , 5237063 (2017).
Qayyum, A. A. et al. Autologous adipose-derived stromal cell treatment for patients with refractory angina (MyStromalCell Trial): 3-years follow-up results. J. Transl. Med. 17 , 360 (2019).
Ngo, A. T. L. et al. Clinically relevant preservation conditions for mesenchymal stem/stromal cells derived from perinatal and adult tissue sources. J. Cell Mol. Med. 25 , 10747–10760 (2021).
Madonna, R., Cevik, C., Nasser, M. & De Caterina, R. Hepatocyte growth factor: molecular biomarker and player in cardioprotection and cardiovascular regeneration. Thromb. Haemost. 107 , 656–661 (2012).
Bartolucci, J. et al. Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: a phase 1/2 randomized controlled trial (RIMECARD Trial [randomized clinical trial of intravenous infusion umbilical cord mesenchymal stem cells on cardiopathy]). Circ. Res. 121 , 1192–1204 (2017).
Ulus, A. T. et al. Intramyocardial transplantation of umbilical cord mesenchymal stromal cells in chronic ischemic cardiomyopathy: a controlled, randomized clinical trial (HUC-HEART trial). Int. J. Stem Cells 13 , 364–376 (2020).
He, X. et al. Effect of intramyocardial grafting collagen scaffold with mesenchymal stromal cells in patients with chronic ischemic heart disease: a randomized clinical trial. JAMA Netw. Open 3 , e2016236 (2020).
Zhang, Q. et al. Signaling pathways and targeted therapy for myocardial infarction. Signal Transduct. Target Ther. 7 , 78 (2022).
Poomani, M. S. et al. Mesenchymal stem cell (MSCs) therapy for ischemic heart disease: a promising frontier. Glob. Heart 17 , 19 (2022).
Xu, W. et al. Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp. Biol. Med. 229 , 623–631 (2004).
Jeong, J. O. et al. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ. Res. 108 , 1340–1347 (2011).
Denu, R. A. et al. Fibroblasts and mesenchymal stromal/stem cells are phenotypically indistinguishable. Acta Haematol. 136 , 85–97 (2016).
Birbrair, A. & Frenette, P. S. Niche heterogeneity in the bone marrow. Ann. N. Y Acad. Sci. 1370 , 82–96 (2016).
Pinho, S. & Frenette, P. S. Haematopoietic stem cell activity and interactions with the niche. Nat. Rev. Mol. Cell Biol. 20 , 303–320 (2019).
Ono, N. et al. Vasculature-associated cells expressing nestin in developing bones encompass early cells in the osteoblast and endothelial lineage. Dev. Cell 29 , 330–339 (2014).
Sugiyama, T., Kohara, H., Noda, M. & Nagasawa, T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25 , 977–988 (2006).
Ehninger, A. & Trumpp, A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J. Exp. Med. 208 , 421–428 (2011).
Golan, K., Kollet, O., Markus, R. P. & Lapidot, T. Daily light and darkness onset and circadian rhythms metabolically synchronize hematopoietic stem cell differentiation and maintenance: the role of bone marrow norepinephrine, tumor necrosis factor, and melatonin cycles. Exp. Hematol. 78 , 1–10 (2019).
Cheng, X. et al. The role of SDF-1/CXCR4/CXCR7 in neuronal regeneration after cerebral ischemia. Front. Neurosci. 11 , 590 (2017).
Zou, Y. R., Kottmann, A. H., Kuroda, M., Taniuchi, I. & Littman, D. R. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 393 , 595–599 (1998).
Mao, W., Yi, X., Qin, J., Tian, M. & Jin, G. CXCL12 inhibits cortical neuron apoptosis by increasing the ratio of Bcl-2/Bax after traumatic brain injury. Int. J. Neurosci. 124 , 281–290 (2014).
Wang, Q. et al. Stromal cell-derived factor 1alpha decreases beta-amyloid deposition in Alzheimer’s disease mouse model. Brain Res. 1459 , 15–26 (2012).
Yellowley, C. CXCL12/CXCR4 signaling and other recruitment and homing pathways in fracture repair. Bonekey Rep. 2 , 300 (2013).
Li, J. et al. CXCL12 promotes spinal nerve regeneration and functional recovery after spinal cord injury. Neuroreport 32 , 450–457 (2021).
Gensel, J. C., Kigerl, K. A., Mandrekar-Colucci, S. S., Gaudet, A. D. & Popovich, P. G. Achieving CNS axon regeneration by manipulating convergent neuro-immune signaling. Cell Tissue Res. 349 , 201–213 (2012).
Matsushita, T. et al. Mesenchymal stem cells transmigrate across brain microvascular endothelial cell monolayers through transiently formed inter-endothelial gaps. Neurosci. Lett. 502 , 41–45 (2011).
Schmidt, A. et al. Mesenchymal stem cells transmigrate over the endothelial barrier. Eur. J. Cell Biol. 85 , 1179–1188 (2006).
Yarygin, K. N. et al. Cell therapy of stroke: do the intra-arterially transplanted mesenchymal stem cells cross the blood-brain barrier? Cells 10 , 2997 (2021).
Schack, L. M. et al. Expression of CD24 in human bone marrow-derived mesenchymal stromal cells is regulated by TGFbeta3 and induces a myofibroblast-like genotype. Stem Cells Int. 2016 , 1319578 (2016).
Ruster, B. et al. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood 108 , 3938–3944 (2006).
Pluchino, N. et al. CXCR4 or CXCR7 antagonists treat endometriosis by reducing bone marrow cell trafficking. J. Cell Mol. Med. 24 , 2464–2474 (2020).
Kowalski, K. et al. Stem cells migration during skeletal muscle regeneration—the role of Sdf-1/Cxcr4 and Sdf-1/Cxcr7 axis. Cell Adh. Migr. 11 , 384–398 (2017).
Liu, L. et al. From blood to the brain: can systemically transplanted mesenchymal stem cells cross the blood-brain barrier? Stem Cells Int. 2013 , 435093 (2013).
Lozito, T. P. & Tuan, R. S. Mesenchymal stem cells inhibit both endogenous and exogenous MMPs via secreted TIMPs. J. Cell Physiol. 226 , 385–396 (2011).
Lozito, T. P., Jackson, W. M., Nesti, L. J. & Tuan, R. S. Human mesenchymal stem cells generate a distinct pericellular zone of MMP activities via binding of MMPs and secretion of high levels of TIMPs. Matrix Biol. 34 , 132–143 (2014).
Menge, T. et al. Mesenchymal stem cells regulate blood-brain barrier integrity through TIMP3 release after traumatic brain injury. Sci. Transl. Med. 4 , 161ra150 (2012).
Franklin, R. J. M. & Ffrench-Constant, C. Regenerating CNS myelin—from mechanisms to experimental medicines. Nat. Rev. Neurosci. 18 , 753–769 (2017).
Brick, R. M., Sun, A. X. & Tuan, R. S. Neurotrophically induced mesenchymal progenitor cells derived from induced pluripotent stem cells enhance neuritogenesis via neurotrophin and cytokine production. Stem Cells Transl. Med. 7 , 45–58 (2018).
Zupanc, H. R. H., Alexander, P. G. & Tuan, R. S. Neurotrophic support by traumatized muscle-derived multipotent progenitor cells: role of endothelial cells and vascular endothelial growth factor-A. Stem Cell Res. Ther. 8 , 226 (2017).
Liu, Y. & Olsen, B. R. Distinct VEGF functions during bone development and homeostasis. Arch. Immunol. Ther. Exp. 62 , 363–368 (2014).
Kangari, P., Talaei-Khozani, T., Razeghian-Jahromi, I. & Razmkhah, M. Mesenchymal stem cells: amazing remedies for bone and cartilage defects. Stem Cell Res. Ther. 11 , 492 (2020).
Liu, Y. et al. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J. Clin. Investig. 122 , 3101–3113 (2012).
Berendsen, A. D. & Olsen, B. R. How vascular endothelial growth factor-A (VEGF) regulates differentiation of mesenchymal stem cells. J. Histochem Cytochem. 62 , 103–108 (2014).
Garcia, K. O. et al. Therapeutic effects of the transplantation of VEGF overexpressing bone marrow mesenchymal stem cells in the hippocampus of murine model of Alzheimer’s disease. Front. Aging Neurosci. 6 , 30 (2014).
Hohman, T. J., Bell, S. P. & Jefferson, A. L., Alzheimer’s Disease Neuroimaging, I. The role of vascular endothelial growth factor in neurodegeneration and cognitive decline: exploring interactions with biomarkers of Alzheimer disease. JAMA Neurol. 72 , 520–529 (2015).
Zhang, W. et al. Neuroprotective effects of SOX5 against ischemic stroke by regulating VEGF/PI3K/AKT pathway. Gene 767 , 145148 (2021).
Jin, K. et al. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl Acad. Sci. USA 99 , 11946–11950 (2002).
Bao, X. J. et al. Transplantation of Flk-1+ human bone marrow-derived mesenchymal stem cells promotes behavioral recovery and anti-inflammatory and angiogenesis effects in an intracerebral hemorrhage rat model. Int. J. Mol. Med. 31 , 1087–1096 (2013).
Bao, X. et al. Transplantation of Flk-1+ human bone marrow-derived mesenchymal stem cells promotes angiogenesis and neurogenesis after cerebral ischemia in rats. Eur. J. Neurosci. 34 , 87–98 (2011).
Pelletier, J. et al. VEGF-A promotes both pro-angiogenic and neurotrophic capacities for nerve recovery after compressive neuropathy in rats. Mol. Neurobiol. 51 , 240–251 (2015).
Hobson, M. I., Green, C. J. & Terenghi, G. VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy. J. Anat. 197 (Pt 4), 591–605 (2000).
Hayakawa, K. et al. Vascular endothelial growth factor regulates the migration of oligodendrocyte precursor cells. J. Neurosci. 31 , 10666–10670 (2011).
Pei, G., Xu, L., Huang, W. & Yin, J. The protective role of microRNA-133b in restricting hippocampal neurons apoptosis and inflammatory injury in rats with depression by suppressing CTGF. Int. Immunopharmacol. 78 , 106076 (2020).
Xu, H. et al. Mesenchymal stem cell-derived exosomal microRNA-133b suppresses glioma progression via Wnt/beta-catenin signaling pathway by targeting EZH2. Stem Cell Res. Ther. 10 , 381 (2019).
Xin, H. et al. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells 31 , 2737–2746 (2013).
Kigerl, K. A. et al. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 29 , 13435–13444 (2009).
Knoller, N. et al. Clinical experience using incubated autologous macrophages as a treatment for complete spinal cord injury: phase I study results. J. Neurosurg. Spine 3 , 173–181 (2005).
Yagura, K. et al. The enhancement of CCL2 and CCL5 by human bone marrow-derived mesenchymal stem/stromal cells might contribute to inflammatory suppression and axonal extension after spinal cord injury. PLoS ONE 15 , e0230080 (2020).
Zhong, Z. et al. Bone marrow mesenchymal stem cells upregulate PI3K/AKT pathway and down-regulate NF-kappaB pathway by secreting glial cell-derived neurotrophic factors to regulate microglial polarization and alleviate deafferentation pain in rats. Neurobiol. Dis. 143 , 104945 (2020).
Zhong, Z. et al. Adipose-derived stem cells modulate BV2 microglial M1/M2 polarization by producing GDNF. Stem Cells Dev. 29 , 714–727 (2020).
Dong, B. et al. Exosomes from human umbilical cord mesenchymal stem cells attenuate the inflammation of severe steroid-resistant asthma by reshaping macrophage polarization. Stem Cell Res. Ther. 12 , 204 (2021).
Li, X. et al. Umbilical cord tissue-derived mesenchymal stem cells induce T lymphocyte apoptosis and cell cycle arrest by expression of indoleamine 2, 3-dioxygenase. Stem Cells Int. 2016 , 7495135 (2016).
Wang, A. Y. L. et al. Human Wharton’s jelly mesenchymal stem cell-mediated sciatic nerve recovery is associated with the upregulation of regulatory T cells. Int. J. Mol. Sci. 21 , 6310 (2020).
Noone, C., Kihm, A., English, K., O’Dea, S. & Mahon, B. P. IFN-gamma stimulated human umbilical-tissue-derived cells potently suppress NK activation and resist NK-mediated cytotoxicity in vitro. Stem Cells Dev. 22 , 3003–3014 (2013).
Li, X. et al. Immunomodulatory effects of mesenchymal stem cells in peripheral nerve injury. Stem Cell Res. Ther. 13 , 18 (2022).
Shang, Y., Guan, H. & Zhou, F. Biological characteristics of umbilical cord mesenchymal stem cells and its therapeutic potential for hematological disorders. Front. Cell Dev. Biol. 9 , 570179 (2021).
Mennan, C. et al. Isolation and characterisation of mesenchymal stem cells from different regions of the human umbilical cord. Biomed. Res. Int. 2013 , 916136 (2013).
D’Addio, F. et al. The link between the PDL1 costimulatory pathway and Th17 in fetomaternal tolerance. J. Immunol. 187 , 4530–4541 (2011).
Amable, P. R., Teixeira, M. V., Carias, R. B., Granjeiro, J. M. & Borojevic, R. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton’s jelly. Stem Cell Res. Ther. 5 , 53 (2014).
de Witte, S. F. H. et al. Immunomodulation by therapeutic mesenchymal stromal cells (MSC) is triggered through phagocytosis of MSC by monocytic cells. Stem Cells 36 , 602–615 (2018).
Li, Y. et al. Cell-cell contact with proinflammatory macrophages enhances the immunotherapeutic effect of mesenchymal stem cells in two abortion models. Cell Mol. Immunol. 16 , 908–920 (2019).
De Paepe, M. E., Wong, T., Chu, S. & Mao, Q. Stromal cell-derived factor-1 (SDF-1) expression in very preterm human lungs: potential relevance for stem cell therapy for bronchopulmonary dysplasia. Exp. Lung Res. 46 , 146–156 (2020).
Wynn, R. F. et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood 104 , 2643–2645 (2004).
Ryu, C. H. et al. Migration of human umbilical cord blood mesenchymal stem cells mediated by stromal cell-derived factor-1/CXCR4 axis via Akt, ERK, and p38 signal transduction pathways. Biochem Biophys. Res. Commun. 398 , 105–110 (2010).
Yang, C. et al. The biological changes of umbilical cord mesenchymal stem cells in inflammatory environment induced by different cytokines. Mol. Cell Biochem. 446 , 171–184 (2018).
Seedorf, G. et al. Hepatocyte growth factor as a downstream mediator of vascular endothelial growth factor-dependent preservation of growth in the developing lung. Am. J. Physiol. Lung Cell Mol. Physiol. 310 , L1098–L1110 (2016).
Chen, X. Y. et al. Therapeutic potential of human umbilical cord-derived mesenchymal stem cells in recovering from murine pulmonary emphysema under cigarette smoke exposure. Front. Med. 8 , 713824 (2021).
Katsha, A. M. et al. Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase-induced emphysema model. Mol. Ther. 19 , 196–203 (2011).
Kyurkchiev, D. et al. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J. Stem Cells 6 , 552–570 (2014).
Ren, Z. et al. Human umbilical-cord mesenchymal stem cells inhibit bacterial growth and alleviate antibiotic resistance in neonatal imipenem-resistant Pseudomonas aeruginosa infection. Innate Immun. 26 , 215–221 (2020).
Liu, J. et al. Type 2 alveolar epithelial cells differentiated from human umbilical cord mesenchymal stem cells alleviate mouse pulmonary fibrosis through beta-catenin-regulated cell apoptosis. Stem Cells Dev. 30 , 660–670 (2021).
Moodley, Y. et al. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am. J. Pathol. 175 , 303–313 (2009).
Li, D. Y., Li, R. F., Sun, D. X., Pu, D. D. & Zhang, Y. H. Mesenchymal stem cell therapy in pulmonary fibrosis: a meta-analysis of preclinical studies. Stem Cell Res. Ther. 12 , 461 (2021).
Lam, G., Zhou, Y., Wang, J. X. & Tsui, Y. P. Targeting mesenchymal stem cell therapy for severe pneumonia patients. World J. Stem Cells 13 , 139–154 (2021).
Chen, K. et al. Human umbilical cord mesenchymal stem cells hUC-MSCs exert immunosuppressive activities through a PGE2-dependent mechanism. Clin. Immunol. 135 , 448–458 (2010).
Ren, G. et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2 , 141–150 (2008).
Loy, H. et al. Therapeutic implications of human umbilical cord mesenchymal stromal cells in attenuating influenza A(H5N1) virus-associated acute lung injury. J. Infect. Dis. 219 , 186–196 (2019).
Gazdhar, A. et al. Targeted gene transfer of hepatocyte growth factor to alveolar type II epithelial cells reduces lung fibrosis in rats. Hum. Gene Ther. 24 , 105–116 (2013).
Wang, W. et al. Therapeutic mechanisms of mesenchymal stem cells in acute respiratory distress syndrome reveal potentials for Covid-19 treatment. J. Transl. Med. 19 , 198 (2021).
Chu, K. A. et al. Reversal of bleomycin-induced rat pulmonary fibrosis by a xenograft of human umbilical mesenchymal stem cells from Wharton’s jelly. Theranostics 9 , 6646–6664 (2019).
Chen, Q. H. et al. Mesenchymal stem cells regulate the Th17/Treg cell balance partly through hepatocyte growth factor in vitro. Stem Cell Res. Ther. 11 , 91 (2020).
Li, L. et al. Human umbilical cord-derived mesenchymal stem cells downregulate inflammatory responses by shifting the Treg/Th17 profile in experimental colitis. Pharmacology 92 , 257–264 (2013).
Zheng, L., Wang, S., Yang, H. & Lyu, X. [Research progress of mesenchymal stem cells attenuating acute respiratory distress syndrome by regulating the balance of M1/M2 macrophage polarization]. Zhonghua Wei Zhong Bing. Ji Jiu Yi Xue 33 , 509–512 (2021).
Fasshauer, M. & Bluher, M. Adipokines in health and disease. Trends Pharm. Sci. 36 , 461–470 (2015).
Kershaw, E. E. & Flier, J. S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 89 , 2548–2556 (2004).
Kurylowicz, A. & Kozniewski, K. Anti-inflammatory strategies targeting metaflammation in type 2 diabetes. Molecules 25 , 2224 (2020).
Liu, J. et al. Homing and restorative effects of bone marrow-derived mesenchymal stem cells on cisplatin injured ovaries in rats. Mol. Cells 37 , 865–872 (2014).
Jalalie, L. et al. Distribution of the CM-Dil-labeled human umbilical cord vein mesenchymal stem cells migrated to the cyclophosphamide-injured ovaries in C57BL/6 mice. Iran. Biomed. J. 23 , 200–208 (2019).
Takehara, Y. et al. The restorative effects of adipose-derived mesenchymal stem cells on damaged ovarian function. Lab. Investig. 93 , 181–193 (2013).
Richards, J. S., Ren, Y. A., Candelaria, N., Adams, J. E. & Rajkovic, A. Ovarian Follicular Theca Cell Recruitment, Differentiation, and Impact on Fertility: 2017 Update. Endocr. Rev. 39 , 1–20 (2018).
Young, J. M. & McNeilly, A. S. Theca: the forgotten cell of the ovarian follicle. Reproduction 140 , 489–504 (2010).
Trzyna, A. & Banas-Zabczyk, A. Adipose-derived stem cells secretome and its potential application in “stem cell-free therapy”. Biomolecules 11 , 878 (2021).
Ding, C. et al. Human amniotic mesenchymal stem cells improve ovarian function in natural aging through secreting hepatocyte growth factor and epidermal growth factor. Stem Cell Res. Ther. 9 , 55 (2018).
Kedenko, L. et al. Genetic polymorphisms at SIRT1 and FOXO1 are associated with carotid atherosclerosis in the SAPHIR cohort. BMC Med. Genet. 15 , 112 (2014).
Shojafar, E., Soleimani Mehranjani, M. & Shariatzadeh, S. M. A. Adipose derived mesenchymal stem cells improve the structure and function of autografted mice ovaries through reducing oxidative stress and inflammation: a stereological and biochemical analysis. Tissue Cell 56 , 23–30 (2019).
Liu, M. et al. Small extracellular vesicles derived from embryonic stem cells restore ovarian function of premature ovarian failure through PI3K/AKT signaling pathway. Stem Cell Res. Ther. 11 , 3 (2020).
Li, Z., Zhang, M., Tian, Y., Li, Q. & Huang, X. Mesenchymal stem cells in premature ovarian insufficiency: mechanisms and prospects. Front. Cell Dev. Biol. 9 , 718192 (2021).
Forghani, A. et al. Differentiation of adipose tissue-derived CD34+/CD31- cells into endothelial cells in vitro. Regen. Eng. Transl. Med 6 , 101–110 (2020).
Baer, P. C. Adipose-derived stem cells and their potential to differentiate into the epithelial lineage. Stem Cells Dev. 20 , 1805–1816 (2011).
Wang, C. et al. Differentiation of adipose-derived stem cells into contractile smooth muscle cells induced by transforming growth factor-beta1 and bone morphogenetic protein-4. Tissue Eng. Part A 16 , 1201–1213 (2010).
Chen, L. et al. Adipose-derived stem cells promote diabetic wound healing via the recruitment and differentiation of endothelial progenitor cells into endothelial cells mediated by the VEGF-PLCgamma-ERK pathway. Arch. Biochem Biophys. 692 , 108531 (2020).
Dekoninck, S. & Blanpain, C. Stem cell dynamics, migration and plasticity during wound healing. Nat. Cell Biol. 21 , 18–24 (2019).
Qian, L., Pi, L., Fang, B. R. & Meng, X. X. Adipose mesenchymal stem cell-derived exosomes accelerate skin wound healing via the lncRNA H19/miR-19b/SOX9 axis. Lab. Investig. 101 , 1254–1266 (2021).
Fujiwara, O. et al. Adipose-derived stem cells improve grafted burn wound healing by promoting wound bed blood flow. Burns Trauma 8 , tkaa009 (2020).
Chen, T. et al. Efficient and sustained IGF-1 expression in the adipose tissue-derived stem cells mediated via a lentiviral vector. J. Mol. Histol. 46 , 1–11 (2015).
Zhou, K. et al. Immunosuppression of human adipose-derived stem cells on T cell subsets via the reduction of NF-kappaB activation mediated by PD-L1/PD-1 and Gal-9/TIM-3 pathways. Stem Cells Dev. 27 , 1191–1202 (2018).
Moll, G. et al. Intravascular mesenchymal stromal/stem cell therapy product diversification: time for new clinical guidelines. Trends Mol. Med. 25 , 149–163 (2019).
He, X. et al. Spontaneous apoptosis of cells in therapeutic stem cell preparation exert immunomodulatory effects through release of phosphatidylserine. Signal Transduct. Target Ther. 6 , 270 (2021).
Lukomska, B. et al. Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells Int. 2019 , 9628536 (2019).
Li, C., Zhao, H. & Wang, B. Challenges for mesenchymal stem cell-based therapy for COVID-19. Drug Des. Devel Ther. 14 , 3995–4001 (2020).
Nguyen Thanh, L. et al. Outcomes of bone marrow mononuclear cell transplantation combined with interventional education for autism spectrum disorder. Stem Cells Transl. Med. 10 , 14–26 (2020).
Nguyen Thanh, L. et al. Can autologous adipose-derived mesenchymal stem cell transplantation improve sexual function in people with sexual functional deficiency? Stem Cell Rev. Rep. 17 , 2153–2163 (2021).
Caplan, A. I. Mesenchymal stem cells: time to change the name! Stem Cells Transl. Med. 6 , 1445–1451 (2017).
de Windt, T. S., Vonk, L. A. & Saris, D. B. F. Response to: Mesenchymal stem cells: time to change the name! Stem Cells Transl. Med. 6 , 1747–1748 (2017).
Boregowda, S. V., Booker, C. N. & Phinney, D. G. Mesenchymal stem cells: the moniker fits the science. Stem Cells 36 , 7–10 (2018).
Masterson, C. & O’Toole, D. The mesenchymal stromal cell magic bullet finds yet another target. Stem Cell Res. Ther. 5 , 82 (2014).
Murray, I. R. et al. Rogue stem cell clinics. Bone Jt. J. 102-B , 148–154 (2020).
Lyons, S., Salgaonkar, S. & Flaherty, G. T. International stem cell tourism: a critical literature review and evidence-based recommendations. Int. Health 14 , 132–141 (2022).
He, C. et al. Mesenchymal stem cell-based treatment in autoimmune liver diseases: underlying roles, advantages and challenges. Ther. Adv. Chronic Dis. 12 , 2040622321993442 (2021).
Bertheuil, N. et al. Adipose mesenchymal stromal cells: definition, immunomodulatory properties, mechanical isolation and interest for plastic surgery. Ann. Chir. Plast. Esthet. 64 , 1–10 (2019).
Chen, Y., Yu, Q., Hu, Y. & Shi, Y. Current research and use of mesenchymal stem cells in the therapy of autoimmune diseases. Curr. Stem Cell Res. Ther. 14 , 579–582 (2019).
Han, Y. et al. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target Ther. 7 , 92 (2022).
Rahmani, A. et al. Mesenchymal stem cell-derived extracellular vesicle-based therapies protect against coupled degeneration of the central nervous and vascular systems in stroke. Ageing Res. Rev. 62 , 101106 (2020).
Zhou, W. et al. Single-cell profiles and clinically useful properties of human mesenchymal stem cells of adipose and bone marrow origin. Am. J. Sports Med. 47 , 1722–1733 (2019).
Pachler, K. et al. A good manufacturing practice-grade standard protocol for exclusively human mesenchymal stromal cell-derived extracellular vesicles. Cytotherapy 19 , 458–472 (2017).
Borger, V., Staubach, S., Dittrich, R., Stambouli, O. & Giebel, B. Scaled isolation of mesenchymal stem/stromal cell-derived extracellular vesicles. Curr. Protoc. Stem Cell Biol. 55 , e128 (2020).
Nikfarjam, S., Rezaie, J., Zolbanin, N. M. & Jafari, R. Mesenchymal stem cell derived-exosomes: a modern approach in translational medicine. J. Transl. Med. 18 , 449 (2020).
Monticelli, S. & Natoli, G. Short-term memory of danger signals and environmental stimuli in immune cells. Nat. Immunol. 14 , 777–784 (2013).
Venkatesha, S. et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 12 , 642–649 (2006).
Bernardo, M. E. & Fibbe, W. E. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 13 , 392–402 (2013).
Liu, G. Y. et al. Short-term memory of danger signals or environmental stimuli in mesenchymal stem cells: implications for therapeutic potential. Cell Mol. Immunol. 13 , 369–378 (2016).
Diez-Tejedor, E. et al. Reparative therapy for acute ischemic stroke with allogeneic mesenchymal stem cells from adipose tissue: a safety assessment: a phase II randomized, double-blind, placebo-controlled, single-center, pilot clinical trial. J. Stroke Cerebrovasc. Dis. 23 , 2694–2700 (2014).
Laskowitz, D. T. et al. Allogeneic umbilical cord blood infusion for adults with ischemic stroke: clinical outcomes from a phase I safety study. Stem Cells Transl. Med. 7 , 521–529 (2018).
Jeon, S. R. et al. Treatment of spinal cord injury with bone marrow-derived, cultured autologous mesenchymal stem cells. Tissue Eng. Regenerative Med. 7 , 316–322 (2010).
Park, J. H. et al. Long-term results of spinal cord injury therapy using mesenchymal stem cells derived from bone marrow in humans. Neurosurgery 70 , 1238–1247 (2012).
Saito, F. et al. Administration of cultured autologous bone marrow stromal cells into cerebrospinal fluid in spinal injury patients: a pilot study. Restor. Neurol. Neurosci. 30 , 127–136 (2012).
El-Kheir, W. A. et al. Autologous bone marrow-derived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell Transpl. 23 , 729–745 (2014).
Karamouzian, S., Nematollahi-Mahani, S. N., Nakhaee, N. & Eskandary, H. Clinical safety and primary efficacy of bone marrow mesenchymal cell transplantation in subacute spinal cord injured patients. Clin. Neurol. Neurosurg. 114 , 935–939 (2012).
Pal, R. et al. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: a pilot clinical study. Cytotherapy 11 , 897–911 (2009).
Mendonca, M. V. et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res. Ther. 5 , 126 (2014).
Vaquero, J. et al. Intrathecal administration of autologous mesenchymal stromal cells for spinal cord injury: safety and efficacy of the 100/3 guideline. Cytotherapy 20 , 806–819 (2018).
Dai, G. et al. Transplantation of autologous bone marrow mesenchymal stem cells in the treatment of complete and chronic cervical spinal cord injury. Brain Res. 1533 , 73–79 (2013).
Jiang, P. C. et al. A clinical trial report of autologous bone marrow-derived mesenchymal stem cell transplantation in patients with spinal cord injury. Exp. Ther. Med. 6 , 140–146 (2013).
Jarocha, D., Milczarek, O., Wedrychowicz, A., Kwiatkowski, S. & Majka, M. Continuous improvement after multiple mesenchymal stem cell transplantations in a patient with complete spinal cord injury. Cell Transpl. 24 , 661–672 (2015).
Huang, L. et al. A randomized, placebo-controlled trial of human umbilical cord blood mesenchymal stem cell infusion for children with cerebral palsy. Cell Transpl. 27 , 325–334 (2018).
Karussis, D. et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch. Neurol. 67 , 1187–1194 (2010).
Yamout, B. et al. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. J. Neuroimmunol. 227 , 185–189 (2010).
Mohajeri, M., Farazmand, A., Mohyeddin Bonab, M., Nikbin, B. & Minagar, A. FOXP3 gene expression in multiple sclerosis patients pre- and post mesenchymal stem cell therapy. Iran. J. Allergy Asthma Immunol. 10 , 155–161 (2011).
Odinak, M. M. et al. [Transplantation of mesenchymal stem cells in multiple sclerosis]. Zh . Nevrol. Psikhiatr Im. S S Korsakova 111 , 72–76 (2011).
Bonab, M. M. et al. Autologous mesenchymal stem cell therapy in progressive multiple sclerosis: an open label study. Curr. Stem Cell Res Ther. 7 , 407–414 (2012).
Mohyeddin Bonab, M. et al. Evaluation of cytokines in multiple sclerosis patients treated with mesenchymal stem cells. Arch. Med Res. 44 , 266–272 (2013).
Llufriu, S. et al. Randomized placebo-controlled phase II trial of autologous mesenchymal stem cells in multiple sclerosis. PLoS ONE 9 , e113936 (2014).
Harris, V. K., Vyshkina, T. & Sadiq, S. A. Clinical safety of intrathecal administration of mesenchymal stromal cell-derived neural progenitors in multiple sclerosis. Cytotherapy 18 , 1476–1482 (2016).
Dahbour, S. et al. Mesenchymal stem cells and conditioned media in the treatment of multiple sclerosis patients: clinical, ophthalmological and radiological assessments of safety and efficacy. CNS Neurosci. Ther. 23 , 866–874 (2017).
Meng, M. et al. Umbilical cord mesenchymal stem cell transplantation in the treatment of multiple sclerosis. Am. J. Transl. Res. 10 , 212–223 (2018).
Fernandez, O. et al. Adipose-derived mesenchymal stem cells (AdMSC) for the treatment of secondary-progressive multiple sclerosis: a triple blinded, placebo controlled, randomized phase I/II safety and feasibility study. PLoS ONE 13 , e0195891 (2018).
Alvarez-Fuente, M. et al. Off-label mesenchymal stromal cell treatment in two infants with severe bronchopulmonary dysplasia: clinical course and biomarkers profile. Cytotherapy 20 , 1337–1344 (2018).
Edessy, M. et al. Autologous stem cells therapy, The first baby of idiopathic premature ovarian failure. Acta Med. Int. 3 , 19–23 (2016).
Gabr, H., Elkheir, W. & El-Gazzar, A. Autologous stem cell transplantation in patients with idiopathic premature ovarian failure. J. Tissue Sci. Eng. 7 , 27 (2016).
Bakhtiary, M. et al. Comparison of transplantation of bone marrow stromal cells (BMSC) and stem cell mobilization by granulocyte colony stimulating factor after traumatic brain injury in rat. Iran. Biomed. J. 14 , 142–149 (2010).
Zhou, Z. et al. Comparison of mesenchymal stromal cells from human bone marrow and adipose tissue for the treatment of spinal cord injury. Cytotherapy 15 , 434–448 (2013).
Yousefifard, M. et al. Human bone marrow-derived and umbilical cord-derived mesenchymal stem cells for alleviating neuropathic pain in a spinal cord injury model. Stem Cell Res. Ther. 7 , 36 (2016).
Takahashi, A. et al. Comparison of mesenchymal stromal cells isolated from murine adipose tissue and bone marrow in the treatment of spinal cord injury. Cell Transpl. 27 , 1126–1139 (2018).
Hao, T. et al. Comparison of bone marrow-vs. adipose tissue-derived mesenchymal stem cells for attenuating liver fibrosis. Exp. Ther. Med. 14 , 5956–5964 (2017).
Zare, H., Jamshidi, S., Dehghan, M. M., Saheli, M. & Piryaei, A. Bone marrow or adipose tissue mesenchymal stem cells: Comparison of the therapeutic potentials in mice model of acute liver failure. J. Cell Biochem 119 , 5834–5842 (2018).
Arminan, A. et al. Mesenchymal stem cells provide better results than hematopoietic precursors for the treatment of myocardial infarction. J. Am. Coll. Cardiol. 55 , 2244–2253 (2010).
Gaebel, R. et al. Cell origin of human mesenchymal stem cells determines a different healing performance in cardiac regeneration. PLoS ONE 6 , e15652 (2011).
Dayan, V. et al. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res. Cardiol. 106 , 1299–1310 (2011).
Lopez, Y. et al. Wharton’s jelly or bone marrow mesenchymal stromal cells improve cardiac function following myocardial infarction for more than 32 weeks in a rat model: a preliminary report. Curr. Stem Cell Res. Ther. 8 , 46–59 (2013).
Rasmussen, J. G. et al. Comparison of human adipose-derived stem cells and bone marrow-derived stem cells in a myocardial infarction model. Cell Transpl. 23 , 195–206 (2014).
Abd Emami, B. et al. Mechanical and chemical predifferentiation of mesenchymal stem cells into cardiomyocytes and their effectiveness on acute myocardial infarction. Artif. Organs 42 , E114–E126 (2018).
Omar, A. M., Meleis, A. E., Arfa, S. A., Zahran, N. M. & Mehanna, R. A. Comparative study of the therapeutic potential of mesenchymal stem cells derived from adipose tissue and bone marrow on acute myocardial infarction model. Oman Med. J. 34 , 534–543 (2019).
Download references
Acknowledgements
The authors would like to thank the Vingroup Scientific Research and Clinical Application Fund (grant number: PRO. 19.47) for supporting this work. All figures were created with Biorender.com. This work is supported by the Vingroup Scientific Research and Clinical Application Fund (Grant number: PRO.19.47).
Author information
Authors and affiliations.
Department of Research and Development, Vinmec Research Institute of Stem Cell and Gene Technology, Vinmec Healthcare System, Hanoi, Vietnam
Duc M. Hoang, Trung Q. Bach, Quyen T. Nguyen, Trang T. K. Phan, Giang H. Nguyen, Phuong T. T. Le, Van T. Hoang & Liem Thanh Nguyen
Department of Cellular Therapy, Vinmec High-Tech Center, Vinmec Healthcare System, Hanoi, Vietnam
Phuong T. Pham & Anh T. L. Ngo
Institute for Science & Technology in Medicine, Keele University, Keele, UK
Nicholas R. Forsyth
Department of Biology, Stanford University, Stanford, CA, USA
Michael Heke
You can also search for this author in PubMed Google Scholar
Contributions
D.M.H.: conception and design, manuscript writing, administrative support, data analysis and interpretation, and final approval of the manuscript. P.T.P.: manuscript writing (BM- and UC-MSC sections) and data analysis and interpretation. T.Q.B.: manuscript writing (BM- and UC-MSC sections) and data analysis and interpretation. A.T.L.N.: manuscript writing (UC-MSC section), figure presentation, and data analysis and interpretation. Q.T.N., T.T.K.P., G.H.N., P.T.T.L., and V.T.H.: manuscript writing and data analysis and interpretation. N.R.F. and M.H.: manuscript writing and editing and data analysis and interpretation. L.T.N.: manuscript writing, administrative support, and final approval of the manuscript. All authors have read and approved the article.
Corresponding author
Correspondence to Duc M. Hoang .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Hoang, D.M., Pham, P.T., Bach, T.Q. et al. Stem cell-based therapy for human diseases. Sig Transduct Target Ther 7 , 272 (2022). https://doi.org/10.1038/s41392-022-01134-4
Download citation
Received : 15 March 2022
Revised : 19 July 2022
Accepted : 21 July 2022
Published : 06 August 2022
DOI : https://doi.org/10.1038/s41392-022-01134-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Adipose-derived stem cell-based optimization strategies for musculoskeletal regeneration: recent advances and perspectives.
- Chenrui Yuan
Stem Cell Research & Therapy (2024)
Safety and efficacy of autologous adipose tissue-derived stem cell transplantation in aging-related low-grade inflammation patients: a single-group, open-label, phase I clinical trial
- Ngoc-Huynh Ton Nguyen
- Hao Thanh Phan
- Nhung Hai Truong
Trials (2024)
Unveiling heterogeneity in MSCs: exploring marker-based strategies for defining MSC subpopulations
- Bowei Liang
- Jianyong Xu
Journal of Translational Medicine (2024)
CD317+ MSCs expanded with chemically defined media have enhanced immunological anti-inflammatory activities
Safety and efficacy of umbilical cord tissue-derived mesenchymal stem cells in the treatment of patients with aging frailty: a phase i/ii randomized, double-blind, placebo-controlled study.
- Yingqian Zhu
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Advertisement
Progress in research on mesenchymal stem cells and their extracellular vesicles for treating fibrosis in systemic sclerosis
- Published: 17 July 2023
- Volume 23 , pages 2997–3009, ( 2023 )
Cite this article

- Yu Xiao 1 , 2 ,
- Zhongzhou Huang 1 , 2 ,
- Yingyu Wang 1 , 2 ,
- Ji Yang 3 ,
- Weiguo Wan 1 , 2 ,
- Hejian Zou 1 , 2 &
- Xue Yang 1 , 2
497 Accesses
1 Altmetric
Explore all metrics
Systemic sclerosis (SSc) refers to an autoimmune disease characterized by immune dysfunction, vascular endothelial damage, and multi-organ fibrosis. Thus far, this disease is incurable, and its high mortality rate is significantly correlated with fibrotic events. Fibrosis has been confirmed as a difficult clinical treatment area that should be urgently treated in clinical medicine. Mesenchymal stem cells (MSCs) exhibit immunomodulatory, pro-angiogenic, and anti-fibrotic functions. MSCs-derived extracellular vesicles (EVs) have aroused rising interest as a cellular component that retains the functions of MSCs while circumventing the possible adverse effects of MSCs. Moreover, EVs have great potential in treating SSc. In this study, the current research progress on MSCs and their EVs for treating fibrosis in SSc was reviewed, with an aim to provide some reference for future MSCs and their EVs in treating SSc.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
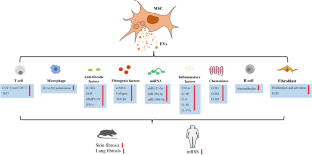
Similar content being viewed by others

Mesenchymal stem cell-based therapy for autoimmune diseases: emerging roles of extracellular vesicles
Mesenchymal stem cells alleviate systemic sclerosis by inhibiting the recruitment of pathogenic macrophages
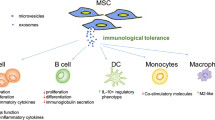
Novel insights into MSC-EVs therapy for immune diseases
Data availability.
All data are available from the corresponding author.
Abbreviations
Systemic sclerosis
Mesenchymal stem cells
Extracellular vesicles
Bone Marrow-derived stem cells
Adipose-derived mesenchymal stem cells
Tumor necrosis factor-stimulated gene 6
Tumor susceptibility gene 101
Heat shock protein
Hepatocyte growth factor
Low molecular heparin
Hypochlorous acid
Advanced oxidation protein products
Chronic graft versus host disease
Modified rodnan skin scores
Health assessment questionnaire disease activity index
Plasma exchange
Good manufacturing practices
Denton CP, Khanna D. Systemic sclerosis. Lancet (London, England). 2017;390(10103):1685–99.
PubMed Google Scholar
Jerjen R, Nikpour M, Krieg T, et al. Systemic sclerosis in adults. Part I: clinical features and pathogenesis. J Am Acad Dermatol. 2022;87(5):937–54.
Bairkdar M, Rossides M, Westerlind H, et al. Incidence and prevalence of systemic sclerosis globally: a comprehensive systematic review and meta-analysis. Rheumatology (Oxford). 2021;60(7):3121–33.
Elhai M, Meune C, Avouac J, et al. Trends in mortality in patients with systemic sclerosis over 40 years: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford). 2012;51(6):1017–26.
Ramos-Casals M, Fonollosa-Pla V, Brito-Zerón P, et al. Targeted therapy for systemic sclerosis: how close are we? Nat Rev Rheumatol. 2010;6(5):269–78.
CAS PubMed Google Scholar
Martin-Lopez M, Carreira PE. Antifibrotics in systemic sclerosis. Best Pract Res Clin Rheumatol. 2021;35(3):101671.
Varrica C, Dias HS, Reis C, et al. Targeted delivery in scleroderma fibrosis. Autoimmun Rev. 2021;20(2):102730.
Li A, Guo F, Pan Q, et al. Mesenchymal stem cell therapy: hope for patients with systemic lupus erythematosus. Front Immunol. 2021;12:728190.
CAS PubMed PubMed Central Google Scholar
Voswinkel J, Francois S, Simon JM, et al. Use of mesenchymal stem cells (MSC) in chronic inflammatory fistulizing and fibrotic diseases: a comprehensive review. Clin Rev Allergy Immunol. 2013;45(2):180–92.
Casiraghi F, Remuzzi G, Abbate M, et al. Multipotent mesenchymal stromal cell therapy and risk of malignancies. Stem Cell Rev Rep. 2013;9(1):65–79.
Rani S, Ryan AE, Griffin MD, et al. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther. 2015;23(5):812–23.
Lv FJ, Tuan RS, Cheung KM, et al. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32(6):1408–19.
Galipeau J, Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824–33.
Ménard C, Tarte K. Immunoregulatory properties of clinical grade mesenchymal stromal cells: evidence, uncertainties, and clinical application. Stem Cell Res Ther. 2013;4(3):64.
PubMed PubMed Central Google Scholar
Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4(5):267–74.
Zhou SL, Zheng C, Su JQ, et al. Isolation and identification of human umbilical cord and placenta-derived stem cells and their component analysis. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2015;23(6):1684–91.
Mebarki M, Abadie C, Larghero J, et al. Human umbilical cord-derived mesenchymal stem/stromal cells: a promising candidate for the development of advanced therapy medicinal products. Stem Cell Res Ther. 2021;12(1):152.
Puissant B, Barreau C, Bourin P, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129(1):118–29.
Ménard C, Dulong J, Roulois D, et al. Integrated transcriptomic, phenotypic, and functional study reveals tissue-specific immune properties of mesenchymal stromal cells. Stem Cells. 2020;38(1):146–59.
Loisel S, Dulong J, MéNARD C, et al. Brief report: proteasomal indoleamine 2,3-dioxygenase degradation reduces the immunosuppressive potential of clinical grade-mesenchymal stromal cells undergoing replicative senescence. Stem Cells. 2017;35(5):1431–6.
Shi Y, Wang Y, Li Q, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14(8):493–507.
Maumus M, Guérit D, Toupet K, et al. Mesenchymal stem cell-based therapies in regenerative medicine: applications in rheumatology. Stem Cell Res Ther. 2011;2(2):14.
Farge D, Loisel S, Lansiaux P, et al. Mesenchymal stromal cells for systemic sclerosis treatment. Autoimmun Rev. 2021;20(3):102755.
Chen W, Xia ZK, Zhang MH, et al. Adipose tissue-derived stem cells ameliorates dermal fibrosis in a mouse model of scleroderma. Asian Pac J Trop Med. 2017;10(1):52–6.
Yang Y, Zhu S, Li Y, et al. Human umbilical cord mesenchymal stem cells ameliorate skin fibrosis development in a mouse model of bleomycin-induced systemic sclerosis. Exp Ther Med. 2020;20(6):257.
Moroncini G, Paolini C, Orlando F, et al. Mesenchymal stromal cells from human umbilical cord prevent the development of lung fibrosis in immunocompetent mice. PLoS ONE. 2018;13(6):e0196048.
Suzuka T, Kotani T, Saito T, et al. Therapeutic effects of adipose-derived mesenchymal stem/stromal cells with enhanced migration ability and hepatocyte growth factor secretion by low-molecular-weight heparin treatment in bleomycin-induced mouse models of systemic sclerosis. Arthritis Res Ther. 2022;24(1):228.
Jin J, Ou Q, Wang Z, et al. BMSC-derived extracellular vesicles intervened the pathogenic changes of scleroderma in mice through miRNAs. Stem Cell Res Ther. 2021;12(1):327.
Li M, Zhang HP, Wang XY, et al. Mesenchymal stem cell-derived exosomes ameliorate dermal fibrosis in a murine model of bleomycin-induced scleroderma. Stem Cells Dev. 2021;30(19):981–90.
Maria AT, Toupet K, Bony C, et al. Antifibrotic, antioxidant, and immunomodulatory effects of mesenchymal stem cells in HOCl-induced systemic sclerosis. Arthritis Rheumatol (Hoboken, NJ). 2016;68(4):1013–25.
CAS Google Scholar
Maria ATJ, Toupet K, Maumus M, et al. Fibrosis development in HOCl-induced systemic sclerosis: a multistage process hampered by mesenchymal stem cells. Front Immunol. 2018;9:2571.
Jin X, Hou J, Zheng K, et al. Umbilical cord mesenchymal stem cells for inhibiting the fibrosis and autoimmune development in HOCl-induced systemic scleroderma mouse model. Int J Stem Cells. 2021;14(3):262–74.
Elessawi DF, Gabr H, Badawy MMM, et al. Therapeutic potential of mesenchymal stem cells for scleroderma induced in mouse model. Tissue Cell. 2021;73:101671.
Maria AT, Toupet K, Maumus M, et al. Human adipose mesenchymal stem cells as potent anti-fibrosis therapy for systemic sclerosis. J Autoimmun. 2016;70:31–9.
Akiyama K, Chen C, Wang D, et al. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10(5):544–55.
Chen C, Wang D, Moshaverinia A, et al. Mesenchymal stem cell transplantation in tight-skin mice identifies miR-151-5p as a therapeutic target for systemic sclerosis. Cell Res. 2017;27(4):559–77.
Lim JY, Ryu DB, Lee SE, et al. Mesenchymal stem cells (MSCs) attenuate cutaneous sclerodermatous graft-versus-host disease (Scl-GVHD) through inhibition of immune cell infiltration in a mouse model. J Invest Dermatol. 2017;137(9):1895–904.
Okamura A, Matsushita T, Komuro A, et al. Adipose-derived stromal/stem cells successfully attenuate the fibrosis of scleroderma mouse models. Int J Rheum Dis. 2020;23(2):216–25.
Zhao F, Zhang YF, Liu YG, et al. Therapeutic effects of bone marrow-derived mesenchymal stem cells engraftment on bleomycin-induced lung injury in rats. Transplant Proc. 2008;40(5):1700–5.
Moodley Y, Atienza D, Manuelpillai U, et al. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol. 2009;175(1):303–13.
Wu Y, Huang S, Enhe J, et al. Bone marrow-derived mesenchymal stem cell attenuates skin fibrosis development in mice. Int Wound J. 2014;11(6):701–10.
Yamamoto T, Takagawa S, Katayama I, et al. Animal model of sclerotic skin. I: Local injections of bleomycin induce sclerotic skin mimicking scleroderma. J Invest Dermatol. 1999;112(4):456–62.
Beyer C, Schett G, Distler O, et al. Animal models of systemic sclerosis: prospects and limitations. Arthritis Rheum. 2010;62(10):2831–44.
Cahill EF, Kennelly H, Carty F, et al. Hepatocyte growth factor is required for mesenchymal stromal cell protection against bleomycin-induced pulmonary fibrosis. Stem Cells Transl Med. 2016;5(10):1307–18.
Sakiyama R, Fukuta K, Matsumoto K, et al. Stimulation of hepatocyte growth factor production by heparin-derived oligosaccharides. J Biochem. 2007;141(5):653–60.
Servettaz A, Goulvestre C, Kavian N, et al. Selective oxidation of DNA topoisomerase 1 induces systemic sclerosis in the mouse. J Immunol. 2009;182(9):5855–64.
Green MC, Sweet HO, Bunker LE. Tight-skin, a new mutation of the mouse causing excessive growth of connective tissue and skeleton. Am J Pathol. 1976;82(3):493–512.
Jagasia MH, Greinix HT, Arora M, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. 2015;21(3):389-401.e1.
Jaffee BD, Claman HN. Chronic graft-versus-host disease (GVHD) as a model for scleroderma. I. Description of model systems. Cell Immunol. 1983;77(1):1–12.
Yang X, Liu C, Fujino M, et al. A modified graft-versus-host-induced model for systemic sclerosis, with pulmonary fibrosis in Rag2-deficient mice. FEBS Open Bio. 2017;7(9):1316–27.
Claman HN, Jaffee BD, Huff JC, et al. Chronic graft-versus-host disease as a model for scleroderma. II. Mast cell depletion with deposition of immunoglobulins in the skin and fibrosis. Cell Immunol. 1985;94(1):73–84.
Christopeit M, Schendel M, FöLL J, et al. Marked improvement of severe progressive systemic sclerosis after transplantation of mesenchymal stem cells from an allogeneic haploidentical-related donor mediated by ligation of CD137L. Leukemia. 2008;22(5):1062–4.
Keyszer G, Christopeit M, Fick S, et al. Treatment of severe progressive systemic sclerosis with transplantation of mesenchymal stromal cells from allogeneic related donors: report of five cases. Arthritis Rheum. 2011;63(8):2540–2.
Guiducci S, Porta F, Saccardi R, et al. Autologous mesenchymal stem cells foster revascularization of ischemic limbs in systemic sclerosis: a case report. Ann Intern Med. 2010;153(10):650–4.
Scuderi N, Ceccarelli S, Onesti MG, et al. Human adipose-derived stromal cells for cell-based therapies in the treatment of systemic sclerosis. Cell Transplant. 2013;22(5):779–95.
Wehbe T, Abi Saab M, Abi Chahine N, et al. Mesenchymal stem cell therapy for refractory scleroderma: a report of 2 cases. Stem Cell Investig. 2016;3:48.
Zhang H, Liang J, Tang X, et al. Sustained benefit from combined plasmapheresis and allogeneic mesenchymal stem cells transplantation therapy in systemic sclerosis. Arthritis Res Ther. 2017;19(1):165.
Wang J, Cai J, Zhang Q, et al. Fat transplantation induces dermal adipose regeneration and reverses skin fibrosis through dedifferentiation and redifferentiation of adipocytes. Stem Cell Res Ther. 2022;13(1):499.
Cras A, Farge D, Carmoi T, et al. Update on mesenchymal stem cell-based therapy in lupus and scleroderma. Arthritis Res Ther. 2015;17:301.
Czirják L, Foeldvari I, Müller-Ladner U. Skin involvement in systemic sclerosis. Rheumatology (Oxford). 2008;47(5):v44–5.
Cui J, Jin L, Ding M, et al. Efficacy and safety of mesenchymal stem cells in the treatment of systemic sclerosis: a systematic review and meta-analysis. Stem Cell Res Ther. 2022;13(1):118.
Volarevic V, Markovic BS, Gazdic M, et al. Ethical and safety issues of stem cell-based therapy. Int J Med Sci. 2018;15(1):36–45.
Varderidou-Minasian S, Lorenowicz MJ. Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: challenges and opportunities. Theranostics. 2020;10(13):5979–97.
van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–28.
Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9(1):63.
Rozier P, Maumus M, Bony C, et al. Extracellular vesicles are more potent than adipose mesenchymal stromal cells to exert an anti-fibrotic effect in an in vitro model of systemic sclerosis. Int J Mol Sci. 2021;22(13):6834.
Google Scholar
Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017;35(4):851–8.
Milbank E, Dragano NRV, González-García I, et al. Small extracellular vesicle-mediated targeting of hypothalamic AMPK α 1 corrects obesity through BAT activation. Nat Metab. 2021;3(10):1415–31.
Zhuang WZ, Lin YH, Su LJ, et al. Mesenchymal stem/stromal cell-based therapy: mechanism, systemic safety and biodistribution for precision clinical applications. J Biomed Sci. 2021;28(1):28.
Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16(7):748–59.
Rockel JS, Rabani R, Viswanathan S. Anti-fibrotic mechanisms of exogenously-expanded mesenchymal stromal cells for fibrotic diseases. Semin Cell Dev Biol. 2020;101:87–103.
Rozier P, Maumus M, Maria ATJ, et al. Lung fibrosis Is improved by extracellular vesicles from IFN γ -primed mesenchymal stromal cells in murine systemic sclerosis. Cells. 2021;10(10):2727.
Rozier P, Maumus M, Maria ATJ, et al. Mesenchymal stromal cells-derived extracellular vesicles alleviate systemic sclerosis via miR-29a-3p. J Autoimmun. 2021;121:102660.
Baral H, Uchiyama A, Yokoyama Y, et al. Antifibrotic effects and mechanisms of mesenchymal stem cell-derived exosomes in a systemic sclerosis mouse model: possible contribution of miR-196b-5p. J Dermatol Sci. 2021;104(1):39–47.
Yu Y, Shen L, Xie X, et al. The therapeutic effects of exosomes derived from human umbilical cord mesenchymal stem cells on scleroderma. Tissue Eng Regen Med. 2022;19(1):141–50.
Guo L, Lai P, Wang Y, et al. Extracellular vesicles derived from mesenchymal stem cells prevent skin fibrosis in the cGVHD mouse model by suppressing the activation of macrophages and B cells immune response. Int Immunopharmacol. 2020;84:106541.
Hostettler KE, Gazdhar A, Khan P, et al. Multipotent mesenchymal stem cells in lung fibrosis. PLoS ONE. 2017;12(8):e0181946.
Toledo DM, Pioli PA. Macrophages in systemic sclerosis: novel insights and therapeutic implications. Curr Rheumatol Rep. 2019;21(7):31.
Sarvar DP, Effatpanah H, Akbarzadehlaleh P, et al. Mesenchymal stromal cell-derived extracellular vesicles: novel approach in hematopoietic stem cell transplantation. Stem Cell Res Ther. 2022;13(1):202.
Download references
Acknowledgements
The authors acknowledge the support from the National Natural Science Foundation of China (No. 81871277) and innovative research team of high-level local universities in Shanghai-Clinical and basic research on the prevention and treatment of some inflammatory diseases by integrative medicine.
This study was supported by the National Natural Science Foundation of China (No. 81871277) and innovative research team of high-level local universities in Shanghai-Clinical and basic research on the prevention and treatment of some inflammatory diseases by integrative medicine.
Author information
Authors and affiliations.
Department of Rheumatology, Huashan Hospital, Fudan University, Shanghai, China
Yu Xiao, Zhongzhou Huang, Yingyu Wang, Weiguo Wan, Hejian Zou & Xue Yang
Institute of Rheumatology, Immunology and Allergy, Fudan University, Shanghai, China
Department of Dermatology, Zhongshan Hospital, Fudan University, Shanghai, China
You can also search for this author in PubMed Google Scholar
Contributions
These authors contributed equally: YX, ZH and YW. All authors read and approved the final version.
Corresponding authors
Correspondence to Hejian Zou or Xue Yang .
Ethics declarations
Competing interests.
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All of the authors approved the manuscript for publication.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Xiao, Y., Huang, Z., Wang, Y. et al. Progress in research on mesenchymal stem cells and their extracellular vesicles for treating fibrosis in systemic sclerosis. Clin Exp Med 23 , 2997–3009 (2023). https://doi.org/10.1007/s10238-023-01136-8
Download citation
Received : 30 March 2023
Accepted : 02 July 2023
Published : 17 July 2023
Issue Date : November 2023
DOI : https://doi.org/10.1007/s10238-023-01136-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Scleroderma, systemic
- Mesenchymal stem cells, extracellular vesicles
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 24 May 2024
Horizontal mitochondrial transfer as a novel bioenergetic tool for mesenchymal stromal/stem cells: molecular mechanisms and therapeutic potential in a variety of diseases
- Roberto Iorio 1 na1 ,
- Sabrina Petricca 1 na1 ,
- Vincenzo Mattei 2 &
- Simona Delle Monache ORCID: orcid.org/0000-0002-8153-915X 1
Journal of Translational Medicine volume 22 , Article number: 491 ( 2024 ) Cite this article
16 Accesses
Metrics details
Intercellular mitochondrial transfer (MT) is a newly discovered form of cell-to-cell signalling involving the active incorporation of healthy mitochondria into stressed/injured recipient cells, contributing to the restoration of bioenergetic profile and cell viability, reduction of inflammatory processes and normalisation of calcium dynamics. Recent evidence has shown that MT can occur through multiple cellular structures and mechanisms: tunneling nanotubes (TNTs), via gap junctions (GJs), mediated by extracellular vesicles (EVs) and other mechanisms (cell fusion, mitochondrial extrusion and migrasome-mediated mitocytosis) and in different contexts, such as under physiological (tissue homeostasis and stemness maintenance) and pathological conditions (hypoxia, inflammation and cancer). As Mesenchimal Stromal/ Stem Cells (MSC)-mediated MT has emerged as a critical regulatory and restorative mechanism for cell and tissue regeneration and damage repair in recent years, its potential in stem cell therapy has received increasing attention. In particular, the potential therapeutic role of MSCs has been reported in several articles, suggesting that MSCs can enhance tissue repair after injury via MT and membrane vesicle release. For these reasons, in this review, we will discuss the different mechanisms of MSCs-mediated MT and therapeutic effects on different diseases such as neuronal, ischaemic, vascular and pulmonary diseases. Therefore, understanding the molecular and cellular mechanisms of MT and demonstrating its efficacy could be an important milestone that lays the foundation for future clinical trials.
Mitochondria are highly dynamic and multifunctional compartments that play a pivotal role in oxidative bioenergetic metabolism and in maintaining cellular homeostasis. In addition to energy production, mitochondria perform many other key functions regulating fatty acid β-oxidation, iron homeostasis, Ca 2+ metabolism, heme and steroid hormones biosynthesis, innate immunity, redox homeostasis, and cellular waste management [ 1 , 2 , 3 ]. They are key sensors of multiple types of cellular stress [ 4 , 5 , 6 ] and crucial determinants in cell survival and death [ 7 ]. To fulfill its multiple tasks, mitochondrial network is dynamically redesigned by distinct processes (collectively referred to as mitochondrial quality control, MQC), including fission and fusion events, intracellular mitochondrial movement, selective removal of damaged mitochondria through mitophagy [ 8 , 9 , 10 ], and mitochondrial biogenesis, that orchestrate the overall shape, size, distribution, and connectivity of mitochondria. As signaling platforms, mitochondria communicate extensively with other cellular compartments [ 11 ] and can operate outside their intracellular confines exchanging information between cells, and even across organ systems [ 12 ]. Interestingly, different forms of circulating mitochondria (e.g., wrapped Mitos, mitochondria transported in vesicles or in platelets; free Mitos, mitochondria without a protective vesicle membrane) with distinct effects on immune cells have been found in both blood and cerebrospinal fluid (CSF) [ 8 , 13 , 14 , 15 , 16 ].
It is not surprising that the concomitant increase of mitochondrial DNA (mtDNA) mutations and reactive oxygen species (ROS) generation, during the aging process, exacerbate mitochondrial dysfunction and dysregulates MQC, contributing to the pathogenesis of multiple age-associated diseases [ 17 , 18 ], recently termed as non‐communicable diseases (NCDs) [ 19 ].
In this view, mitochondria-targeted therapeutic approaches have been explored over the past decade as potential treatments for tissue revitalization and homeostasis.
The phenomenon of intercellular transport of mitochondria between mammalian cells, also known as horizontal transfer of mitochondria, has recently attracted a renewed attention from the scientific community, representing an intriguing reparative strategy [ 20 , 21 ]. Intercellular mitochondrial transfer (MT) is a novel form of cell-to-cell signalling involving the active incorporation of healthy mitochondria into stressed/injured recipient cells [ 22 ], contributing to restore the bioenergetic profile (ATP and mitochondrial membrane potential) and cell viability, to increase the mtDNA content, as well as to reduce inflammatory processes and normalize calcium dynamics [ 23 ].
MT can occur via multiple distinct molecular mechanisms, including tunnelling nanotubes (TNTs), extracellular vesicles (EVs), gap junction channels (GJCs), and other non-traditional routes, such as cell fusion and mitochondrial extrusion [ 24 , 25 ] (Fig. 1 ).

Different mechanisms and routes of intercellular mitochondrial transfer. Exposure to stress signals (e.g., dysfunctional mitochondria, mitoDAMPs, and NAD + ) triggers the transfer of healthy mitochondria from donor cells to stressed/injured recipient cells via the activation of distinct signaling pathways and different routes, such as TNTs (1), EVs (2), and GJs (3). Key participants in the development of TNTs are F-Actin, microtubule, and intermediate filaments. Microtubule-based mitochondrial movement is mediated by Miro/TRAK complex attached to KIF5 kinesin/dynein molecular motors, whereas myosin motors are involved in mitochondrial transport based on actin. The NAD + /CD38/cADPR/Ca 2+ pathway is involved in the release of EVs. In the presence of NAD + , CD38 can synthetize cADPR through its cyclase activity. As second messenger, cADPR triggers the release of the intracellular Ca 2+ from the Endoplasmic reticulum by acting on Ryanodine Receptor (RyR). Therefore, the calcium-mediated activation of exocyst complex leads to the release of vesicles into the extracellular space. Importantly, the endocytosis of EVs may also occur via these mechanisms. Cx43-GJs are often located to one end of specific TNTs and the transcellular transfer of mitochondria may occur via gap junction internalization
The mitochondrial donation from mesenchymal stromal/stem cells (MSCs) has gained increasing attention in recent years [ 26 ], suggesting its potential in stem cell therapy [ 21 ]. MSCs-mediated MT is emerging as a critical regulatory mechanism for cell and tissue regeneration, and damage repair, where a remarkable restoration of cellular bioenergetics and a reduction in oxidative stress have been reported [ 26 , 27 ]. MSCs, as reported by Viswanathan et al., are to be better referred as Mesenchymal Stromal/Stem Cells (MSCs) given the prevalence of stromal cells with respect to the smaller fraction of true "mesenchymal stem cells” ( https://doi.org/10.1016/j.jcyt.2019.08.002 ). MSCs have the ability to maintain tissue homeostasis and renewal via regulating functional activity of parenchymal cells, including haematopoietic stem cells (HSCs) [ 28 , 29 ]. They can be isolated and expanded in vitro from almost all tissues, including bone marrow (BM), dental pulp (DP), umbilical cord (UC), adipose tissue (AD), placenta, and other sources [ 30 , 31 , 32 , 33 ]. Moreover, they have pleiotropic ability to differentiate into mesodermal lineage cells, including adipocytes, chondrocytes and osteocytes, or ectodermal lineage cells, such as neuronal and neuroglial cells [ 34 ]. In physiological conditions, the original microenvironment characteristics of MSCs (intercellular matrix, other cell types, soluble factors and humoral components) dictate their cellular fate [ 35 ]. On the other hand, MSCs reciprocally interact with the microenvironment through distinct processes, including immunomodulation and tissue repair. Therefore, MSCs display remarkable tissue regenerative properties given their ability to receive distinct signals from the surrounding tissues (e.g., damage-associated molecular patterns (DAMPs) and ROS) and constantly set up intricate intercellular communication networks with stressed/damaged cells.
Mitochondrial exchange and its beneficial effects were firstly reported by Spees and colleagues [ 36 ] in A549 ρ° cells with mtDNA deletion or defects. Specifically, the acquisition of healthy mitochondria in A549 ρ° cells following active interaction with human MSCs resulted in increasing levels of oxygen consumption, membrane potential, and intracellular ATP. Subsequent investigations in different in vitro and in vivo models have revealed the possibility of using mitochondria derived from MSCs for therapeutic purposes (e.g., stroke, lung and acute respiratory disorders, brain injury, muscle sepsis, and cardiac diseases), thus representing a novel strategy to treat many NCDs. Features such as immune privilege, significant migratory capacity to injured sites, fine-tuned redox balance, and low oxidative damage levels, as well as low energy demand (mitochondria in MSCs are quiescent and exhibit low activity level) render MSCs as elective donor cells in delivering functional mitochondria to diseased cells [ 37 , 38 , 39 , 40 , 41 , 42 ]. Furthermore, MSCs isolated from different tissue types (BM; AD; DP; UC; Wharton’s jelly, WJ) exhibit distinct bioenergetic signatures that influence their MT capacity, with AD-MSCs and BM-MSCs showing higher MT levels to cardiomyocytes than DP-MSCs and WJ-MSCs [ 37 ].
On the other hand, it has also been shown that MSCs-mediated MT plays a critical role in pathological states. In this sense, recent evidence indicates a dark side of MSCs in increasing malignancy of cancer cells, supporting tumor microenvironment and cancer progression, and providing metabolic flexibility and chemotherapy resistance [ 37 , 43 , 44 , 45 ].
In this review, we highlight the therapeutic potential of MT from MSCs in restoring the bioenergetic metabolism and cell functionality/viability into stressed/injured recipient cells. Therefore, we provide a comprehensive overview of the molecular signals triggering MT, as well as the major routes and mechanisms mediating MT. Finally, recent advances in MSCs regenerative properties through MT process in different NCDs are proposed.
Intercellular mitochondrial transfer via multiple cellular structures and mechanisms
MT has been demonstrated in various contexts under physiological (e.g., in tissue homeostasis and stemness maintenance) and pathological conditions (e.g., hypoxia, inflammation, and cancer). MT is a highly regulated multistep process that requires the spatiotemporal orchestration of many factors, including distinct triggering events/signals, molecular processes governing the formation of transfer machinery, and regulatory elements that modulate mitochondrial transfer speed and duration. In this section we will discuss the current state of knowledge regarding molecular stress signals and modes of MT, as well as signaling pathways regulating the different patterns of mitochondrial exchange.
Mitochondrial transfer trigger signals
Depending on different forms and activation states, mitochondria can affect the functions of neighbouring cells performing complex activities as mediators of regenerative and anti-inflammatory effects or trigger factors of inflammatory reactions [ 8 , 46 , 47 , 48 ]. Multiple stress conditions, including oxygen–glucose deprivation [ 49 , 50 ] drug-induced oxidative stress [ 51 , 52 ] and inflammation [ 53 , 54 ] generate harmed and fragmented extracellular mitochondria that trigger MT between cells. Therefore, the tissue regenerative properties of MSCs via the acquisition of active mitochondria by injured cells may be prompted by stress signals such as dysfunctional mitochondria and DAMPs of mitochondrial origin (mitoDAMPs), including ROS, DNA, cardiolipin, ATP, N-formyl peptides, transcription factor A mitochondria (TFAM), Cytochrome C (Cyt-C), succinate, and Ca 2+ (Fig. 1 ).
Consistent with this hypothesis, in an in vivo model of myocardial infarction, mitochondria released from damaged cells operate as potential DAMPs in MSCs, inducing the activation of eme-oxygenase-1 (HO-1) signaling pathway and mitochondrial biogenesis. These events enhance MT and consequently potentiate the rescue ability of MSCs to damaged cells [ 55 ]. In acute myeloid leukemia (AML) cells, NADPH oxidase-2 (NOX2)-derived ROS regulates MT from the BM-MSCs to the AML through TNTs [ 56 ]. In line with this, the generation of oxidative stress in HSCs triggers MT from BM-MSCs. Specifically, infection by Gram-negative bacteria drives MT from the BM-MSCs to HSCs through a ROS-dependent mechanism involving the activation of p53 and its downstream Akt/PI3K/mTOR pathway, and Connexin 43 (CX43) Gap Junctions [ 57 , 58 ]. Of note, the p53-dependent activation of Akt/PI3K/mTOR pathway also leads to the overexpression of Tumor Necrosis Factor Alpha Induced Protein 2 (TNFαip2) and the formation of TNTs. As described above, MSCs also promotes the transfer of depolarized mitochondria to macrophages in response to ROS generation [ 58 ].
In addition to ROS, the Cyt-C released in stressed cells can also stimulate MT. Therefore, in UV-damaged PC12 cells at early stage of apoptosis loss of Cyt-C from injured mitochondria activates MT to healthy cells through TNTs formation, thereby leading to the recovery of apoptotic PC12 cells [ 59 ].
CD38 is a multifunctional transmembrane glycoprotein responsible for the biosynthesis of two calcium-mobilizing second messengers, cyclic ADP-ribose (cADPR) and nicotinic acid adenine dinucleotide phosphate. Interestingly, CD38 may also play a role in promoting MT in different cellular models. Therefore, by a calcium-dependent mechanism involving CD38 and cADPR signalling, astrocytes promote the mitochondrial exchange to nearby neurons via microvesicles (MVs), thereby contributing to activation of neuroprotective and neurorecovery mechanisms after ischemic stroke [ 60 ]. In this case, CD38 expression may be related to the excessive release of excitotoxic glutamate from ischemic neuron, suggesting for this neurotransmitter a potential role in promoting astrocytes-mediated MT. A subsequent study further demonstrated that CD38/cADPR signaling and mitochondrial Rho GTPases (Miro) 1 and 2 contribute to the MT between astrocytes, and from neuronal cells into astrocytes [ 61 ]. CD38 has been indicated to exert a critical function during bone formation [ 62 ]. Very recently, it has been demonstrated that CD38/cADPR signaling plays a crucial role in mediating the differentiation and maturation of osteoblasts and osteoprogenitor cells through stimulating the secretion of mitochondria and mitochondrial-derived EVs [ 63 ].
Tunnelling nanotubes (TNTs) as a major route for mitochondria exchange
Multiple in vitro and in vivo models have highlighted the elective role played by TNTs in MT. Specifically, MT can occur from MSCs to lung macrophages via TNTs, resulting in higher phagocytic activity of macrophage cells in a model of acute respiratory distress syndrome [ 64 ]. In an in vitro simulated ischemia/reperfusion model, MSCs move mitochondria to injured H9c2 cardiomyoblasts via TNTs, protecting cardiac cells against the apoptosis [ 65 ]. A bidirectional MT between MSCs and vascular smooth muscle cells via TNTs upregulates MSCs proliferation [ 66 ]. Also, MT via TNTs occurs from human BM-MSCs and endothelial cells experiencing chemotherapy stress, resulting in recovery of injured endothelial cells [ 67 ]. Mitochondria move between BM-MSCs and myeloma cells via TNTs, as described above [ 56 , 68 ]. TNTs-mediated mitochondria exchange also form between donor cells other than MSCs, including PC12 cells [ 59 ], astrocytes [ 69 ], lung epithelial cells [ 70 ], cancer cells [ 71 ], retinal pigment epithelium [ 72 ], and trabecular meshwork cells [ 73 ].
Structural features of TNTs
According to the initial description, TNTs are open-ended membranous channels directly connecting cytoplasm of cells over long distances in a homotypic and heterotypic fashion. They are non-surface adherent and have a structure of 50–900 nm (with an average of 200 nm) in width and an average length between 20 and 100 mm.
TNTs contain F-actin cytoskeletal filaments that allow the bidirectional and unidirectional exchange between cells of various‐sized cargoes, including small molecules, nucleic acids and proteins (e.g., tau, α-synuclein, and huntingtin), organelles (e.g., vesicles, lysosomes, endoplasmic reticulum (ER), mitochondria, and autophagosomes), and even virus (herpesvirus and SARS-CoV-2) and bacteria [ 74 , 75 , 76 , 77 , 78 ], suggesting their role in in coordinating metabolism and signalling events in a wide-range of physiological processes and pathological conditions. Of note, this transfer function represents a crucial distinctive characteristic between TNTs and filopodia. The membrane structure of TNTs is very heterogeneous and cell type-specific. Therefore, close-ended TNTs (exhibiting gap junctions), that allow transfer of electrical signals, have also been identified in different in vitro and in vivo conditions [ 72 , 79 , 80 , 81 ]. In this regard, 3 types of close-ended TNTs have been observed, such as “ hand-shake ”, “ invaginated ”, and “ resting ” [ 82 ].
In addition, two types of open-ended TNTs according to the different distribution of cytoskeletal elements have been described: “thin” TNTs (< 700 nm in diameter), containing only F-Actin, and “thick” TNTs (> 700 nm in diameter), with F-Actin, microtubule, and intermediate filaments [ 83 ]. In neuronal and stromal cell lines, cryo-correlative light and electron microscopy (cryo-CLEM) coupled with tomography have also revealed individual TNTs (iTNTs), a bundle of small open-ended tubes (up of two to 11) that run parallel, and sometimes braided together, along the entire length of the TNTs [ 82 , 84 ]. These structures exhibit N-Cadherin as molecular linker connecting adjacent iTNTs. In addition to confer mechanical stability, it acts as guidance during growing iTNTs. Differently from single TNT, iTNTs allow for a bidirectional transfer of cargoes, including vesicles and mitochondria.
Mechanisms of TNT formation and protein regulators
Thus far, we have learned that the formation of TNTs can be generated via two different mechanisms that can occur simultaneously, or change dependently on the environmental conditions, though the underlying molecular process still remains under active investigation. These mechanisms include the “ cell dislodgement”, wherein cells initially in contact leave behind a tubular connection when they move apart, and the “ protrusion-elongation mechanism ” where the cell extends a filopodia-like protrusion to another cell located at some distance. In this regard, the main mechanism responsible for TNTs biogenesis seems to be cell dislodgement, as reported by live imaging analyses in different cell types [ 85 , 86 ]. However, the protrusion-elongation mechanism is typical of post-mitotic cells with low migratory phenotype, such as neurons and epithelial cells [ 87 ].
As mentioned above, the biogenesis of TNTs involves stress-signaling (e.g., p53 and MAP kinase) and pro-survival (e.g., EGFR, Akt, ROCK, PAK, MAP/ERK, PI3K or mTOR) pathways. Downstream effectors of these signaling pathways are proteins related to both membrane recycling and cytoskeletal remodeling.
Interestingly, the Rab family of small GTPases regulates many steps of membrane trafficking and also participate in actin cytoskeleton remodeling [ 88 , 89 ]. Therefore, transport and recycling of vesicles regulated by the small GTPases Rab11a and Rab8a promote TNTs formation in different cell systems [ 88 , 90 ]. Specifically, VAMP3 (vesicle-associated membrane protein 3, often called v-SNARES) operates downstream of Rab8a to regulate TNTs biogenesis [ 88 ]. Depletion of Ras association domain-containing protein 1A, a master regulator of cellular homeostasis and cytoskeleton, results in Rab11 accumulation and the subsequent release of exosome, thereby leading to TNTs formation [ 91 ]. Furthermore, Rab35 and its downstream effectors, such as ACAP2, ARF6-GDP, and EHD1 operate in a cascade mechanism to promote TNTs biogenesis in neuronal cells [ 92 ].
F-actin remodeling processes are responsible for initiating TNT protrusion by acting against the tensile strength of the plasma membrane, thus allowing it to deform and generate forces for tube growth. In this regard, distinct mechanisms involving specific sets of actin regulatory proteins are implicated, such as M-Sec (also known as TNFAIP2) [ 93 , 94 , 95 ] the exocyst complex, leukocyte specific transcript 1 (LST1) [ 96 ], the unconventional Myosin X (Myo10) [ 97 ], the actin bundler epidermal growth receptor substrate 8 (Eps8) and insulin receptor tyrosine kinase substrate protein 53 kDa (IRSp53), and small GTPases (e.g., Miro1/2, Rac1, Cdc42 and RalA) [ 98 ].
The cytosolic protein M-Sec acts as a key regulator of TNTs biogenesis through interaction with the small GTPase Ral in the mouse macrophage-like cell line RAW264.7 [ 93 ]. In this context, the transmembrane MHC class III protein LST1 may operate as a membrane scaffold for the generation of multi-molecular complex that controls the formation of TNTs. Thus, LST1 promotes TNTs formation by recruiting RalA and the actin-crosslinking protein filamin to the plasma membrane. It also stimulates the binding of RalA with two components of exocyst complex, Sec5 and Exo84, thereby leading to actin cytoskeletal remodeling and membrane protrusion. Meanwhile, the interaction of LST1 with M-Sec, myosin, and myoferlin may be involved in the process of mitochondrial anchoring and transfer [ 96 ]. In this regard, the recruitment of cytosolic M-Sec to the plasma membrane during the initial phase of TNT formation may occur through its direct binding to phosphatidylinositol (4,5)-bisphosphate [PI(4,5)P 2 ] or phosphatidylinositol (3,4,5)-trisphosphate [PI(3,4,5)P 3 ] [ 94 ]. Nucleolin, an RNA-binding protein, is essential for TNT formation in various types of mammalian cells [ 99 ]. Specifically, the binding of nucleolin to 14-3-3ζ mRNA modulates phospho-cofilin levels, leading to its inactivation, F-actin polymerization, and TNTs biogenesis.
In addition to the Rab35 signaling, Wingles-related integration site (Wnt)/Ca 2+ pathway, an intracellular cascade that is implicated in actin cytoskeleton remodeling, promotes TNTs biogenesis and stability in CAD (mouse catecholaminergic neuronal cell line) cells and primary neurons through the modulation of the interaction between the β isoform of Ca 2+ /calmodulin‐dependent protein kinase II and actin [ 100 ].
The I-Bin/Amphiphysin/Rvs (BAR)-domain protein IRSp53 is an essential spatio-temporal coordinator of plasma membrane protrusions (through promoting negative membrane curvature) that couples Rho-GTPase signaling to cytoskeleton remodeling and membrane dynamics. As a signaling platform, IRSp53 operates under the control of activatory (Cdc42, Eps8) or inhibitory (14-3-3) inputs as well as downstream effectors, and recruits to the plasma membrane various actin regulatory proteins, such as Eps8, Ena/VASP4, Wiskott-Aldrich syndrome protein (WASP), N-WASP, mDia2, WASP family verprolin-homologous 2 (WAVE2), and others [ 101 , 102 ].
Therefore, Delage and colleagues [ 98 ] have shown that Cdc42/IRSp53/VASP network negatively regulates TNTs formation and vesicular transport in neuronal cells, suggesting that Cdc42-dependent pathways are mainly involved in filopodia formation, rather than TNTs generation. By contrast, Eps8 (that reduces filopodia formation) is a positive regulator of TNTs biogenesis as its overexpression leads to increases in the extent of TNT connections and cargo transfer. Interestingly, the inhibition of actin related protein 2/3 complex (Arp2/3; a seven-subunit protein complex that mediates the formation of branched networks of filamentous actin, crucial for both filopodia and lamellipodia formation) promotes TNTs biogenesis and actin polymerization over longer distance in neuronal cells [ 82 ]. In particular, Arp2/3 inhibition enhances the co-expression of Eps8 and IRSp53, thus favoring their synergistic interaction. This event promotes the reorganization of the actin cytoskeleton, thereby leading to the switch of the system towards the extension of straight F-actin formation rather than the formation of branched networks [ 87 ] (Fig. 2 ). In addition, another recent study has demonstrated that the inhibition of ROCK, a downstream effector of Rho/Rac/Cdc42, stimulates TNTs formation via Myosin II mediated F-actin modulation [ 103 ].

Current model of TNTs formation via Eps8-IRSp53 interaction. In the cellular environment, the assembly of actin can be organized into two general types of architectures in balance with each other, such as branched actin networks and closely packed parallel arrays. The inhibition of Arp2/3-dependent branched filaments enhances the synergic activity of Eps8-IRSp53 complex at the membrane causing the shift of the actin balance in favour of polymerization of long, linear filaments for TNTs outgrowth. In this context, Eps8 seems to play a crucial role, possibly through renewed interactions with proteins and signaling pathways involved in actin dynamics, including phospho-regulators of cofilin (e.g., slingshot protein phosphatases and LIM-kinases), and coronins (e.g., Coro2b). Therefore, changes in Eps8’s interactome would lead to drastic reduction of actin filament turnover and disassembly of Arp2/3 networks, while promoting the extension and organization of actin
However, the same Rho GTPase signaling pathways operating in neuronal cells may act in a different way depending on the cell type. Indeed, observations in macrophages indicate that the blockage of Arp2/3 results in a decrease of TNTs formation [ 104 ]. Moreover, pathways converging on Arp2/3, including Cdc-42-WASP and Rac1-WAVE2 contribute together to TNT biogenesis. Consistent with this, in human trabecular meshwork cells (HTMCs; endothelial-like cells), the inactivation of Arp2/3 complex causes a reduction in TNT number and length, as well as vesicles transfer [ 73 ]. On the contrary, disassembly of actin stress fibers formation using Rho kinases inhibitors increased the number of TNTs and vesicle transfer.
Myo10 is an unconventional myosin that has critical functions in TNTs and filopodia formation [ 97 , 105 ]. In particular, neuronal TNTs biogenesis requires both the N-terminal head (the myosin motor domain that can bind to F-actin) and C-terminal tail domains (that can bind to several molecules, such as PI(3,4,5)P 3 , microtubules and β-integrins) of Myo10. Myo10 operates downstream of Cdc42 and promotes TNTs formation independent of VASP (a strong inducer of dorsal filopodia) and through a mechanism independent of integrins and substrate attachment, suggesting that Myo10-driven TNTs may arise from a different mechanism than the one that drives the formation of dorsal filopodia [ 98 , 105 , 106 ].
In neurons, the extracellular protein S100A4 and its receptor RAGE (Receptor for Advanced Glycation End Product) play crucial role in directing TNTs biogenesis [ 107 ]. Under oxidative stress, p53-caspase-3 axis determines the cleavage and depletion of S100A4, thereby leading to a concentration gradient between neurons and astrocytes that drives the extension direction of TNTs from neurons (with low concentration of S100A4) to astrocytes (with high concentration of S100A4).
Role of Miro proteins in mitochondrial transfer via TNTs
Miro 1 and 2 proteins are embedded in the outer mitochondrial membrane and involved in the structure of the mitochondrial contact-site and cristae organizing system complex (that connects outer and inner-mitochondrial membrane), Ca 2+ metabolism, mitochondria-ER communication, and mitophagy. Besides these functions, Miro proteins have critical role in regulating the mitochondrial spatial positioning and transport acting as adaptors that link mitochondria to cytoskeleton-associated motor proteins [ 108 , 109 ]. Generally, microtubules and actin microfilament system mediate long- and short-distance transport of mitochondria, respectively. In this regard, movement of mitochondria based on microtubules involves the interaction of Miro/TRAK (Trafficking Kinesin Protein) complex with different motor proteins, such as Kinesin, dynein, and kinesin superfamily KIF5 [ 110 , 111 ], while the actin-based mitochondrial movement is mediated by myosin family members, including myosin II, V, VI, and XIX [ 112 , 113 ].
Recent studies demonstrate the essential role of Miro proteins in TNTs‐mediated mitochondrial [ 38 ] transport [ 114 , 115 , 116 , 117 , 118 ]. Specifically, overexpression of Miro1 promotes MT from donor MSCs to epithelial injured cells, leading to the rescue of epithelial functions. On the contrary, Miro1 knockdown inhibits MSC-mediated MT [ 114 ]. Consistent with this, upregulation of Miro1 in MSCs increases the metabolic/bioenergetic benefits of MT following neuronal oxidative stress and mitochondrial damage, while decreasing Miro1 expression reduces these effects [ 115 , 117 ]. Also, MT via TNTs from astrocytes to neurons rescues neurons from cisplatin-induced damage. Furthermore, siRNA-mediated knockdown of Miro1 in astrocytes reduces MT, thus preventing the normalization of neuronal calcium dynamics [ 116 ].
Mitochondrial transfer via gap junctions (GJs)
MT can also occur in a Cx43-dependent manner since the deletion of Cx43 in donor/acceptor cells usually negatively affects the process [ 119 , 120 , 121 ]. Canonically, the formation of GJs or hemichannels across the plasma membrane by six Cx43 monomers allows uni- and bidirectional transfer of ions, small molecules (e.g., glucose, prostaglandins, microRNAs, and secondary messengers), and organelles between cells, thereby regulating intracellular mechanisms of signaling and several cellular functions [ 50 , 122 ]. In line with this context, the MT from BM-MSCs to injured alveolar epithelium requires the Ca 2+ exchanges between the two cells via Cx43-GJs, in the lipopolysaccharide (LPS)-induced acute lung injury mouse model [ 119 ]. In addition, MT from hematopoietic progenitors to BM-MSCs is required to induce the metabolic recovery of recipient BM-MSCs following irradiation and is cell-contact dependent and mediated by Cx43 [ 121 ].
Besides this classical function, Cx43-GJs may play further physiological roles, such as the regulation of mitochondrial functions and mediation of MT through the formation of TNTs and GJs internalization. In particular, Cx43 has also been detected within mitochondria [ 123 ] and seems to play a crucial role in mitochondrial calcium homeostasis and cell survival [ 124 ]. Cx43 is also implicated in forming connections between TNTs [ 125 , 126 ] and marked reduction of TNTs is observed following knocking out the Cx43 genes in human trabecular meshwork [ 127 ]. Also, Cx43-mediated TNTs formation is crucial for MT from human induced pluripotent stem cell (iPSC)-derived MSCs to the injured bronchial epithelial cells and results in the inhibition of asthma inflammation [ 120 ]. Importantly, a novel mechanism for MT mediated by Cx43-GJs has been reported [ 128 ]. Specifically, this process requires a distinct form of GJs turnover involving the engulfment of GJs (a form of trogocytosis). Thus, following GJs internalization whole mitochondria and endosomes are incorporated into vesicles (named connexosomes/annular gap junctions) and transferred between neighbouring granulosa cells.
Mitochondrial transfer mediated by extracellular vesicles (EVs)
EVs are heterogeneous, phospholipid-enclosed structures that play a pivotal role in cell-to-cell communication at a longer range [ 129 ]. They are released by any cells into the extracellular space under different physiological and pathological conditions and encompass small EVs or exosomes (diameter of 30–200 nm), microvesicles (MVs or ectosomes; diameter of 100–1000 nm), and apoptotic bodies or apoptosomes (> 1 μm in size) [ 130 , 131 ] Very recently, a specific subset of small EVs of mitochondrial origin has been identified in mouse and human brains named “mitovesicles” [ 132 , 133 , 134 ]. Mitochondria encapsulated into EVs can be transferred between cells to maintain the survival of metabolically compromised cells [ 58 ], to regulate immune responses [ 135 ], or to maintain tissue homeostasis [ 136 ]. In a mouse model of focal cerebral ischemia, MT by EVs between astrocytes and neurons acts as a survival mechanism protecting neurons from glucose deprivation and hypoxia [ 60 ]. EVs-mediated MT from renal scattered tubular cells (STC-like cells) to injured tubular epithelial cells (TEC) exerts protective effects, resulting in attenuating renal stenosis and recovering mitochondrial respiration [ 137 ]. Very recently, it has been demonstrated that mitochondria containing MVs (diameter of about 185 nm) derived from a human brain microvascular endothelial cell line significantly increase ATP levels, mitochondrial respiration, and glycolytic capacities of the ischemic primary human brain microvascular endothelial cells [ 138 ]. Interestingly, EVs-based interorgan transport of mitochondria may protect the heart through hormetic responses [ 139 ]. Specifically, circulating EVs derived from energetically stressed adipocytes, and containing oxidatively damaged mitochondria are taken up by cardiomyocytes, where they promote oxidative stress, resulting in a metabolic and redox adaptation of the heart that may confer protection against a future lethal stress.
In in vitro models of acute respiratory distress syndrome (ARDS), EVs-mediated MT from MSCs to human macrophages promotes phagocytosis and abolishes proinflammatory cytokine secretion [e.g., TNF-α] by macrophages, thereby mitigating lung injury [ 140 ]. Furthermore, in a model of allergic airway disease, myeloid-derived regulatory cells (MDRCs) encapsulate and transfer mitochondria to peripheral T cells via EVs, thereby affecting their bioenergetic and/or redox profile [ 141 , 142 ]. Active mitochondria (in EVs and isolated) released by platelets stimulates the pro-angiogenic activity of MSCs via metabolic remodeling, including increased de novo fatty acid synthesis [ 143 ]. In this context, the clathrin-dependent endocytosis is the mechanism by which mitochondria are internalized by acceptor cells. Accordingly, EVs from MSCs are emerging as a novel nano-strategy approach to attenuate mitochondrial damage and improving TFAM-mtDNA complex stability, essential for regenerative capability of different tissues [ 144 ].
Other routes of mitochondrial transfer: cell fusion, mitochondrial extrusion, and migrasome-mediated mitocytosis
Although most data to date suggests that MT can occur via TNTs, Cx43-GJs, and EVs, various other routes have been proposed, including cell fusion, mitochondrial extrusion, and the migrasome-mediated mitocytosis [ 21 , 145 ].
Cell fusion involves the physical merging of two or more cellular membranes, which would hypothetically allow the exchange of multiple protein complexes and even organelles, such as mitochondria [ 146 ]. Although cell fusion is infrequent under normal conditions, it may result in a marked mitochondrial delivery into recipient cells following injury and inflammation [ 147 ], hypoxia-induced apoptosis [ 148 ], or irradiation [ 149 ]. Cell fusion between human MSCs and cardiomyocytes results in MT into cardiomyocytes, and the resulting hybrid cells are reprogrammed toward a progenitor-like state [ 150 ]. Stem cells can fuse with other cellular models, including hepatocytes [ 151 ] and neurons [ 152 ], generating hybrid phenotypes which summarize distinct characteristics of both cells [ 153 ].
MT can also involve the extrusion or internalization of free mitochondria or mitochondrial components without membranous carriers. Under stress conditions, these events are crucial for regulating mitochondrial turnover and homeostasis [ 145 , 154 , 155 ]. Uncouplers of oxidative phosphorylation strongly stimulate the complete release of fragmented mitochondria from highly glycolytic HeLa cells via a mitoptosis process [ 154 , 156 , 157 ]. An analogous mechanism is also confirmed in in vivo models, including cardiomyocytes [ 158 ] and neurons [ 159 , 160 ] suggesting a possible role as “waste removal” [ 145 ]. In Caenorhabditis elegans , under proteotoxic stress, neurons release dysfunctional mitochondria and protein aggregates via large membrane‐bound vesicles called “exophers” to support cellular homeostasis and functionality [ 160 ]. Murine cardiomyocytes can remove mitochondria and a significant portion of subcellular components via exopher‐like structures that are finally taken up and eliminated by cardiac macrophages. This process would support heart homeostasis by preventing extracellular accumulation of waste material, autophagic block, and inflammasome activation [ 158 ].
In hepatocytes and fibroblasts, the extrusion of mitochondria is also promoted by LPS through a process resembling secretory autophagy [ 161 ]. TNF-α induces the release of naked mitochondria into the extracellular spaces [ 155 ]. Similarly, massive mitochondrial extrusion has been shown in cell models of TNF‐α-induced necroptosis [ 162 ]. In this regard, free mitochondria would act as a specific danger signal to trigger inflammatory processes. Therefore, activated platelets can also release active mitochondria, both within membranous carriers and as free compartments, able to induce inflammation [ 163 ].
Importantly, MT may also occur via migrasomes, vesicular structures that grow on the tips and intersections of retraction fibers of migrating cells via tetraspanin microdomains [ 164 , 165 ]. Therefore, to maintain cellular homeostasis, damaged mitochondria would be transported to the cell periphery before disposal by mitocytosis, an important mitochondrial quality-control process [ 166 ]. This process would deliver damaged mitochondria to surrounding cells [ 167 ].
Mesenchymal stem cells-mediated therapeutic effects of MT on different diseases
MT-mediated by MSCs may be used in restoring the bioenergetic metabolism and cell functionality and may be a useful tool for several diseases’ treatment. The MSCs potential therapeutic role has reported in several papers with a proposed mechanism which suggests that MSCs may enhance tissue repair after injury by MT and shedding of membrane vesicles [ 168 ]. MT is a strategy investigated in several kinds of cells such as pulmonary, cardiac, renal, corneal epithelium, and brain cortical [ 37 ]. Rustom et al. reported mitochondrial donation via a new form of cell-to-cell interaction based on TNTs [ 169 ]. Moreover, Jang et al. described intercellular mitochondrial transportation from MSCs to corneal endothelial cells, photoreceptors, and retinal pigment cells [ 170 ] and that recipient cells exhibited increased mitochondrial respiratory abilities. In the same paper Jang et al. reported that direct contact is a prerequisite for TNT formation and identified F-actin-based TNTs bridging MSCs and recipient cells. Several authors reported that mitochondrial dysfunction is associated also with several neurological diseases such as stroke, spinal cord injury and Alzheimer diseases (AD) [ 171 , 172 ]. Moreover, there are evidence that MT may be a new approach to restoring mitochondrial functions and that the MT can be used to correct a range of problems caused by mitochondrial dysfunction [ 173 , 174 ].
MT transfer and neuronal diseases
In several retinal and corneal diseases has been shown that mitochondrial dysfunction is a critical phenomenon [ 92 , 175 ]. For example, it has observed that the increase of mitochondrial fission and mitochondrial DNA damage in retinal vasculature precede apoptosis of retinal endothelial cells in diabetic retinopathy [ 176 , 177 ]. Therefore, several authors thought that targeting mitochondrial dysfunction may be an approach to prevent the development and progression of both retinal and corneal degeneration. Jiang et al. showed that intercellular mitochondrial transport is a vital mechanism for regeneration of corneal epithelial cells and retinal ganglion cells [ 51 , 178 ]. Moreover, Jiang et al. describe intercellular mitochondrial transportation from MSCs to corneal endothelial cells, photoreceptors, and retinal pigment cells. In this paper they showed that the cell that receive the mitochondrion increased respiratory abilities and elevated expression of mitochondrial structure and function related gene [ 170 ]. Mitochondrial dysfunction has observed in Leber’s hereditary optic neuropathy (LHON). Recent evidence showed that LHON derives from a genetic mutation in mitochondria, which evolves to optic atrophy, which gives rise to visual acuity and blindness. Thus, the replacement of mitochondria may be a possible solution, which can be achieved by MT utilizing mesenchymal stem cells or their conditioned media derivate [ 179 ]. Several authors investigated a possible role of MT in AD.
AD is a chronic neurodegenerative disease and manifests symptoms such as: short-term memory loss, visual-spatial perception disorders and impaired language and executive functions [ 180 ]. Several papers showed that MSCs could inhibit amyloid β-peptide (Aβ) generation and promote its effective clearance, alter amyloid precursor protein (APP) processing, decrease tau phosphorylation, and increase proteasomal activity resulting in reduced accumulation of ubiquitin-conjugated proteins [ 181 , 182 , 183 ]. In AD has been observed the presence of Aβ in the mitochondria. This presence of Aβ generates hyperphosphorylated tau proteins by interacting with mitochondrial Drp1 protein, which, in turn, disrupts microtubule function and induces neural toxicity [ 184 , 185 ]. Moreover, mitochondrial Aβ can also interact with Aβ-binding alcohol dehydrogenase (ABAD), leading to mitochondrial dysfunction and the production of ROS [ 186 ]. The main consequence of mitochondrial dysfunction, ROS accumulation, and increased oxidative stress are main factors involved in AD pathogenesis. Several studies showed that MSCs may promote microglia and autophagy-mediated clearance of protein aggregates as Aβ [ 187 , 188 , 189 ]. MSCs can protect neurons from cell death by secretion of some neuroprotective factors or by MT [ 52 , 190 , 191 ]. Zhan et al. showed that UC-MSC-CM significantly decreased tau phosphorylated at the Thr181 level, which increased in AD-alleviated intracellular and mitochondrial oxidative stress of okadaic acid (OA)-treated SH-SY5Y cells. In addition, UC-MSC-CM suppressed apoptosis and improved mitochondrial function in OA-treated SH-SY5Y cells. In this paper, Zhan et al. showed that UC-MSC-CM exerted protective effects relying on or partly extracellular vesicle (EV) MT from UC-MSCs to OA-treated SH-SY5Y cells [ 191 ]. Moreover, it observed that UC-MSC-CM decreased the level of p181-tau in the AD cell model, improved cell viability, and suppressed apoptosis in OA-treated Sh-SY5Y cells. In addition, they showed that UC-MSC-CM improved mitochondrial functions in OA-treated SH-SY5Y cells [ 192 ].
Spinal cord injury (SCI) is a destructive neurological disease that causes major motor, sensory and autonomic dysfunction that in some cases can lead blood vessels rupture and vasoconstriction reflexive led to an oxygen reduction and consequent mitochondrial damage. In addition, it is possible to observe a series of damages such as mitochondrial permeability, calcium overload, excitatory toxicity, oxidative stress, and increased ROS production [ 193 ]. These phenomenon results in impaired capability to maintain mitochondrial homeostasis with less energy available [ 194 ]. In this context, several authors are evolving new strategies to improve secondary injuries such as repairing or replacing damaged mitochondria, the use of antioxidants, and restoring mitochondrial permeability [ 195 ]. Li et al. showed that either MSCs or MSC-derived mitochondria injected into the injured spinal cord of a rat contusion SCI model significantly improved locomotor functions 6 weeks after injury [ 50 ]. Other authors reported that MSCs are able to improve the secondary injury caused by inflammation, myelin insulation, and assist the angiogenesis process [ 9 , 196 , 197 , 198 , 199 ]. Sykova et al. showed the safety of the use of intravenous and intraarterial delivery of MSCs in SCI patients [ 200 ], while Deng et al. used MSCs coupled with collagen in SCI patients and compared with the control group (only collagen) [ 201 ]. After 12 months the study showed the treatment group vs control group showed significantly improved American Spinal Injury Association scores and better bowel and urinary functions [ 44 ].The ability of cells to interact with other cells via mitochondria has been demonstrated also in other tissues and organs where different modes of MT from MSCs to injured or damaged cells in order to restore or support nonfunctional mitochondria has been discovered [ 168 ]. For example, the active transfer from adult stem cells and somatic cells can rescue aerobic respiration in mammalian cells with non-functional mitochondria [ 36 , 168 ].
MT transfer and ischemic vascular diseases
Another pathology that may benefit from studies on stem cell-mediated MT is stroke and ischemia–reperfusion injury. A stroke occurs when something blocks the blood supply to part of the brain or when a blood vessel in the brain bursts. Therefore, we can identify two types of strokes: ischemic or hemorrhagic. The blockage of one or more arteries is the main characteristic of acute ischemic stroke, with blood flow reduction and cellular dysfunction, damage, and/or death. The revascularization process is necessary for stroke treatment, but the oxygen and nutrient trasport to the damaged tissues may lead to the activation of the innate and adaptive immune responses that may cause secondary damage to the remaining cells [ 202 , 203 ]. The hallmark of ischemia/reperfusion process is a mitochondrial dysfunction, ATP production decrease, ROS increase and cellular death [ 204 ]. During this process, cell switchs to anaerobic metabolism with a consequent indirect increase of Ca 2+ [ 205 ]. This Ca 2+ overload and oxidative stress lead to the opening of mitochondrial permeability transition pore in the inner mitochondrial membrane and an increase in ROS production [ 206 , 207 ]. Therefore, MT from other cells could represent a useful tool in management of pathological damage caused by mitochondrial dysfunction in ischemia–reperfusion injury. It has been shown that several brain cells as neurons, astrocytes, endothelial cells, and MSCs are able to transfer mitochondria [ 171 ]. In preclinical studies, Liu et al. showed that human bone marrow MSCs could save endothelial cells during hypoxia and nutrients deprivation-induced stress. MSCs could abolish apoptosis in the endothelial cells induced by dysfunctional mitochondria during hypoxia by shifting the functional mitochondria from MSCs through TNTs-like cell protrusions [ 204 ]. Babenko et al. showed that BM-MSCs are able to save astrocytes and PC12 cells during hypoxia and glucose deprivation induced by oxidative stress and mitochondrial damage. Moreover, the same author reported that in the same in vivo study BM-MSCs improve the neurological impairments of cerebral ischemia rats and that this phenomenon is mediated by MT from BM-MSCs via TNTs. Moreover, it has been reported that BM-MSCs with overexpression of Miro1 improve the recovery of ischemic rats [ 115 , 208 ]. Transplantation of healthy mitochondria has been studied as a solution in rescuing injured cells and tissue for treatment of others different pathologies connected to ischemia vascular diseases. The first studies have been conducted in animal models of ischemia–reperfusion [ 209 ]. For example, Mc Cully et al. have been demonstrated that administering mitochondria isolated from the left ventricle of the rabbit to the site of partial ischemia–reperfusion allowed a significant reduction of myocardial infarction and apoptosis markers with a consequent recovery of myocardial infarction [ 210 ]. Then, Masuzawa et al. demonstrated in their work the internalization of the transplanted mitochondria and enhanced myocardial energetics despite the lack of demonstration of internalization (by tunneling nanotubes) mechanism [ 211 ]. Similar results have been obtained in rat models of ischemia reperfusion. Kaza et al., administering mitochondria in in vivo rat models prior to reperfusion, obtained a decrease of Infarct Size and Area at Risk (IS/AAR) index and altogether enhanced myocardia and cell viability [ 212 ]. Similarly, Guariento and coworkers and Blitzer et al. investigated on the therapeutic use single or multiple intracoronary doses of isolated mitochondria before ischemia–reperfusion episodes [ 213 , 214 ]. The first clinical application of mitochondrial transplantation was carried out to treat myocardial ischemia–reperfusion injury in pediatric patients of Boston Children’s Hospital (United States) [ 215 ]. The role of MT has been demonstrated also in SENECA trials where induced patients derived cardiomyocytes (iCM) co-cultured with MSCs, thanks to MSCs EV release, improved their viability and physiology, reducing ROS production and preserving mitochondrial biogenesis. Moreover, some authors suggested a mechanism by which EV from MSCs resulted enriched in mitochondria that probably were transferred to iCM [ 216 ]). Also Liu and colleagued have been demonstrated a mechanism by which MSCs can be used as a novel treatment of ischemic vascular disease; they demonstrated a transfer of mitochondria via a tunnelling nanotube-like structure from stem cells to injured human umbilical vein endothelial cells [ 204 ].
MT transfer and respiratory and pulmonary diseases
The effect of dysfunctional mitochondria has been reported also in respiratory and pulmonary diseases. Among these, Chronic Obstructive Pulmonary Disease (COPD) induced by cigarette smoke (CS) is an example of lung diseases characterized by inflammation and damage of cells. In particular, cigarette smoke induces mitochondrial disfunctions in lung epithelial cells. MSCs and MSC-derived exosomes have been proposed as therapeutic intervention of COPD induced by cigarette smoke as reported by Maremanda et al. which demonstrated as BEAS2B-mMSC co-cultures showed protective response against the CSE-altered mitochondrial respiration parameters, confirming the beneficial effect of MSC towards human bronchial lung epithelial cells [ 217 ]. Morrison et al. studying the mechanisms of MSCs effects in ARDS demonstrated that MSCs are able to induce in this environment an anti-inflammatory and highly phagocytic macrophage phenotype through EV-mediated MT. In particular, alveolar macrophage treated with MSC-derived EVs ameliorate lung injury in vivo. Transfer of functional mitochondria via EVs determined an increase in Oxidative phosphorylation which led to enhanced phagocytosis and a decrease of TNF-a and IL-8 levels of secretion by macrophages in vitro and in vivo [ 140 ]. Similarly, Jackson et al. reported that human bone marrow derived MSCs transfer their mitochondria to macrophages both in vivo and in vitro via TNT and micro vesicle secretion. This leads to enhanced macrophage phagocytosis and improved bioenergetics. So mitochondrial donation represented a novel mechanism to explain the antimicrobial effect of MSCs in a condition determined by bacterial infection providing additional evidence about their therapeutic use in acute, inflammatory lung diseases [ 64 ]. Moreover, the effect of mitochondrial transplantation or donation has been reported in other cell pathologies including renal and liver diseases. For example, Kubat et al. observed good therapeutic effects of mitochondrial transplantation in a nephrotoxicity model [ 218 ]. Otherwise, Lu et al. demonstrated a nanotherapeutic effect of UC-MSC-EVs on inhibiting local NETs formation by transferring functional mitochondria to intrahepatic neutrophils and repairing their mitochondrial function [ 219 ]. In addition, Bi et al. reported a functional therapeutic effect of mitochondrial transfer to combat Non-alcoholic fatty liver disease [ 220 ]. By the way, MT has been observed in lung diseases also associated with bioenergetic impairment and dysfunctional mitochondria such as allergic airway inflammation, ARDS or asthma but what signalling events can trigger this mechanism remain elusive.
Conclusions
Mitochondrial transfer and the mechanisms by which mitochondria enter recipient cells are concepts that have gained much attention in recent years. In particular, intercellular communication and mitochondrial transfer in MSCs promise interesting therapeutic results in various pathological conditions. In general, the active transfer of mitochondria from mesenchymal cells to somatic cells could, for instance, restore aerobic respiration in cells with non-functional mitochondria. So, the possible transplantation of healthy mitochondria has been studied as a solution in rescuing injured cells and tissue for treatment of several pathologies such as pulmonary, cardiac, renal, corneal epithelium, and brain cortical. Therefore, understanding the molecular and cellular mechanisms of mitochondrial transfer/transplantation and demonstrating its efficacy could be an important milestone that lays the foundation for future clinical trials.

Availability of data and materials
Not applicable.
Abbreviations
Actin related protein 2/3 complex
Acute myeloid leukemia
Acute respiratory distress syndrome
Alzheimer diseases
Bone marrow
Cerebrospinal fluid
Chronic Obstructive Pulmonary Disease
Connexin 43
Cytochrome C
Damage-associated molecular patterns
Dental pulp
Endoplasmic reticulum
- Extracellular vesicles
Gap junctions
Haematopoietic stem cells
Human induced pluripotent stem cell
Human trabecular meshwork cells
I-Bin/Amphiphysin/Rvs (BAR)-domain protein
Individual TNTs
Induced patients derived cardiomyocytes
Leber’s hereditary optic neuropathy
Mesenchymal stem cells
Microvesicles
Mitochondrial contact-site and cristae organizing system
Mitochondrial DNA
Mitochondrial quality control
Mitochondrial transfer
Myeloid-derived regulatory cells
NADPH oxidase-2
Non‐communicable diseases
Outer mitochondrial membrane
Phosphatidylinositol (4;5)-bisphosphate
Reactive oxygen species
Receptor for Advanced Glycation End Product
Spinal cord injury
TNF-α-induced protein 2
Transcription factor A mitochondria
Tubular epithelial cells
Tumor necrosis factor
- Tunnelling nanotubes
Umbilical cord
WASP family verprolin-homologous 2
Wharton’s jelly
Spinelli JB, Haigis MC. The multifaceted contributions of mitochondria to cellular metabolism. Nat Cell Biol. 2018;20(7):745–54.
Article CAS PubMed PubMed Central Google Scholar
Pfanner N, Warscheid B, Wiedemann N. Mitochondrial proteins: from biogenesis to functional networks. Nat Rev Mol Cell Biol. 2019;20(5):267–84.
Chakrabarty RP, Chandel NS. Mitochondria as signaling organelles control mammalian stem cell fate. Cell Stem Cell. 2021;28(3):394–408.
Iorio R, Castellucci A, Rossi G, Cinque B, Cifone MG, Macchiarelli G, et al. Mancozeb affects mitochondrial activity, redox status and ATP production in mouse granulosa cells. Toxicol In Vitro. 2015;30(1):438–45.
Article CAS PubMed Google Scholar
Petricca S, Flati V, Celenza G, Di Gregorio J, Lizzi AR, Luzi C, et al. Tebuconazole and econazole act synergistically in mediating mitochondrial stress, energy imbalance, and sequential activation of autophagy and apoptosis in mouse sertoli TM4 cells: possible role of AMPK/ULK1 axis. Toxicol Sci. 2019;169(1):209–23.
Di Gregorio J, Petricca S, Iorio R, Toniato E, Flati V. Mitochondrial and metabolic alterations in cancer cells. Eur J Cell Biol. 2022;101(3): 151225.
Article PubMed Google Scholar
Bock FJ, Tait SWG. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol. 2020;21(2):85–100.
Miliotis S, Nicolalde B, Ortega M, Yepez J, Caicedo A. Forms of extracellular mitochondria and their impact in health. Mitochondrion. 2019;48:16–30.
Zhou M, Yu Y, Luo X, Wang J, Lan X, Liu P, et al. Myocardial ischemia-reperfusion injury: therapeutics from a mitochondria-centric perspective. Cardiology. 2021;146(6):781–92.
Dong Y, Zhuang XX, Wang YT, Tan J, Feng D, Li M, et al. Chemical mitophagy modulators: drug development strategies and novel regulatory mechanisms. Pharmacol Res. 2023;194: 106835.
Picard M, Shirihai OS. Mitochondrial signal transduction. Cell Metab. 2022;34(11):1620–53.
Aguirre-Gutierrez J, Berenguer E, Menor IO, Bauman D, Corral-Rivas JJ, Nava-Miranda MG, et al. Functional susceptibility of tropical forests to climate change. Nat Ecol Evol. 2022;6(7):878.
Al Amir Dache Z, Otandault A, Tanos R, Pastor B, Meddeb R, Sanchez C, et al. Blood contains circulating cell-free respiratory competent mitochondria. FASEB J. 2020;34(3):3616–30.
Song X, Hu W, Yu H, Wang H, Zhao Y, Korngold R, et al. Existence of circulating mitochondria in human and animal peripheral blood. Int J Mol Sci. 2020;21(6):2122.
Stephens OR, Grant D, Frimel M, Wanner N, Yin M, Willard B, et al. Characterization and origins of cell-free mitochondria in healthy murine and human blood. Mitochondrion. 2020;54:102–12.
Chou SH, Lan J, Esposito E, Ning M, Balaj L, Ji X, et al. Extracellular mitochondria in cerebrospinal fluid and neurological recovery after subarachnoid hemorrhage. Stroke. 2017;48(8):2231–7.
Amorim JA, Coppotelli G, Rolo AP, Palmeira CM, Ross JM, Sinclair DA. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat Rev Endocrinol. 2022;18(4):243–58.
Article PubMed PubMed Central Google Scholar
Lima T, Li TY, Mottis A, Auwerx J. Pleiotropic effects of mitochondria in aging. Nature aging. 2022;2(3):199–213.
Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–60.
Shanmughapriya S, Langford D, Natarajaseenivasan K. Inter and Intracellular mitochondrial trafficking in health and disease. Ageing Res Rev. 2020;62: 101128.
Liu D, Gao Y, Liu J, Huang Y, Yin J, Feng Y, et al. Intercellular mitochondrial transfer as a means of tissue revitalization. Signal Transduct Target Ther. 2021;6(1):65.
Al Amir Dache Z, Thierry AR. Mitochondria-derived cell-to-cell communication. Cell Rep. 2023;42(7):112728.
Liu Z, Sun Y, Qi Z, Cao L, Ding S. Mitochondrial transfer/transplantation: an emerging therapeutic approach for multiple diseases. Cell Biosci. 2022;12(1):66.
Tiash S, Brestoff JR, Crewe C. A guide to studying mitochondria transfer. Nat Cell Biol. 2023;25(11):1551–3.
Clemente-Suarez VJ, Martin-Rodriguez A, Yanez-Sepulveda R, Tornero-Aguilera JF. Mitochondrial transfer as a novel therapeutic approach in disease diagnosis and treatment. Int J Mol Sci. 2023;24(10):8848.
Boukelmoune N, Chiu GS, Kavelaars A, Heijnen CJ. Mitochondrial transfer from mesenchymal stem cells to neural stem cells protects against the neurotoxic effects of cisplatin. Acta Neuropathol Commun. 2018;6(1):139.
Rodriguez AM, Nakhle J, Griessinger E, Vignais ML. Intercellular mitochondria trafficking highlighting the dual role of mesenchymal stem cells as both sensors and rescuers of tissue injury. Cell Cycle. 2018;17(6):712–21.
Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–34.
Kfoury Y, Scadden DT. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell. 2015;16(3):239–53.
Martellucci S, Santacroce C, Manganelli V, Santilli F, Piccoli L, Cassetta M, et al. Isolation, propagation, and prion protein expression during neuronal differentiation of human dental pulp stem cells. J Vis Exp JoVE. 2019;145:e59282.
Google Scholar
Delle Monache S, Pulcini F, Santilli F, Martellucci S, Santacroce C, Fabrizi J, et al. Hypoxia induces DPSC differentiation versus a neurogenic phenotype by the paracrine mechanism. Biomedicines. 2022;10(5):1056.
Galipeau J, Sensebe L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824–33.
Pittenger MF, Discher DE, Peault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regenerative medicine. 2019;4:22.
Salehinejad P, Moshrefi M, Eslaminejad T. An overview on mesenchymal stem cells derived from extraembryonic tissues: supplement sources and isolation methods. Stem Cells Cloning Adv Appl. 2020;13:57–65.
Liu J, Gao J, Liang Z, Gao C, Niu Q, Wu F, et al. Mesenchymal stem cells and their microenvironment. Stem Cell Res Ther. 2022;13(1):429.
Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A. 2006;103(5):1283–8.
Paliwal S, Chaudhuri R, Agrawal A, Mohanty S. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J Biomed Sci. 2018;25(1):31.
Yang J, Liu L, Oda Y, Wada K, Ago M, Matsuda S, et al. Extracellular vesicles and Cx43-gap junction channels are the main routes for mitochondrial transfer from ultra-purified mesenchymal stem cells, RECs. Int J Mol Sci. 2023;24(12):10294.
Zhang F, Zheng X, Zhao F, Li L, Ren Y, Li L, et al. TFAM-Mediated mitochondrial transfer of MSCs improved the permeability barrier in sepsis-associated acute lung injury. Apoptosis. 2023;28(7–8):1048–59.
Malekpour K, Hazrati A, Soudi S, Hashemi SM. Mechanisms behind therapeutic potentials of mesenchymal stem cell mitochondria transfer/delivery. J Control Release. 2023;354:755–69.
Fahey M, Bennett M, Thomas M, Montney K, Vivancos-Koopman I, Pugliese B, et al. Mesenchymal stromal cells donate mitochondria to articular chondrocytes exposed to mitochondrial, environmental, and mechanical stress. Sci Rep. 2022;12(1):21525.
Thomas MA, Fahey MJ, Pugliese BR, Irwin RM, Antonyak MA, Delco ML. Human mesenchymal stromal cells release functional mitochondria in extracellular vesicles. Front Bioeng Biotechnol. 2022;10: 870193.
Aponte PM, Caicedo A. Stemness in cancer: stem cells, cancer stem cells, and their microenvironment. Stem Cells Int. 2017;2017:5619472.
Chen YC, Gonzalez ME, Burman B, Zhao X, Anwar T, Tran M, et al. Mesenchymal stem/stromal cell engulfment reveals metastatic advantage in breast cancer. Cell Rep. 2019;27(13):3916-26 e5.
Sundstrom T, Prestegarden L, Azuaje F, Aasen SN, Rosland GV, Varughese JK, et al. Inhibition of mitochondrial respiration prevents BRAF-mutant melanoma brain metastasis. Acta Neuropathol Commun. 2019;7(1):55.
Patergnani S, Bouhamida E, Leo S, Pinton P, Rimessi A. Mitochondrial oxidative stress and “mito-inflammation”: actors in the diseases. Biomedicines. 2021;9(2):216.
Garg M, Johri S, Chakraborty K. Immunomodulatory role of mitochondrial DAMPs: a missing link in pathology? FEBS J. 2023;290(18):4395–418.
Marchi S, Guilbaud E, Tait SWG, Yamazaki T, Galluzzi L. Mitochondrial control of inflammation. Nat Rev Immunol. 2023;23(3):159–73.
Hayakawa K, Chan SJ, Mandeville ET, Park JH, Bruzzese M, Montaner J, et al. Protective effects of endothelial progenitor cell-derived extracellular mitochondria in brain endothelium. Stem cells. 2018;36(9):1404–10.
Li H, Wang C, He T, Zhao T, Chen YY, Shen YL, et al. Mitochondrial transfer from bone marrow mesenchymal stem cells to motor neurons in spinal cord injury rats via gap junction. Theranostics. 2019;9(7):2017–35.
Jiang D, Gao F, Zhang Y, Wong DS, Li Q, Tse HF, et al. Mitochondrial transfer of mesenchymal stem cells effectively protects corneal epithelial cells from mitochondrial damage. Cell Death Dis. 2016;7(11): e2467.
Wang J, Liu X, Qiu Y, Shi Y, Cai J, Wang B, et al. Cell adhesion-mediated mitochondria transfer contributes to mesenchymal stem cell-induced chemoresistance on T cell acute lymphoblastic leukemia cells. J Hematol Oncol. 2018;11(1):11.
Zhang Y, Yu Z, Jiang D, Liang X, Liao S, Zhang Z, et al. iPSC-MSCs with high intrinsic MIRO1 and sensitivity to TNF-alpha yield efficacious mitochondrial transfer to rescue anthracycline-induced cardiomyopathy. Stem cell reports. 2016;7(4):749–63.
Luz-Crawford P, Hernandez J, Djouad F, Luque-Campos N, Caicedo A, Carrere-Kremer S, et al. Mesenchymal stem cell repression of Th17 cells is triggered by mitochondrial transfer. Stem Cell Res Ther. 2019;10(1):232.
Mahrouf-Yorgov M, Augeul L, Da Silva CC, Jourdan M, Rigolet M, Manin S, et al. Mesenchymal stem cells sense mitochondria released from damaged cells as danger signals to activate their rescue properties. Cell Death Differ. 2017;24(7):1224–38.
Marlein CR, Zaitseva L, Piddock RE, Robinson SD, Edwards DR, Shafat MS, et al. NADPH oxidase-2 derived superoxide drives mitochondrial transfer from bone marrow stromal cells to leukemic blasts. Blood. 2017;130(14):1649–60.
Mistry JJ, Marlein CR, Moore JA, Hellmich C, Wojtowicz EE, Smith JGW, et al. ROS-mediated PI3K activation drives mitochondrial transfer from stromal cells to hematopoietic stem cells in response to infection. Proc Natl Acad Sci USA. 2019;116(49):24610–9.
Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472.
Wang X, Gerdes HH. Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ. 2015;22(7):1181–91.
Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535(7613):551–5.
Gao L, Zhang Z, Lu J, Pei G. Mitochondria are dynamically transferring between human neural cells and alexander disease-associated GFAP mutations impair the astrocytic transfer. Front Cell Neurosci. 2019;13:316.
Moridera K, Uchida S, Tanaka S, Menuki K, Utsunomiya H, Yamaoka K, et al. Skeletal unloading reduces cluster of differentiation (CD) 38 expression in the bone marrow and osteoblasts of mice. J Orthopaed Sci. 2020;25(2):331–7.
Article Google Scholar
Suh J, Kim NK, Shim W, Lee SH, Kim HJ, Moon E, et al. Mitochondrial fragmentation and donut formation enhance mitochondrial secretion to promote osteogenesis. Cell Metab. 2023;35(2):345-60 e7.
Jackson MV, Morrison TJ, Doherty DF, McAuley DF, Matthay MA, Kissenpfennig A, et al. Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem cells. 2016;34(8):2210–23.
Han H, Hu J, Yan Q, Zhu J, Zhu Z, Chen Y, et al. Bone marrow-derived mesenchymal stem cells rescue injured H9c2 cells via transferring intact mitochondria through tunneling nanotubes in an in vitro simulated ischemia/reperfusion model. Mol Med Rep. 2016;13(2):1517–24.
Vallabhaneni KC, Haller H, Dumler I. Vascular smooth muscle cells initiate proliferation of mesenchymal stem cells by mitochondrial transfer via tunneling nanotubes. Stem Cells Dev. 2012;21(17):3104–13.
Feng Y, Zhu R, Shen J, Wu J, Lu W, Zhang J, et al. Human bone marrow mesenchymal stem cells rescue endothelial cells experiencing chemotherapy stress by mitochondrial transfer via tunneling nanotubes. Stem Cells Dev. 2019;28(10):674–82.
Marlein CR, Piddock RE, Mistry JJ, Zaitseva L, Hellmich C, Horton RH, et al. CD38-driven mitochondrial trafficking promotes bioenergetic plasticity in multiple myeloma. Can Res. 2019;79(9):2285–97.
Article CAS Google Scholar
Rostami J, Holmqvist S, Lindstrom V, Sigvardson J, Westermark GT, Ingelsson M, et al. Human astrocytes transfer aggregated alpha-synuclein via tunneling nanotubes. J Neurosci. 2017;37(49):11835–53.
Kumar A, Kim JH, Ranjan P, Metcalfe MG, Cao W, Mishina M, et al. Influenza virus exploits tunneling nanotubes for cell-to-cell spread. Sci Rep. 2017;7:40360.
Lu J, Zheng X, Li F, Yu Y, Chen Z, Liu Z, et al. Tunneling nanotubes promote intercellular mitochondria transfer followed by increased invasiveness in bladder cancer cells. Oncotarget. 2017;8(9):15539–52.
Chinnery HR, Keller KE. Tunneling nanotubes and the eye: intercellular communication and implications for ocular health and disease. Biomed Res Int. 2020;2020:7246785.
Keller KE, Bradley JM, Sun YY, Yang YF, Acott TS. Tunneling nanotubes are novel cellular structures that communicate signals between trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2017;58(12):5298–307.
Li RF, Zhang W, Man QW, Zhao YF, Zhao Y. Tunneling nanotubes mediate intercellular communication between endothelial progenitor cells and osteoclast precursors. J Mol Histol. 2019;50(5):483–91.
Panasiuk M, Rychlowski M, Derewonko N, Bienkowska-Szewczyk K. Tunneling nanotubes as a novel route of cell-to-cell spread of herpesviruses. J Virol. 2018;92(10):10–1128.
de Rooij B, Polak R, Stalpers F, Pieters R, den Boer ML. Tunneling nanotubes facilitate autophagosome transfer in the leukemic niche. Leukemia. 2017;31(7):1651–4.
Tiwari V, Koganti R, Russell G, Sharma A, Shukla D. Role of tunneling nanotubes in viral infection, neurodegenerative disease, and cancer. Front Immunol. 2021;12: 680891.
Pepe A, Pietropaoli S, Vos M, Barba-Spaeth G, Zurzolo C. Tunneling nanotubes provide a route for SARS-CoV-2 spreading. Sci Adv. 2022;8(29):eabo0171.
Wang X, Bukoreshtliev NV, Gerdes HH. Developing neurons form transient nanotubes facilitating electrical coupling and calcium signaling with distant astrocytes. PLoS ONE. 2012;7(10): e47429.
Chinnery HR, Pearlman E, McMenamin PG. Cutting edge: membrane nanotubes in vivo: a feature of MHC class II+ cells in the mouse cornea. J Immunol. 2008;180(9):5779–83.
Alarcon-Martinez L, Villafranca-Baughman D, Quintero H, Kacerovsky JB, Dotigny F, Murai KK, et al. Interpericyte tunnelling nanotubes regulate neurovascular coupling. Nature. 2020;585(7823):91–5.
Sartori-Rupp A, Cordero Cervantes D, Pepe A, Gousset K, Delage E, Corroyer-Dulmont S, et al. Correlative cryo-electron microscopy reveals the structure of TNTs in neuronal cells. Nat Commun. 2019;10(1):342.
Resnik N, Erman A, Veranic P, Kreft ME. Triple labelling of actin filaments, intermediate filaments and microtubules for broad application in cell biology: uncovering the cytoskeletal composition in tunneling nanotubes. Histochem Cell Biol. 2019;152(4):311–7.
Kolba MD, Dudka W, Zareba-Koziol M, Kominek A, Ronchi P, Turos L, et al. Tunneling nanotube-mediated intercellular vesicle and protein transfer in the stroma-provided imatinib resistance in chronic myeloid leukemia cells. Cell Death Dis. 2019;10(11):817.
Reichert D, Scheinpflug J, Karbanova J, Freund D, Bornhauser M, Corbeil D. Tunneling nanotubes mediate the transfer of stem cell marker CD133 between hematopoietic progenitor cells. Exp Hematol. 2016;44(11):1092-112 e2.
Saenz-de-Santa-Maria I, Bernardo-Castineira C, Enciso E, Garcia-Moreno I, Chiara JL, Suarez C, et al. Control of long-distance cell-to-cell communication and autophagosome transfer in squamous cell carcinoma via tunneling nanotubes. Oncotarget. 2017;8(13):20939–60.
Henderson JM, Ljubojevic N, Chaze T, Castaneda D, Battistella A, Gianetto QG, et al. A balance between actin and Eps8/IRSp53 utilization in branched versus linear actin networks determines tunneling nanotube formation. BioRXiv. 2022.
Zhu S, Bhat S, Syan S, Kuchitsu Y, Fukuda M, Zurzolo C. Rab11a-Rab8a cascade regulates the formation of tunneling nanotubes through vesicle recycling. J Cell Sci. 2018;131(19):jcs215889.
Kuhns S, Seixas C, Pestana S, Tavares B, Nogueira R, Jacinto R, et al. Rab35 controls cilium length, function and membrane composition. EMBO Rep. 2019;20(10): e47625.
Zhu H, Xue C, Xu X, Guo Y, Li X, Lu J, et al. Rab8a/Rab11a regulate intercellular communications between neural cells via tunneling nanotubes. Cell Death Dis. 2016;7(12): e2523.
Dubois F, Jean-Jacques B, Roberge H, Benard M, Galas L, Schapman D, et al. A role for RASSF1A in tunneling nanotube formation between cells through GEFH1/Rab11 pathway control. Cell Commun Signal. 2018;16(1):66.
Bhat S, Ljubojevic N, Zhu S, Fukuda M, Echard A, Zurzolo C. Rab35 and its effectors promote formation of tunneling nanotubes in neuronal cells. Sci Rep. 2020;10(1):16803.
Hase K, Kimura S, Takatsu H, Ohmae M, Kawano S, Kitamura H, et al. M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nat Cell Biol. 2009;11(12):1427–32.
Kimura S, Yamashita M, Yamakami-Kimura M, Sato Y, Yamagata A, Kobashigawa Y, et al. Distinct roles for the N- and C-terminal regions of M-Sec in plasma membrane deformation during tunneling nanotube formation. Sci Rep. 2016;6:33548.
Lotfi S, Nasser H, Noyori O, Hiyoshi M, Takeuchi H, Koyanagi Y, et al. M-Sec facilitates intercellular transmission of HIV-1 through multiple mechanisms. Retrovirology. 2020;17(1):20.
Schiller C, Diakopoulos KN, Rohwedder I, Kremmer E, von Toerne C, Ueffing M, et al. LST1 promotes the assembly of a molecular machinery responsible for tunneling nanotube formation. J Cell Sci. 2013;126(Pt 3):767–77.
CAS PubMed Google Scholar
Gousset K, Marzo L, Commere PH, Zurzolo C. Myo10 is a key regulator of TNT formation in neuronal cells. J Cell Sci. 2013;126(Pt 19):4424–35.
Delage E, Cervantes DC, Penard E, Schmitt C, Syan S, Disanza A, et al. Differential identity of Filopodia and Tunneling Nanotubes revealed by the opposite functions of actin regulatory complexes. Sci Rep. 2016;6:39632.
Dagar S, Pushpa K, Pathak D, Samaddar S, Saxena A, Banerjee S, et al. Nucleolin regulates 14-3-3zeta mRNA and promotes cofilin phosphorylation to induce tunneling nanotube formation. FASEB J. 2021;35(1): e21199.
Vargas JY, Loria F, Wu YJ, Cordova G, Nonaka T, Bellow S, et al. The Wnt/Ca(2+) pathway is involved in interneuronal communication mediated by tunneling nanotubes. EMBO J. 2019;38(23): e101230.
Kast DJ, Dominguez R. Mechanism of IRSp53 inhibition by 14-3-3. Nat Commun. 2019;10(1):483.
Bisi S, Marchesi S, Rizvi A, Carra D, Beznoussenko GV, Ferrara I, et al. IRSp53 controls plasma membrane shape and polarized transport at the nascent lumen in epithelial tubules. Nat Commun. 2020;11(1):3516.
Scheiblich H, Dansokho C, Mercan D, Schmidt SV, Bousset L, Wischhof L, et al. Microglia jointly degrade fibrillar alpha-synuclein cargo by distribution through tunneling nanotubes. Cell. 2021;184(20):5089-106 e21.
Hanna SJ, McCoy-Simandle K, Miskolci V, Guo P, Cammer M, Hodgson L, et al. The role of Rho-GTPases and actin polymerization during macrophage tunneling nanotube biogenesis. Sci Rep. 2017;7(1):8547.
Bohil AB, Robertson BW, Cheney RE. Myosin-X is a molecular motor that functions in filopodia formation. Proc Natl Acad Sci USA. 2006;103(33):12411–6.
Kerber ML, Cheney RE. Myosin-X: a MyTH-FERM myosin at the tips of filopodia. J Cell Sci. 2011;124(Pt 22):3733–41.
Sun X, Wang Y, Zhang J, Tu J, Wang XJ, Su XD, et al. Tunneling-nanotube direction determination in neurons and astrocytes. Cell Death Dis. 2012;3(12): e438.
Eberhardt EL, Ludlam AV, Tan Z, Cianfrocco MA. Miro: a molecular switch at the center of mitochondrial regulation. Protein Sci. 2020;29(6):1269–84.
Nahacka Z, Zobalova R, Dubisova M, Rohlena J, Neuzil J. Miro proteins connect mitochondrial function and intercellular transport. Crit Rev Biochem Mol Biol. 2021;56(4):401–25.
van Spronsen M, Mikhaylova M, Lipka J, Schlager MA, van den Heuvel DJ, Kuijpers M, et al. TRAK/Milton motor-adaptor proteins steer mitochondrial trafficking to axons and dendrites. Neuron. 2013;77(3):485–502.
Birsa N, Norkett R, Higgs N, Lopez-Domenech G, Kittler JT. Mitochondrial trafficking in neurons and the role of the Miro family of GTPase proteins. Biochem Soc Trans. 2013;41(6):1525–31.
Oeding SJ, Majstrowicz K, Hu XP, Schwarz V, Freitag A, Honnert U, et al. Identification of Miro1 and Miro2 as mitochondrial receptors for myosin XIX. J Cell Sci. 2018;131(17):jcs219469.
Lopez-Domenech G, Covill-Cooke C, Ivankovic D, Halff EF, Sheehan DF, Norkett R, et al. Miro proteins coordinate microtubule- and actin-dependent mitochondrial transport and distribution. EMBO J. 2018;37(3):321–36.
Ahmad T, Mukherjee S, Pattnaik B, Kumar M, Singh S, Kumar M, et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014;33(9):994–1010.
CAS PubMed PubMed Central Google Scholar
Babenko VA, Silachev DN, Popkov VA, Zorova LD, Pevzner IB, Plotnikov EY, et al. Miro1 enhances mitochondria transfer from multipotent mesenchymal stem cells (MMSC) to neural cells and improves the efficacy of cell recovery. Molecules. 2018;23(3):687.
English K, Shepherd A, Uzor NE, Trinh R, Kavelaars A, Heijnen CJ. Astrocytes rescue neuronal health after cisplatin treatment through mitochondrial transfer. Acta Neuropathol Commun. 2020;8(1):36.
Tseng N, Lambie SC, Huynh CQ, Sanford B, Patel M, Herson PS, et al. Mitochondrial transfer from mesenchymal stem cells improves neuronal metabolism after oxidant injury in vitro: The role of Miro1. J Cereb Blood Flow Metab. 2021;41(4):761–70.
Wang F, Chen X, Cheng H, Song L, Liu J, Caplan S, et al. MICAL2PV suppresses the formation of tunneling nanotubes and modulates mitochondrial trafficking. EMBO Rep. 2021;22(7): e52006.
Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759–65.
Yao Y, Fan XL, Jiang D, Zhang Y, Li X, Xu ZB, et al. Connexin 43-mediated mitochondrial transfer of iPSC-MSCs alleviates asthma inflammation. Stem cell reports. 2018;11(5):1120–35.
Golan K, Singh AK, Kollet O, Bertagna M, Althoff MJ, Khatib-Massalha E, et al. Bone marrow regeneration requires mitochondrial transfer from donor Cx43-expressing hematopoietic progenitors to stroma. Blood. 2020;136(23):2607–19.
Thuringer D, Jego G, Berthenet K, Hammann A, Solary E, Garrido C. Gap junction-mediated transfer of miR-145-5p from microvascular endothelial cells to colon cancer cells inhibits angiogenesis. Oncotarget. 2016;7(19):28160–8.
Boengler K, Leybaert L, Ruiz-Meana M, Schulz R. Connexin 43 in mitochondria: what do we really know about its function? Front Physiol. 2022;13: 928934.
Gadicherla AK, Wang N, Bulic M, Agullo-Pascual E, Lissoni A, De Smet M, et al. Mitochondrial Cx43 hemichannels contribute to mitochondrial calcium entry and cell death in the heart. Basic Res Cardiol. 2017;112(3):27.
Osswald M, Jung E, Sahm F, Solecki G, Venkataramani V, Blaes J, et al. Brain tumour cells interconnect to a functional and resistant network. Nature. 2015;528(7580):93–8.
Tishchenko A, Azorin DD, Vidal-Brime L, Munoz MJ, Arenas PJ, Pearce C, et al. Cx43 and associated cell signaling pathways regulate tunneling nanotubes in breast cancer cells. Cancers. 2020;12(10):2798.
Li X. Gap junction protein connexin43 and tunneling nanotubes in human trabecular meshwork cells. Int J Physiol Pathophysiol Pharmacol. 2019;11(5):212–9.
Norris RP. Transfer of mitochondria and endosomes between cells by gap junction internalization. Traffic. 2021;22(6):174–9.
Dixson AC, Dawson TR, Di Vizio D, Weaver AM. Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nat Rev Mol Cell Biol. 2023;24(7):454–76.
Welsh JA, Holloway JA, Wilkinson JS, Englyst NA. Extracellular vesicle flow cytometry analysis and standardization. Front Cell Dev Biol. 2017;5:78.
Isaac R, Reis FCG, Ying W, Olefsky JM. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 2021;33(9):1744–62.
D’Acunzo P, Perez-Gonzalez R, Kim Y, Hargash T, Miller C, Alldred MJ, et al. Mitovesicles are a novel population of extracellular vesicles of mitochondrial origin altered in Down syndrome. Sci Adv. 2021;7(7):eabe5085.
D’Acunzo P, Ungania JM, Kim Y, Barreto BR, DeRosa S, Pawlik M, et al. Cocaine perturbs mitovesicle biology in the brain. J Extracell Vesicles. 2023;12(1): e12301.
Kim Y, Perez-Gonzalez R, Miller C, Kurz M, D’Acunzo P, Goulbourne CN, et al. Sex differentially alters secretion of brain extracellular vesicles during aging: a potential mechanism for maintaining brain homeostasis. Neurochem Res. 2022;47(11):3428–39.
She Z, Xie M, Hun M, Abdirahman AS, Li C, Wu F, et al. Immunoregulatory effects of mitochondria transferred by extracellular vesicles. Front Immunol. 2020;11: 628576.
Brestoff JR, Wilen CB, Moley JR, Li Y, Zou W, Malvin NP, et al. Intercellular mitochondria transfer to macrophages regulates white adipose tissue homeostasis and is impaired in obesity. Cell Metab. 2021;33(2):270-82 e8.
Zou X, Kwon SH, Jiang K, Ferguson CM, Puranik AS, Zhu X, et al. Renal scattered tubular-like cells confer protective effects in the stenotic murine kidney mediated by release of extracellular vesicles. Sci Rep. 2018;8(1):1263.
Dave KM, Stolz DB, Venna VR, Quaicoe VA, Maniskas ME, Reynolds MJ, et al. Mitochondria-containing extracellular vesicles (EV) reduce mouse brain infarct sizes and EV/HSP27 protect ischemic brain endothelial cultures. J Control Release. 2023;354:368–93.
Crewe C, Funcke JB, Li S, Joffin N, Gliniak CM, Ghaben AL, et al. Extracellular vesicle-based interorgan transport of mitochondria from energetically stressed adipocytes. Cell Metab. 2021;33(9):1853-68 e11.
Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O’Kane CM, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196(10):1275–86.
Hough KP, Trevor JL, Strenkowski JG, Wang Y, Chacko BK, Tousif S, et al. Exosomal transfer of mitochondria from airway myeloid-derived regulatory cells to T cells. Redox Biol. 2018;18:54–64.
Hough KP, Deshane JS. Exosomes in allergic airway diseases. Curr Allergy Asthma Rep. 2019;19(5):26.
Levoux J, Prola A, Lafuste P, Gervais M, Chevallier N, Koumaiha Z, et al. Platelets facilitate the wound-healing capability of mesenchymal stem cells by mitochondrial transfer and metabolic reprogramming. Cell Metab. 2021;33(2):283–99.
Zhao M, Liu S, Wang C, Wang Y, Wan M, Liu F, et al. Mesenchymal stem cell-derived extracellular vesicles attenuate mitochondrial damage and inflammation by stabilizing mitochondrial DNA. ACS Nano. 2021;15(1):1519–38.
Lyamzaev KG, Zinovkin RA, Chernyak BV. Extrusion of mitochondria: garbage clearance or cell-cell communication signals? J Cell Physiol. 2022;237(5):2345–56.
Bagheri HS, Bani F, Tasoglu S, Zarebkohan A, Rahbarghazi R, Sokullu E. Mitochondrial donation in translational medicine; from imagination to reality. J Transl Med. 2020;18(1):367.
Weimann JM, Johansson CB, Trejo A, Blau HM. Stable reprogrammed heterokaryons form spontaneously in Purkinje neurons after bone marrow transplant. Nat Cell Biol. 2003;5(11):959–66.
Noubissi FK, Harkness T, Alexander CM, Ogle BM. Apoptosis-induced cancer cell fusion: a mechanism of breast cancer metastasis. FASEB J. 2015;29(9):4036–45.
Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425(6961):968–73.
Acquistapace A, Bru T, Lesault PF, Figeac F, Coudert AE, le Coz O, et al. Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer. Stem cells. 2011;29(5):812–24.
Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416(6880):542–5.
Cusulin C, Monni E, Ahlenius H, Wood J, Brune JC, Lindvall O, et al. Embryonic stem cell-derived neural stem cells fuse with microglia and mature neurons. Stem cells. 2012;30(12):2657–71.
Murray LMA, Krasnodembskaya AD. Concise review: intercellular communication via organelle transfer in the biology and therapeutic applications of stem cells. Stem cells. 2019;37(1):14–25.
Lyamzaev KG, Nepryakhina OK, Saprunova VB, Bakeeva LE, Pletjushkina OY, Chernyak BV, et al. Novel mechanism of elimination of malfunctioning mitochondria (mitoptosis): formation of mitoptotic bodies and extrusion of mitochondrial material from the cell. Biochem Biophys Acta. 2008;1777(7–8):817–25.
Nakajima A, Kurihara H, Yagita H, Okumura K, Nakano H. Mitochondrial Extrusion through the cytoplasmic vacuoles during cell death. J Biol Chem. 2008;283(35):24128–35.
Lyamzaev KG, Pletjushkina OY, Saprunova VB, Bakeeva LE, Chernyak BV, Skulachev VP. Selective elimination of mitochondria from living cells induced by inhibitors of bioenergetic functions. Biochem Soc Trans. 2004;32(Pt 6):1070–1.
Lyamzaev KG, Knorre DA, Chernyak BV. Mitoptosis, twenty years after. Biochem Biokhimiia. 2020;85(12):1484–98.
Nicolas-Avila JA, Lechuga-Vieco AV, Esteban-Martinez L, Sanchez-Diaz M, Diaz-Garcia E, Santiago DJ, et al. A network of macrophages supports mitochondrial homeostasis in the heart. Cell. 2020;183(1):94-109 e23.
Davis CH, Kim KY, Bushong EA, Mills EA, Boassa D, Shih T, et al. Transcellular degradation of axonal mitochondria. Proc Natl Acad Sci USA. 2014;111(26):9633–8.
Melentijevic I, Toth ML, Arnold ML, Guasp RJ, Harinath G, Nguyen KC, et al. C. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress. Nature. 2017;542(7641):367–71.
Unuma K, Aki T, Funakoshi T, Hashimoto K, Uemura K. Extrusion of mitochondrial contents from lipopolysaccharide-stimulated cells: involvement of autophagy. Autophagy. 2015;11(9):1520–36.
Maeda A, Fadeel B. Mitochondria released by cells undergoing TNF-alpha-induced necroptosis act as danger signals. Cell Death Dis. 2014;5(7): e1312.
Boudreau LH, Duchez AC, Cloutier N, Soulet D, Martin N, Bollinger J, et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood. 2014;124(14):2173–83.
Ma L, Li Y, Peng J, Wu D, Zhao X, Cui Y, et al. Discovery of the migrasome, an organelle mediating release of cytoplasmic contents during cell migration. Cell Res. 2015;25(1):24–38.
Huang Y, Zucker B, Zhang S, Elias S, Zhu Y, Chen H, et al. Migrasome formation is mediated by assembly of micron-scale tetraspanin macrodomains. Nat Cell Biol. 2019;21(8):991–1002.
Jiao H, Jiang D, Hu X, Du W, Ji L, Yang Y, et al. Mitocytosis, a migrasome-mediated mitochondrial quality-control process. Cell. 2021;184(11):2896–910.
Yu S, Yu L. Migrasome biogenesis and functions. FEBS J. 2022;289(22):7246–54.
Savukinas UB, Enes SR, Sjoland AA, Westergren-Thorsson G. Concise review: the bystander effect: mesenchymal stem cell-mediated lung repair. Stem cells. 2016;34(6):1437–44.
Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303(5660):1007–10.
Jiang D, Chen FX, Zhou H, Lu YY, Tan H, Yu SJ, et al. Bioenergetic crosstalk between mesenchymal stem cells and various ocular cells through the intercellular trafficking of mitochondria. Theranostics. 2020;10(16):7260–72.
Han D, Zheng X, Wang X, Jin T, Cui L, Chen Z. Mesenchymal stem/stromal cell-mediated mitochondrial transfer and the therapeutic potential in treatment of neurological diseases. Stem cells international. 2020;2020:8838046.
Regmi S, Liu DD, Shen M, Kevadiya BD, Ganguly A, Primavera R, et al. Mesenchymal stromal cells for the treatment of Alzheimer’s disease: Strategies and limitations. Front Mol Neurosci. 2022;15:1011225.
Ben-Shachar D, Laifenfeld D. Mitochondria, synaptic plasticity, and schizophrenia. Int Rev Neurobiol. 2004;59:273–96.
Sun T, Ma QH. Repairing neural injuries using human umbilical cord blood. Mol Neurobiol. 2013;47(3):938–45.
Jurkunas UV. Fuchs endothelial corneal dystrophy through the prism of oxidative stress. Cornea. 2018;37(Suppl 1):S50–4.
Kumar B, Kowluru A, Kowluru RA. Lipotoxicity augments glucotoxicity-induced mitochondrial damage in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2015;56(5):2985–92.
Zhong Q, Kowluru RA. Diabetic retinopathy and damage to mitochondrial structure and transport machinery. Invest Ophthalmol Vis Sci. 2011;52(12):8739–46.
Jiang D, Xiong G, Feng H, Zhang Z, Chen P, Yan B, et al. Donation of mitochondria by iPSC-derived mesenchymal stem cells protects retinal ganglion cells against mitochondrial complex I defect-induced degeneration. Theranostics. 2019;9(8):2395–410.
Subramaniam MD, Chirayath RB, Iyer M, Nair AP, Vellingiri B. Mesenchymal stem cells (MSCs) in Leber’s hereditary optic neuropathy (LHON): a potential therapeutic approach for future. Int Ophthalmol. 2022;42(9):2949–64.
Crestini A, Santilli F, Martellucci S, Carbone E, Sorice M, Piscopo P, et al. Prions and neurodegenerative diseases: a focus on Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2022;85(2):503–18.
Lee NK, Park SE, Kwon SJ, Shim S, Byeon Y, Kim JH, et al. Agouti related peptide secreted via human mesenchymal stem cells upregulates proteasome activity in an Alzheimer’s disease model. Sci Rep. 2017;7:39340.
Mendonca CF, Kuras M, Nogueira FCS, Pla I, Hortobagyi T, Csiba L, et al. Proteomic signatures of brain regions affected by tau pathology in early and late stages of Alzheimer’s disease. Neurobiol Dis. 2019;130: 104509.
Neves AF, Camargo C, Premer C, Hare JM, Baumel BS, Pinto M. Intravenous administration of mesenchymal stem cells reduces Tau phosphorylation and inflammation in the 3xTg-AD mouse model of Alzheimer’s disease. Exp Neurol. 2021;341: 113706.
Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Hum Mol Genet. 2011;20(13):2495–509.
Manczak M, Reddy PH. Abnormal interaction between the mitochondrial fission protein Drp1 and hyperphosphorylated tau in Alzheimer’s disease neurons: implications for mitochondrial dysfunction and neuronal damage. Hum Mol Genet. 2012;21(11):2538–47.
Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–95.
Kim JY, Kim DH, Kim JH, Lee D, Jeon HB, Kwon SJ, et al. Soluble intracellular adhesion molecule-1 secreted by human umbilical cord blood-derived mesenchymal stem cell reduces amyloid-beta plaques. Cell Death Differ. 2012;19(4):680–91.
Sha S, Shen X, Cao Y, Qu L. Mesenchymal stem cells-derived extracellular vesicles ameliorate Alzheimer’s disease in rat models via the microRNA-29c-3p/BACE1 axis and the Wnt/beta-catenin pathway. Aging. 2021;13(11):15285–306.
Yokokawa K, Iwahara N, Hisahara S, Emoto MC, Saito T, Suzuki H, et al. Transplantation of mesenchymal stem cells improves amyloid-beta pathology by modifying microglial function and suppressing oxidative stress. J Alzheimer’s Dis JAD. 2019;72(3):867–84.
Cui Y, Ma S, Zhang C, Cao W, Liu M, Li D, et al. Human umbilical cord mesenchymal stem cells transplantation improves cognitive function in Alzheimer’s disease mice by decreasing oxidative stress and promoting hippocampal neurogenesis. Behav Brain Res. 2017;320:291–301.
Zhang Z, Sheng H, Liao L, Xu C, Zhang A, Yang Y, et al. Mesenchymal stem cell-conditioned medium improves mitochondrial dysfunction and suppresses apoptosis in okadaic acid-treated SH-SY5Y cells by extracellular vesicle mitochondrial transfer. J Alzheimer’s Dis JAD. 2020;78(3):1161–76.
Alves AA, Andrade LM, Barbosa AF, Bediaga I, Cernicchiaro G, Guerrer G, et al. The LHCb Detector at the LHC. J Instrum. 2008;3:S08005.
Oyinbo CA. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp. 2011;71(2):281–99.
Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552(Pt 2):335–44.
Scholpa NE, Schnellmann RG. Mitochondrial-based therapeutics for the treatment of spinal cord injury: mitochondrial biogenesis as a potential pharmacological target. J Pharmacol Exp Ther. 2017;363(3):303–13.
Shende P, Subedi M. Pathophysiology, mechanisms and applications of mesenchymal stem cells for the treatment of spinal cord injury. Biomed Pharmacother Biomed Pharmacother. 2017;91:693–706.
Lin L, Lin H, Bai S, Zheng L, Zhang X. Bone marrow mesenchymal stem cells (BMSCs) improved functional recovery of spinal cord injury partly by promoting axonal regeneration. Neurochem Int. 2018;115:80–4.
Yang EZ, Zhang GW, Xu JG, Chen S, Wang H, Cao LL, et al. Multichannel polymer scaffold seeded with activated Schwann cells and bone mesenchymal stem cells improves axonal regeneration and functional recovery after rat spinal cord injury. Acta Pharmacol Sin. 2017;38(5):623–37.
Poudineh M, Wang Z, Labib M, Ahmadi M, Zhang L, Das J, et al. Three-dimensional nanostructured architectures enable efficient neural differentiation of mesenchymal stem cells via mechanotransduction. Nano Lett. 2018;18(11):7188–93.
Sykova E, Jendelova P, Urdzikova L, Lesny P, Hejcl A. Bone marrow stem cells and polymer hydrogels–two strategies for spinal cord injury repair. Cell Mol Neurobiol. 2006;26(7–8):1113–29.
Deng WS, Ma K, Liang B, Liu XY, Xu HY, Zhang J, et al. Collagen scaffold combined with human umbilical cord-mesenchymal stem cells transplantation for acute complete spinal cord injury. Neural Regen Res. 2020;15(9):1686–700.
Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Ischemia/reperfusion. Compr Physiol. 2016;7(1):113–70.
Anzell AR, Maizy R, Przyklenk K, Sanderson TH. Mitochondrial quality control and disease: insights into ischemia-reperfusion injury. Mol Neurobiol. 2018;55(3):2547–64.
Liu K, Ji K, Guo L, Wu W, Lu H, Shan P, et al. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia-reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc Res. 2014;92:10–8.
Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88(2):581–609.
Halestrap AP. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem Soc Trans. 2006;34(Pt 2):232–7.
Luongo TS, Lambert JP, Gross P, Nwokedi M, Lombardi AA, Shanmughapriya S, et al. The mitochondrial Na(+)/Ca(2+) exchanger is essential for Ca(2+) homeostasis and viability. Nature. 2017;545(7652):93–7.
Soundara Rajan T, Gugliandolo A, Bramanti P, Mazzon E. Tunneling nanotubes-mediated protection of mesenchymal stem cells: an update from preclinical studies. Int J Mol Sci. 2020;21(10):3481.
Yamada Y, Ito M, Arai M, Hibino M, Tsujioka T, Harashima H. Challenges in promoting mitochondrial transplantation therapy. Int J Mol Sci. 2020;21(17):6365.
McCully JD, Cowan DB, Pacak CA, Toumpoulis IK, Dayalan H, Levitsky S. Injection of isolated mitochondria during early reperfusion for cardioprotection. Am J Physiol Heart Circ Physiol. 2009;296(1):H94–105.
Masuzawa A, Black KM, Pacak CA, Ericsson M, Barnett RJ, Drumm C, et al. Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2013;304(7):H966–82.
Kaza AK, Wamala I, Friehs I, Kuebler JD, Rathod RH, Berra I, et al. Myocardial rescue with autologous mitochondrial transplantation in a porcine model of ischemia/reperfusion. J Thorac Cardiovasc Surg. 2017;153(4):934–43.
Guariento A, Blitzer D, Doulamis I, Shin B, Moskowitzova K, Orfany A, et al. Preischemic autologous mitochondrial transplantation by intracoronary injection for myocardial protection. J Thorac Cardiovasc Surg. 2020;160(2):e15–29.
Blitzer D, Guariento A, Doulamis IP, Shin B, Moskowitzova K, Barbieri GR, et al. Delayed transplantation of autologous mitochondria for cardioprotection in a porcine model. Ann Thorac Surg. 2020;109(3):711–9.
Emani SM, Piekarski BL, Harrild D, Del Nido PJ, McCully JD. Autologous mitochondrial transplantation for dysfunction after ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2017;154(1):286–9.
O’Brien CG, Ozen MO, Ikeda G, Vaskova E, Jung JH, Bayardo N, et al. Mitochondria-rich extracellular vesicles rescue patient-specific cardiomyocytes from doxorubicin injury: insights into the SENECA trial. JACC CardioOncol. 2021;3(3):428–40.
Maremanda KP, Sundar IK, Rahman I. Protective role of mesenchymal stem cells and mesenchymal stem cell-derived exosomes in cigarette smoke-induced mitochondrial dysfunction in mice. Toxicol Appl Pharmacol. 2019;385: 114788.
Kubat GB, Ozler M, Ulger O, Ekinci O, Atalay O, Celik E, et al. The effects of mesenchymal stem cell mitochondrial transplantation on doxorubicin-mediated nephrotoxicity in rats. J Biochem Mol Toxicol. 2021;35(1): e22612.
Lu T, Zhang J, Cai J, Xiao J, Sui X, Yuan X, et al. Extracellular vesicles derived from mesenchymal stromal cells as nanotherapeutics for liver ischaemia-reperfusion injury by transferring mitochondria to modulate the formation of neutrophil extracellular traps. Biomaterials. 2022;284: 121486.
Bi Y, Guo X, Zhang M, Zhu K, Shi C, Fan B, et al. Bone marrow derived-mesenchymal stem cell improves diabetes-associated fatty liver via mitochondria transformation in mice. Stem Cell Res Ther. 2021;12(1):602.
Download references
No funding.
Author information
Roberto Iorio and Sabrina Petricca contributed equally to the manuscript.
Authors and Affiliations
Department of Biotechnological and Applied Clinical Sciences, University of L’Aquila, Via Vetoio, 67100, L’Aquila, Italy
Roberto Iorio, Sabrina Petricca & Simona Delle Monache
Dipartimento di Scienze della Vita, Della Salute e delle Professioni Sanitarie, Link Campus University, Via del Casale di San Pio V 44, 00165, Rome, Italy
Vincenzo Mattei
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization, I.R., V.M. and S.D.M.; writing—original draft preparation, S.P, I.R., S.D.M. and V.M.; writing—review and editing, S.P., I.R., V.M. and S.D.M.; supervision, I.R., V.M. and S.D.M. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Correspondence to Vincenzo Mattei or Simona Delle Monache .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Iorio, R., Petricca, S., Mattei, V. et al. Horizontal mitochondrial transfer as a novel bioenergetic tool for mesenchymal stromal/stem cells: molecular mechanisms and therapeutic potential in a variety of diseases. J Transl Med 22 , 491 (2024). https://doi.org/10.1186/s12967-024-05047-4
Download citation
Received : 21 December 2023
Accepted : 29 February 2024
Published : 24 May 2024
DOI : https://doi.org/10.1186/s12967-024-05047-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Mitochondria
- Horizontal mitochondrial transfer
- Mesenchymal Stromal/Stem cells
- Neuronal diseases
- Ischemic vascular diseases
Journal of Translational Medicine
ISSN: 1479-5876
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Open access
- Published: 26 February 2019
Stem cells: past, present, and future
- Wojciech Zakrzewski 1 ,
- Maciej Dobrzyński 2 ,
- Maria Szymonowicz 1 &
- Zbigniew Rybak 1
Stem Cell Research & Therapy volume 10 , Article number: 68 ( 2019 ) Cite this article
567k Accesses
856 Citations
54 Altmetric
Metrics details
In recent years, stem cell therapy has become a very promising and advanced scientific research topic. The development of treatment methods has evoked great expectations. This paper is a review focused on the discovery of different stem cells and the potential therapies based on these cells. The genesis of stem cells is followed by laboratory steps of controlled stem cell culturing and derivation. Quality control and teratoma formation assays are important procedures in assessing the properties of the stem cells tested. Derivation methods and the utilization of culturing media are crucial to set proper environmental conditions for controlled differentiation. Among many types of stem tissue applications, the use of graphene scaffolds and the potential of extracellular vesicle-based therapies require attention due to their versatility. The review is summarized by challenges that stem cell therapy must overcome to be accepted worldwide. A wide variety of possibilities makes this cutting edge therapy a turning point in modern medicine, providing hope for untreatable diseases.
Stem cell classification
Stem cells are unspecialized cells of the human body. They are able to differentiate into any cell of an organism and have the ability of self-renewal. Stem cells exist both in embryos and adult cells. There are several steps of specialization. Developmental potency is reduced with each step, which means that a unipotent stem cell is not able to differentiate into as many types of cells as a pluripotent one. This chapter will focus on stem cell classification to make it easier for the reader to comprehend the following chapters.
Totipotent stem cells are able to divide and differentiate into cells of the whole organism. Totipotency has the highest differentiation potential and allows cells to form both embryo and extra-embryonic structures. One example of a totipotent cell is a zygote, which is formed after a sperm fertilizes an egg. These cells can later develop either into any of the three germ layers or form a placenta. After approximately 4 days, the blastocyst’s inner cell mass becomes pluripotent. This structure is the source of pluripotent cells.
Pluripotent stem cells (PSCs) form cells of all germ layers but not extraembryonic structures, such as the placenta. Embryonic stem cells (ESCs) are an example. ESCs are derived from the inner cell mass of preimplantation embryos. Another example is induced pluripotent stem cells (iPSCs) derived from the epiblast layer of implanted embryos. Their pluripotency is a continuum, starting from completely pluripotent cells such as ESCs and iPSCs and ending on representatives with less potency—multi-, oligo- or unipotent cells. One of the methods to assess their activity and spectrum is the teratoma formation assay. iPSCs are artificially generated from somatic cells, and they function similarly to PSCs. Their culturing and utilization are very promising for present and future regenerative medicine.
Multipotent stem cells have a narrower spectrum of differentiation than PSCs, but they can specialize in discrete cells of specific cell lineages. One example is a haematopoietic stem cell, which can develop into several types of blood cells. After differentiation, a haematopoietic stem cell becomes an oligopotent cell. Its differentiation abilities are then restricted to cells of its lineage. However, some multipotent cells are capable of conversion into unrelated cell types, which suggests naming them pluripotent cells.
Oligopotent stem cells can differentiate into several cell types. A myeloid stem cell is an example that can divide into white blood cells but not red blood cells.
Unipotent stem cells are characterized by the narrowest differentiation capabilities and a special property of dividing repeatedly. Their latter feature makes them a promising candidate for therapeutic use in regenerative medicine. These cells are only able to form one cell type, e.g. dermatocytes.
Stem cell biology
A blastocyst is formed after the fusion of sperm and ovum fertilization. Its inner wall is lined with short-lived stem cells, namely, embryonic stem cells. Blastocysts are composed of two distinct cell types: the inner cell mass (ICM), which develops into epiblasts and induces the development of a foetus, and the trophectoderm (TE). Blastocysts are responsible for the regulation of the ICM microenvironment. The TE continues to develop and forms the extraembryonic support structures needed for the successful origin of the embryo, such as the placenta. As the TE begins to form a specialized support structure, the ICM cells remain undifferentiated, fully pluripotent and proliferative [ 1 ]. The pluripotency of stem cells allows them to form any cell of the organism. Human embryonic stem cells (hESCs) are derived from the ICM. During the process of embryogenesis, cells form aggregations called germ layers: endoderm, mesoderm and ectoderm (Fig. 1 ), each eventually giving rise to differentiated cells and tissues of the foetus and, later on, the adult organism [ 2 ]. After hESCs differentiate into one of the germ layers, they become multipotent stem cells, whose potency is limited to only the cells of the germ layer. This process is short in human development. After that, pluripotent stem cells occur all over the organism as undifferentiated cells, and their key abilities are proliferation by the formation of the next generation of stem cells and differentiation into specialized cells under certain physiological conditions.
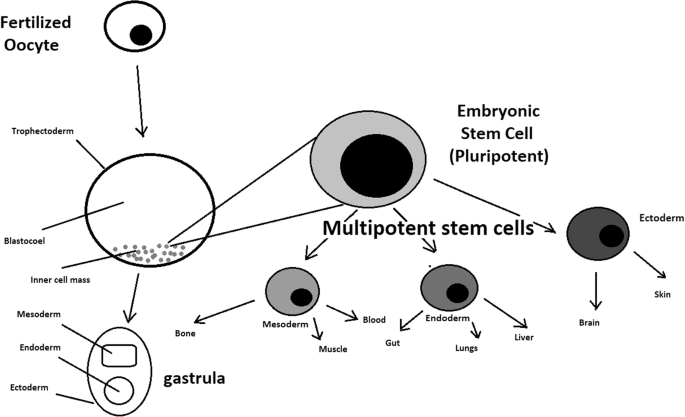
Oocyte development and formation of stem cells: the blastocoel, which is formed from oocytes, consists of embryonic stem cells that later differentiate into mesodermal, ectodermal, or endodermal cells. Blastocoel develops into the gastrula
Signals that influence the stem cell specialization process can be divided into external, such as physical contact between cells or chemical secretion by surrounding tissue, and internal, which are signals controlled by genes in DNA.
Stem cells also act as internal repair systems of the body. The replenishment and formation of new cells are unlimited as long as an organism is alive. Stem cell activity depends on the organ in which they are in; for example, in bone marrow, their division is constant, although in organs such as the pancreas, division only occurs under special physiological conditions.
Stem cell functional division
Whole-body development.
During division, the presence of different stem cells depends on organism development. Somatic stem cell ESCs can be distinguished. Although the derivation of ESCs without separation from the TE is possible, such a combination has growth limits. Because proliferating actions are limited, co-culture of these is usually avoided.
ESCs are derived from the inner cell mass of the blastocyst, which is a stage of pre-implantation embryo ca. 4 days after fertilization. After that, these cells are placed in a culture dish filled with culture medium. Passage is an inefficient but popular process of sub-culturing cells to other dishes. These cells can be described as pluripotent because they are able to eventually differentiate into every cell type in the organism. Since the beginning of their studies, there have been ethical restrictions connected to the medical use of ESCs in therapies. Most embryonic stem cells are developed from eggs that have been fertilized in an in vitro clinic, not from eggs fertilized in vivo.
Somatic or adult stem cells are undifferentiated and found among differentiated cells in the whole body after development. The function of these cells is to enable the healing, growth, and replacement of cells that are lost each day. These cells have a restricted range of differentiation options. Among many types, there are the following:
Mesenchymal stem cells are present in many tissues. In bone marrow, these cells differentiate mainly into the bone, cartilage, and fat cells. As stem cells, they are an exception because they act pluripotently and can specialize in the cells of any germ layer.
Neural cells give rise to nerve cells and their supporting cells—oligodendrocytes and astrocytes.
Haematopoietic stem cells form all kinds of blood cells: red, white, and platelets.
Skin stem cells form, for example, keratinocytes, which form a protective layer of skin.
The proliferation time of somatic stem cells is longer than that of ESCs. It is possible to reprogram adult stem cells back to their pluripotent state. This can be performed by transferring the adult nucleus into the cytoplasm of an oocyte or by fusion with the pluripotent cell. The same technique was used during cloning of the famous Dolly sheep.
hESCs are involved in whole-body development. They can differentiate into pluripotent, totipotent, multipotent, and unipotent cells (Fig. 2 ) [ 2 ].
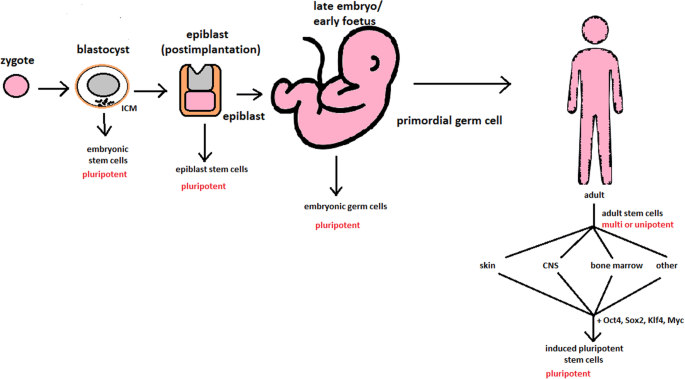
Changes in the potency of stem cells in human body development. Potency ranges from pluripotent cells of the blastocyst to unipotent cells of a specific tissue in a human body such as the skin, CNS, or bone marrow. Reversed pluripotency can be achieved by the formation of induced pluripotent stem cells using either octamer-binding transcription factor (Oct4), sex-determining region Y (Sox2), Kruppel-like factor 4 (Klf4), or the Myc gene
Pluripotent cells can be named totipotent if they can additionally form extraembryonic tissues of the embryo. Multipotent cells are restricted in differentiating to each cell type of given tissue. When tissue contains only one lineage of cells, stem cells that form them are called either called oligo- or unipotent.
iPSC quality control and recognition by morphological differences
The comparability of stem cell lines from different individuals is needed for iPSC lines to be used in therapeutics [ 3 ]. Among critical quality procedures, the following can be distinguished:
Short tandem repeat analysis—This is the comparison of specific loci on the DNA of the samples. It is used in measuring an exact number of repeating units. One unit consists of 2 to 13 nucleotides repeating many times on the DNA strand. A polymerase chain reaction is used to check the lengths of short tandem repeats. The genotyping procedure of source tissue, cells, and iPSC seed and master cell banks is recommended.
Identity analysis—The unintentional switching of lines, resulting in other stem cell line contamination, requires rigorous assay for cell line identification.
Residual vector testing—An appearance of reprogramming vectors integrated into the host genome is hazardous, and testing their presence is a mandatory procedure. It is a commonly used procedure for generating high-quality iPSC lines. An acceptable threshold in high-quality research-grade iPSC line collections is ≤ 1 plasmid copies per 100 cells. During the procedure, 2 different regions, common to all plasmids, should be used as specific targets, such as EBNA and CAG sequences [ 3 ]. To accurately represent the test reactions, a standard curve needs to be prepared in a carrier of gDNA from a well-characterized hPSC line. For calculations of plasmid copies per cell, it is crucial to incorporate internal reference gDNA sequences to allow the quantification of, for example, ribonuclease P (RNaseP) or human telomerase reverse transcriptase (hTERT).
Karyotype—A long-term culture of hESCs can accumulate culture-driven mutations [ 4 ]. Because of that, it is crucial to pay additional attention to genomic integrity. Karyotype tests can be performed by resuscitating representative aliquots and culturing them for 48–72 h before harvesting cells for karyotypic analysis. If abnormalities are found within the first 20 karyotypes, the analysis must be repeated on a fresh sample. When this situation is repeated, the line is evaluated as abnormal. Repeated abnormalities must be recorded. Although karyology is a crucial procedure in stem cell quality control, the single nucleotide polymorphism (SNP) array, discussed later, has approximately 50 times higher resolution.
Viral testing—When assessing the quality of stem cells, all tests for harmful human adventitious agents must be performed (e.g. hepatitis C or human immunodeficiency virus). This procedure must be performed in the case of non-xeno-free culture agents.
Bacteriology—Bacterial or fungal sterility tests can be divided into culture- or broth-based tests. All the procedures must be recommended by pharmacopoeia for the jurisdiction in which the work is performed.
Single nucleotide polymorphism arrays—This procedure is a type of DNA microarray that detects population polymorphisms by enabling the detection of subchromosomal changes and the copy-neutral loss of heterozygosity, as well as an indication of cellular transformation. The SNP assay consists of three components. The first is labelling fragmented nucleic acid sequences with fluorescent dyes. The second is an array that contains immobilized allele-specific oligonucleotide (ASO) probes. The last component detects, records, and eventually interprets the signal.
Flow cytometry—This is a technique that utilizes light to count and profile cells in a heterogeneous fluid mixture. It allows researchers to accurately and rapidly collect data from heterogeneous fluid mixtures with live cells. Cells are passed through a narrow channel one by one. During light illumination, sensors detect light emitted or refracted from the cells. The last step is data analysis, compilation and integration into a comprehensive picture of the sample.
Phenotypic pluripotency assays—Recognizing undifferentiated cells is crucial in successful stem cell therapy. Among other characteristics, stem cells appear to have a distinct morphology with a high nucleus to cytoplasm ratio and a prominent nucleolus. Cells appear to be flat with defined borders, in contrast to differentiating colonies, which appear as loosely located cells with rough borders [ 5 ]. It is important that images of ideal and poor quality colonies for each cell line are kept in laboratories, so whenever there is doubt about the quality of culture, it can always be checked according to the representative image. Embryoid body formation or directed differentiation of monolayer cultures to produce cell types representative of all three embryonic germ layers must be performed. It is important to note that colonies cultured under different conditions may have different morphologies [ 6 ].
Histone modification and DNA methylation—Quality control can be achieved by using epigenetic analysis tools such as histone modification or DNA methylation. When stem cells differentiate, the methylation process silences pluripotency genes, which reduces differentiation potential, although other genes may undergo demethylation to become expressed [ 7 ]. It is important to emphasize that stem cell identity, together with its morphological characteristics, is also related to its epigenetic profile [ 8 , 9 ]. According to Brindley [ 10 ], there is a relationship between epigenetic changes, pluripotency, and cell expansion conditions, which emphasizes that unmethylated regions appear to be serum-dependent.
hESC derivation and media
hESCs can be derived using a variety of methods, from classic culturing to laser-assisted methodologies or microsurgery [ 11 ]. hESC differentiation must be specified to avoid teratoma formation (see Fig. 3 ).
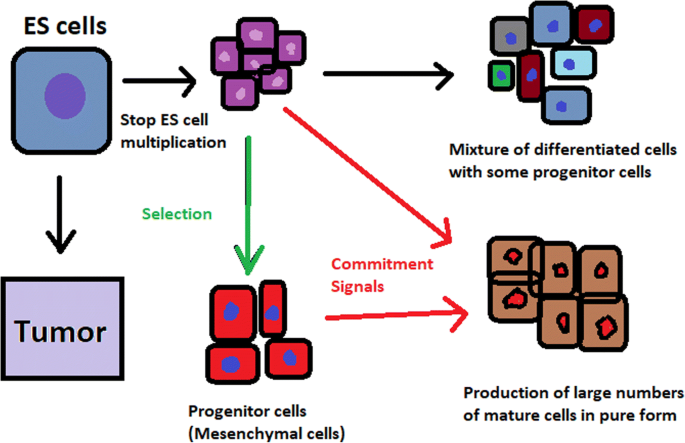
Spontaneous differentiation of hESCs causes the formation of a heterogeneous cell population. There is a different result, however, when commitment signals (in forms of soluble factors and culture conditions) are applied and enable the selection of progenitor cells
hESCs spontaneously differentiate into embryonic bodies (EBs) [ 12 ]. EBs can be studied instead of embryos or animals to predict their effects on early human development. There are many different methods for acquiring EBs, such as bioreactor culture [ 13 ], hanging drop culture [ 12 ], or microwell technology [ 14 , 15 ]. These methods allow specific precursors to form in vitro [ 16 ].
The essential part of these culturing procedures is a separation of inner cell mass to culture future hESCs (Fig. 4 ) [ 17 ]. Rosowski et al. [ 18 ] emphasizes that particular attention must be taken in controlling spontaneous differentiation. When the colony reaches the appropriate size, cells must be separated. The occurrence of pluripotent cells lasts for 1–2 days. Because the classical utilization of hESCs caused ethical concerns about gastrulas used during procedures, Chung et al. [ 19 ] found out that it is also possible to obtain hESCs from four cell embryos, leaving a higher probability of embryo survival. Additionally, Zhang et al. [ 20 ] used only in vitro fertilization growth-arrested cells.
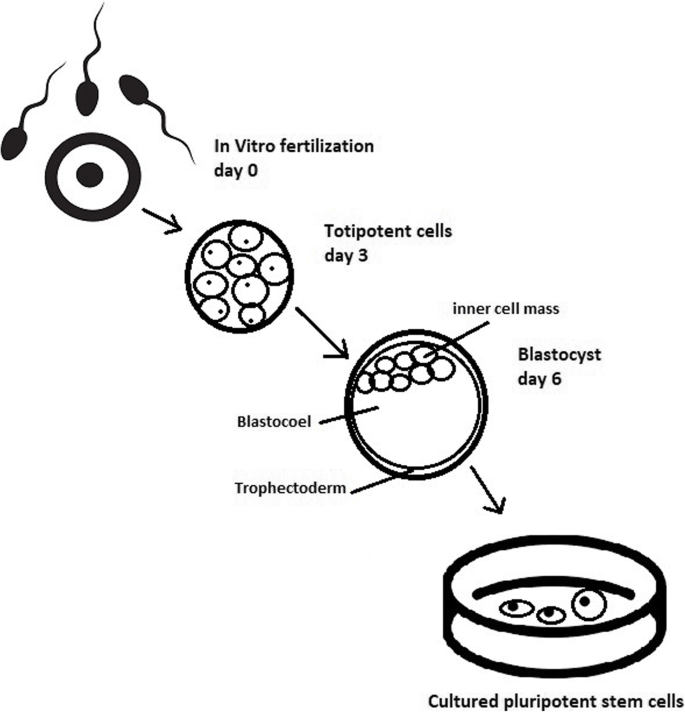
Culturing of pluripotent stem cells in vitro. Three days after fertilization, totipotent cells are formed. Blastocysts with ICM are formed on the sixth day after fertilization. Pluripotent stem cells from ICM can then be successfully transmitted on a dish
Cell passaging is used to form smaller clusters of cells on a new culture surface [ 21 ]. There are four important passaging procedures.
Enzymatic dissociation is a cutting action of enzymes on proteins and adhesion domains that bind the colony. It is a gentler method than the manual passage. It is crucial to not leave hESCs alone after passaging. Solitary cells are more sensitive and can easily undergo cell death; collagenase type IV is an example [ 22 , 23 ].
Manual passage , on the other hand, focuses on using cell scratchers. The selection of certain cells is not necessary. This should be done in the early stages of cell line derivation [ 24 ].
Trypsin utilization allows a healthy, automated hESC passage. Good Manufacturing Practice (GMP)-grade recombinant trypsin is widely available in this procedure [ 24 ]. However, there is a risk of decreasing the pluripotency and viability of stem cells [ 25 ]. Trypsin utilization can be halted with an inhibitor of the protein rho-associated protein kinase (ROCK) [ 26 ].
Ethylenediaminetetraacetic acid ( EDTA ) indirectly suppresses cell-to-cell connections by chelating divalent cations. Their suppression promotes cell dissociation [ 27 ].
Stem cells require a mixture of growth factors and nutrients to differentiate and develop. The medium should be changed each day.
Traditional culture methods used for hESCs are mouse embryonic fibroblasts (MEFs) as a feeder layer and bovine serum [ 28 ] as a medium. Martin et al. [ 29 ] demonstrated that hESCs cultured in the presence of animal products express the non-human sialic acid, N -glycolylneuraminic acid (NeuGc). Feeder layers prevent uncontrolled proliferation with factors such as leukaemia inhibitory factor (LIF) [ 30 ].
First feeder layer-free culture can be supplemented with serum replacement, combined with laminin [ 31 ]. This causes stable karyotypes of stem cells and pluripotency lasting for over a year.
Initial culturing media can be serum (e.g. foetal calf serum FCS), artificial replacement such as synthetic serum substitute (SSS), knockout serum replacement (KOSR), or StemPro [ 32 ]. The simplest culture medium contains only eight essential elements: DMEM/F12 medium, selenium, NaHCO 3, l -ascorbic acid, transferrin, insulin, TGFβ1, and FGF2 [ 33 ]. It is not yet fully known whether culture systems developed for hESCs can be allowed without adaptation in iPSC cultures.
Turning point in stem cell therapy
The turning point in stem cell therapy appeared in 2006, when scientists Shinya Yamanaka, together with Kazutoshi Takahashi, discovered that it is possible to reprogram multipotent adult stem cells to the pluripotent state. This process avoided endangering the foetus’ life in the process. Retrovirus-mediated transduction of mouse fibroblasts with four transcription factors (Oct-3/4, Sox2, KLF4, and c-Myc) [ 34 ] that are mainly expressed in embryonic stem cells could induce the fibroblasts to become pluripotent (Fig. 5 ) [ 35 ]. This new form of stem cells was named iPSCs. One year later, the experiment also succeeded with human cells [ 36 ]. After this success, the method opened a new field in stem cell research with a generation of iPSC lines that can be customized and biocompatible with the patient. Recently, studies have focused on reducing carcinogenesis and improving the conduction system.
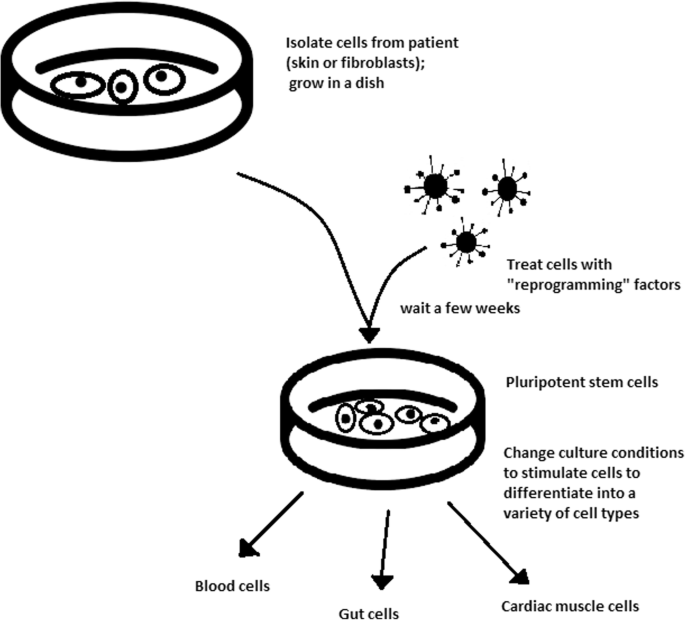
Retroviral-mediated transduction induces pluripotency in isolated patient somatic cells. Target cells lose their role as somatic cells and, once again, become pluripotent and can differentiate into any cell type of human body
The turning point was influenced by former discoveries that happened in 1962 and 1987.
The former discovery was about scientist John Gurdon successfully cloning frogs by transferring a nucleus from a frog’s somatic cells into an oocyte. This caused a complete reversion of somatic cell development [ 37 ]. The results of his experiment became an immense discovery since it was previously believed that cell differentiation is a one-way street only, but his experiment suggested the opposite and demonstrated that it is even possible for a somatic cell to again acquire pluripotency [ 38 ].
The latter was a discovery made by Davis R.L. that focused on fibroblast DNA subtraction. Three genes were found that originally appeared in myoblasts. The enforced expression of only one of the genes, named myogenic differentiation 1 (Myod1), caused the conversion of fibroblasts into myoblasts, showing that reprogramming cells is possible, and it can even be used to transform cells from one lineage to another [ 39 ].
Although pluripotency can occur naturally only in embryonic stem cells, it is possible to induce terminally differentiated cells to become pluripotent again. The process of direct reprogramming converts differentiated somatic cells into iPSC lines that can form all cell types of an organism. Reprogramming focuses on the expression of oncogenes such as Myc and Klf4 (Kruppel-like factor 4). This process is enhanced by a downregulation of genes promoting genome stability, such as p53. Additionally, cell reprogramming involves histone alteration. All these processes can cause potential mutagenic risk and later lead to an increased number of mutations. Quinlan et al. [ 40 ] checked fully pluripotent mouse iPSCs using whole genome DNA sequencing and structural variation (SV) detection algorithms. Based on those studies, it was confirmed that although there were single mutations in the non-genetic region, there were non-retrotransposon insertions. This led to the conclusion that current reprogramming methods can produce fully pluripotent iPSCs without severe genomic alterations.
During the course of development from pluripotent hESCs to differentiated somatic cells, crucial changes appear in the epigenetic structure of these cells. There is a restriction or permission of the transcription of genes relevant to each cell type. When somatic cells are being reprogrammed using transcription factors, all the epigenetic architecture has to be reconditioned to achieve iPSCs with pluripotency [ 41 ]. However, cells of each tissue undergo specific somatic genomic methylation. This influences transcription, which can further cause alterations in induced pluripotency [ 42 ].
Source of iPSCs
Because pluripotent cells can propagate indefinitely and differentiate into any kind of cell, they can be an unlimited source, either for replacing lost or diseased tissues. iPSCs bypass the need for embryos in stem cell therapy. Because they are made from the patient’s own cells, they are autologous and no longer generate any risk of immune rejection.
At first, fibroblasts were used as a source of iPSCs. Because a biopsy was needed to achieve these types of cells, the technique underwent further research. Researchers investigated whether more accessible cells could be used in the method. Further, other cells were used in the process: peripheral blood cells, keratinocytes, and renal epithelial cells found in urine. An alternative strategy to stem cell transplantation can be stimulating a patient’s endogenous stem cells to divide or differentiate, occurring naturally when skin wounds are healing. In 2008, pancreatic exocrine cells were shown to be reprogrammed to functional, insulin-producing beta cells [ 43 ].
The best stem cell source appears to be the fibroblasts, which is more tempting in the case of logistics since its stimulation can be fast and better controlled [ 44 ].
- Teratoma formation assay
The self-renewal and differentiation capabilities of iPSCs have gained significant interest and attention in regenerative medicine sciences. To study their abilities, a quality-control assay is needed, of which one of the most important is the teratoma formation assay. Teratomas are benign tumours. Teratomas are capable of rapid growth in vivo and are characteristic because of their ability to develop into tissues of all three germ layers simultaneously. Because of the high pluripotency of teratomas, this formation assay is considered an assessment of iPSC’s abilities [ 45 ].
Teratoma formation rate, for instance, was observed to be elevated in human iPSCs compared to that in hESCs [ 46 ]. This difference may be connected to different differentiation methods and cell origins. Most commonly, the teratoma assay involves an injection of examined iPSCs subcutaneously or under the testis or kidney capsule in mice, which are immune-deficient [ 47 ]. After injection, an immature but recognizable tissue can be observed, such as the kidney tubules, bone, cartilage, or neuroepithelium [ 30 ]. The injection site may have an impact on the efficiency of teratoma formation [ 48 ].
There are three groups of markers used in this assay to differentiate the cells of germ layers. For endodermal tissue, there is insulin/C-peptide and alpha-1 antitrypsin [ 49 ]. For the mesoderm, derivatives can be used, e.g. cartilage matrix protein for the bone and alcian blue for the cartilage. As ectodermal markers, class III B botulin or keratin can be used for keratinocytes.
Teratoma formation assays are considered the gold standard for demonstrating the pluripotency of human iPSCs, demonstrating their possibilities under physiological conditions. Due to their actual tissue formation, they could be used for the characterization of many cell lineages [ 50 ].
Directed differentiation
To be useful in therapy, stem cells must be converted into desired cell types as necessary or else the whole regenerative medicine process will be pointless. Differentiation of ESCs is crucial because undifferentiated ESCs can cause teratoma formation in vivo. Understanding and using signalling pathways for differentiation is an important method in successful regenerative medicine. In directed differentiation, it is likely to mimic signals that are received by cells when they undergo successive stages of development [ 51 ]. The extracellular microenvironment plays a significant role in controlling cell behaviour. By manipulating the culture conditions, it is possible to restrict specific differentiation pathways and generate cultures that are enriched in certain precursors in vitro. However, achieving a similar effect in vivo is challenging. It is crucial to develop culture conditions that will allow the promotion of homogenous and enhanced differentiation of ESCs into functional and desired tissues.
Regarding the self-renewal of embryonic stem cells, Hwang et al. [ 52 ] noted that the ideal culture method for hESC-based cell and tissue therapy would be a defined culture free of either the feeder layer or animal components. This is because cell and tissue therapy requires the maintenance of large quantities of undifferentiated hESCs, which does not make feeder cells suitable for such tasks.
Most directed differentiation protocols are formed to mimic the development of an inner cell mass during gastrulation. During this process, pluripotent stem cells differentiate into ectodermal, mesodermal, or endodermal progenitors. Mall molecules or growth factors induce the conversion of stem cells into appropriate progenitor cells, which will later give rise to the desired cell type. There is a variety of signal intensities and molecular families that may affect the establishment of germ layers in vivo, such as fibroblast growth factors (FGFs) [ 53 ]; the Wnt family [ 54 ] or superfamily of transforming growth factors—β(TGFβ); and bone morphogenic proteins (BMP) [ 55 ]. Each candidate factor must be tested on various concentrations and additionally applied to various durations because the precise concentrations and times during which developing cells in embryos are influenced during differentiation are unknown. For instance, molecular antagonists of endogenous BMP and Wnt signalling can be used for ESC formation of ectoderm [ 56 ]. However, transient Wnt and lower concentrations of the TGFβ family trigger mesodermal differentiation [ 57 ]. Regarding endoderm formation, a higher activin A concentration may be required [ 58 , 59 ].
There are numerous protocols about the methods of forming progenitors of cells of each of germ layers, such as cardiomyocytes [ 60 ], hepatocytes [ 61 ], renal cells [ 62 ], lung cells [ 63 , 64 ], motor neurons [ 65 ], intestinal cells [ 66 ], or chondrocytes [ 67 ].
Directed differentiation of either iPSCs or ESCs into, e.g. hepatocytes, could influence and develop the study of the molecular mechanisms in human liver development. In addition, it could also provide the possibility to form exogenous hepatocytes for drug toxicity testing [ 68 ].
Levels of concentration and duration of action with a specific signalling molecule can cause a variety of factors. Unfortunately, for now, a high cost of recombinant factors is likely to limit their use on a larger scale in medicine. The more promising technique focuses on the use of small molecules. These can be used for either activating or deactivating specific signalling pathways. They enhance reprogramming efficiency by creating cells that are compatible with the desired type of tissue. It is a cheaper and non-immunogenic method.
One of the successful examples of small-molecule cell therapies is antagonists and agonists of the Hedgehog pathway. They show to be very useful in motor neuron regeneration [ 69 ]. Endogenous small molecules with their function in embryonic development can also be used in in vitro methods to induce the differentiation of cells; for example, retinoic acid, which is responsible for patterning the nervous system in vivo [ 70 ], surprisingly induced retinal cell formation when the laboratory procedure involved hESCs [ 71 ].
The efficacy of differentiation factors depends on functional maturity, efficiency, and, finally, introducing produced cells to their in vivo equivalent. Topography, shear stress, and substrate rigidity are factors influencing the phenotype of future cells [ 72 ].
The control of biophysical and biochemical signals, the biophysical environment, and a proper guide of hESC differentiation are important factors in appropriately cultured stem cells.
Stem cell utilization and their manufacturing standards and culture systems
The European Medicines Agency and the Food and Drug Administration have set Good Manufacturing Practice (GMP) guidelines for safe and appropriate stem cell transplantation. In the past, protocols used for stem cell transplantation required animal-derived products [ 73 ].
The risk of introducing animal antigens or pathogens caused a restriction in their use. Due to such limitations, the technique required an obvious update [ 74 ]. Now, it is essential to use xeno-free equivalents when establishing cell lines that are derived from fresh embryos and cultured from human feeder cell lines [ 75 ]. In this method, it is crucial to replace any non-human materials with xeno-free equivalents [ 76 ].
NutriStem with LN-511, TeSR2 with human recombinant laminin (LN-511), and RegES with human foreskin fibroblasts (HFFs) are commonly used xeno-free culture systems [ 33 ]. There are many organizations and international initiatives, such as the National Stem Cell Bank, that provide stem cell lines for treatment or medical research [ 77 ].
Stem cell use in medicine
Stem cells have great potential to become one of the most important aspects of medicine. In addition to the fact that they play a large role in developing restorative medicine, their study reveals much information about the complex events that happen during human development.
The difference between a stem cell and a differentiated cell is reflected in the cells’ DNA. In the former cell, DNA is arranged loosely with working genes. When signals enter the cell and the differentiation process begins, genes that are no longer needed are shut down, but genes required for the specialized function will remain active. This process can be reversed, and it is known that such pluripotency can be achieved by interaction in gene sequences. Takahashi and Yamanaka [ 78 ] and Loh et al. [ 79 ] discovered that octamer-binding transcription factor 3 and 4 (Oct3/4), sex determining region Y (SRY)-box 2 and Nanog genes function as core transcription factors in maintaining pluripotency. Among them, Oct3/4 and Sox2 are essential for the generation of iPSCs.
Many serious medical conditions, such as birth defects or cancer, are caused by improper differentiation or cell division. Currently, several stem cell therapies are possible, among which are treatments for spinal cord injury, heart failure [ 80 ], retinal and macular degeneration [ 81 ], tendon ruptures, and diabetes type 1 [ 82 ]. Stem cell research can further help in better understanding stem cell physiology. This may result in finding new ways of treating currently incurable diseases.
Haematopoietic stem cell transplantation
Haematopoietic stem cells are important because they are by far the most thoroughly characterized tissue-specific stem cell; after all, they have been experimentally studied for more than 50 years. These stem cells appear to provide an accurate paradigm model system to study tissue-specific stem cells, and they have potential in regenerative medicine.
Multipotent haematopoietic stem cell (HSC) transplantation is currently the most popular stem cell therapy. Target cells are usually derived from the bone marrow, peripheral blood, or umbilical cord blood [ 83 ]. The procedure can be autologous (when the patient’s own cells are used), allogenic (when the stem cell comes from a donor), or syngeneic (from an identical twin). HSCs are responsible for the generation of all functional haematopoietic lineages in blood, including erythrocytes, leukocytes, and platelets. HSC transplantation solves problems that are caused by inappropriate functioning of the haematopoietic system, which includes diseases such as leukaemia and anaemia. However, when conventional sources of HSC are taken into consideration, there are some important limitations. First, there is a limited number of transplantable cells, and an efficient way of gathering them has not yet been found. There is also a problem with finding a fitting antigen-matched donor for transplantation, and viral contamination or any immunoreactions also cause a reduction in efficiency in conventional HSC transplantations. Haematopoietic transplantation should be reserved for patients with life-threatening diseases because it has a multifactorial character and can be a dangerous procedure. iPSC use is crucial in this procedure. The use of a patient’s own unspecialized somatic cells as stem cells provides the greatest immunological compatibility and significantly increases the success of the procedure.
Stem cells as a target for pharmacological testing
Stem cells can be used in new drug tests. Each experiment on living tissue can be performed safely on specific differentiated cells from pluripotent cells. If any undesirable effect appears, drug formulas can be changed until they reach a sufficient level of effectiveness. The drug can enter the pharmacological market without harming any live testers. However, to test the drugs properly, the conditions must be equal when comparing the effects of two drugs. To achieve this goal, researchers need to gain full control of the differentiation process to generate pure populations of differentiated cells.
Stem cells as an alternative for arthroplasty
One of the biggest fears of professional sportsmen is getting an injury, which most often signifies the end of their professional career. This applies especially to tendon injuries, which, due to current treatment options focusing either on conservative or surgical treatment, often do not provide acceptable outcomes. Problems with the tendons start with their regeneration capabilities. Instead of functionally regenerating after an injury, tendons merely heal by forming scar tissues that lack the functionality of healthy tissues. Factors that may cause this failed healing response include hypervascularization, deposition of calcific materials, pain, or swelling [ 84 ].
Additionally, in addition to problems with tendons, there is a high probability of acquiring a pathological condition of joints called osteoarthritis (OA) [ 85 ]. OA is common due to the avascular nature of articular cartilage and its low regenerative capabilities [ 86 ]. Although arthroplasty is currently a common procedure in treating OA, it is not ideal for younger patients because they can outlive the implant and will require several surgical procedures in the future. These are situations where stem cell therapy can help by stopping the onset of OA [ 87 ]. However, these procedures are not well developed, and the long-term maintenance of hyaline cartilage requires further research.
Osteonecrosis of the femoral hip (ONFH) is a refractory disease associated with the collapse of the femoral head and risk of hip arthroplasty in younger populations [ 88 ]. Although total hip arthroplasty (THA) is clinically successful, it is not ideal for young patients, mostly due to the limited lifetime of the prosthesis. An increasing number of clinical studies have evaluated the therapeutic effect of stem cells on ONFH. Most of the authors demonstrated positive outcomes, with reduced pain, improved function, or avoidance of THA [ 89 , 90 , 91 ].
Rejuvenation by cell programming
Ageing is a reversible epigenetic process. The first cell rejuvenation study was published in 2011 [ 92 ]. Cells from aged individuals have different transcriptional signatures, high levels of oxidative stress, dysfunctional mitochondria, and shorter telomeres than in young cells [ 93 ]. There is a hypothesis that when human or mouse adult somatic cells are reprogrammed to iPSCs, their epigenetic age is virtually reset to zero [ 94 ]. This was based on an epigenetic model, which explains that at the time of fertilization, all marks of parenteral ageing are erased from the zygote’s genome and its ageing clock is reset to zero [ 95 ].
In their study, Ocampo et al. [ 96 ] used Oct4, Sox2, Klf4, and C-myc genes (OSKM genes) and affected pancreas and skeletal muscle cells, which have poor regenerative capacity. Their procedure revealed that these genes can also be used for effective regenerative treatment [ 97 ]. The main challenge of their method was the need to employ an approach that does not use transgenic animals and does not require an indefinitely long application. The first clinical approach would be preventive, focused on stopping or slowing the ageing rate. Later, progressive rejuvenation of old individuals can be attempted. In the future, this method may raise some ethical issues, such as overpopulation, leading to lower availability of food and energy.
For now, it is important to learn how to implement cell reprogramming technology in non-transgenic elder animals and humans to erase marks of ageing without removing the epigenetic marks of cell identity.
Cell-based therapies
Stem cells can be induced to become a specific cell type that is required to repair damaged or destroyed tissues (Fig. 6 ). Currently, when the need for transplantable tissues and organs outweighs the possible supply, stem cells appear to be a perfect solution for the problem. The most common conditions that benefit from such therapy are macular degenerations [ 98 ], strokes [ 99 ], osteoarthritis [ 89 , 90 ], neurodegenerative diseases, and diabetes [ 100 ]. Due to this technique, it can become possible to generate healthy heart muscle cells and later transplant them to patients with heart disease.
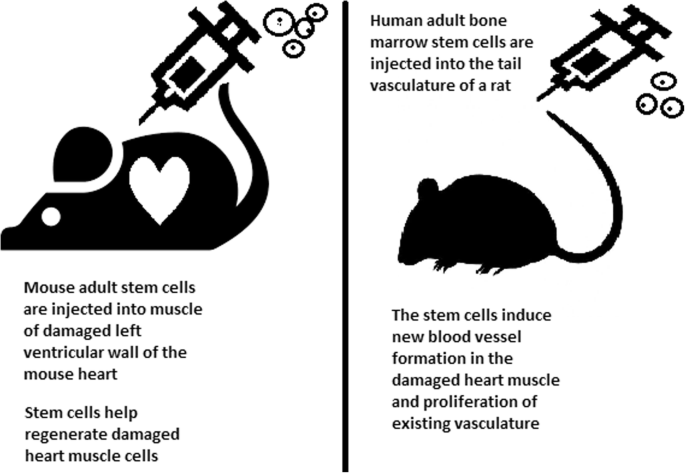
Stem cell experiments on animals. These experiments are one of the many procedures that proved stem cells to be a crucial factor in future regenerative medicine
In the case of type 1 diabetes, insulin-producing cells in the pancreas are destroyed due to an autoimmunological reaction. As an alternative to transplantation therapy, it can be possible to induce stem cells to differentiate into insulin-producing cells [ 101 ].
Stem cells and tissue banks
iPS cells with their theoretically unlimited propagation and differentiation abilities are attractive for the present and future sciences. They can be stored in a tissue bank to be an essential source of human tissue used for medical examination. The problem with conventional differentiated tissue cells held in the laboratory is that their propagation features diminish after time. This does not occur in iPSCs.
The umbilical cord is known to be rich in mesenchymal stem cells. Due to its cryopreservation immediately after birth, its stem cells can be successfully stored and used in therapies to prevent the future life-threatening diseases of a given patient.
Stem cells of human exfoliated deciduous teeth (SHED) found in exfoliated deciduous teeth has the ability to develop into more types of body tissues than other stem cells [ 102 ] (Table 1 ). Techniques of their collection, isolation, and storage are simple and non-invasive. Among the advantages of banking, SHED cells are:
Guaranteed donor-match autologous transplant that causes no immune reaction and rejection of cells [ 103 ]
Simple and painless for both child and parent
Less than one third of the cost of cord blood storage
Not subject to the same ethical concerns as embryonic stem cells [ 104 ]
In contrast to cord blood stem cells, SHED cells are able to regenerate into solid tissues such as connective, neural, dental, or bone tissue [ 105 , 106 ]
SHED can be useful for close relatives of the donor
Fertility diseases
In 2011, two researchers, Katsuhiko Hayashi et al. [ 107 ], showed in an experiment on mice that it is possible to form sperm from iPSCs. They succeeded in delivering healthy and fertile pups in infertile mice. The experiment was also successful for female mice, where iPSCs formed fully functional eggs .
Young adults at risk of losing their spermatogonial stem cells (SSC), mostly cancer patients, are the main target group that can benefit from testicular tissue cryopreservation and autotransplantation. Effective freezing methods for adult and pre-pubertal testicular tissue are available [ 108 ].
Qiuwan et al. [ 109 ] provided important evidence that human amniotic epithelial cell (hAEC) transplantation could effectively improve ovarian function by inhibiting cell apoptosis and reducing inflammation in injured ovarian tissue of mice, and it could be a promising strategy for the management of premature ovarian failure or insufficiency in female cancer survivors.
For now, reaching successful infertility treatments in humans appears to be only a matter of time, but there are several challenges to overcome. First, the process needs to have high efficiency; second, the chances of forming tumours instead of eggs or sperm must be maximally reduced. The last barrier is how to mature human sperm and eggs in the lab without transplanting them to in vivo conditions, which could cause either a tumour risk or an invasive procedure.
Therapy for incurable neurodegenerative diseases
Thanks to stem cell therapy, it is possible not only to delay the progression of incurable neurodegenerative diseases such as Parkinson’s disease, Alzheimer’s disease (AD), and Huntington disease, but also, most importantly, to remove the source of the problem. In neuroscience, the discovery of neural stem cells (NSCs) has nullified the previous idea that adult CNS were not capable of neurogenesis [ 110 , 111 ]. Neural stem cells are capable of improving cognitive function in preclinical rodent models of AD [ 112 , 113 , 114 ]. Awe et al. [ 115 ] clinically derived relevant human iPSCs from skin punch biopsies to develop a neural stem cell-based approach for treating AD. Neuronal degeneration in Parkinson’s disease (PD) is focal, and dopaminergic neurons can be efficiently generated from hESCs. PD is an ideal disease for iPSC-based cell therapy [ 116 ]. However, this therapy is still in an experimental phase ( https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4539501 /). Brain tissue from aborted foetuses was used on patients with Parkinson’s disease [ 117 ]. Although the results were not uniform, they showed that therapies with pure stem cells are an important and achievable therapy.
Stem cell use in dentistry
Teeth represent a very challenging material for regenerative medicine. They are difficult to recreate because of their function in aspects such as articulation, mastication, or aesthetics due to their complicated structure. Currently, there is a chance for stem cells to become more widely used than synthetic materials. Teeth have a large advantage of being the most natural and non-invasive source of stem cells.
For now, without the use of stem cells, the most common periodontological treatments are either growth factors, grafts, or surgery. For example, there are stem cells in periodontal ligament [ 118 , 119 ], which are capable of differentiating into osteoblasts or cementoblasts, and their functions were also assessed in neural cells [ 120 ]. Tissue engineering is a successful method for treating periodontal diseases. Stem cells of the root apical areas are able to recreate periodontal ligament. One of the possible methods of tissue engineering in periodontology is gene therapy performed using adenoviruses-containing growth factors [ 121 ].
As a result of animal studies, dentin regeneration is an effective process that results in the formation of dentin bridges [ 122 ].
Enamel is more difficult to regenerate than dentin. After the differentiation of ameloblastoma cells into the enamel, the former is destroyed, and reparation is impossible. Medical studies have succeeded in differentiating bone marrow stem cells into ameloblastoma [ 123 ].
Healthy dental tissue has a high amount of regular stem cells, although this number is reduced when tissue is either traumatized or inflamed [ 124 ]. There are several dental stem cell groups that can be isolated (Fig. 7 ).
Localization of stem cells in dental tissues. Dental pulp stem cells (DPSCs) and human deciduous teeth stem cells (SHED) are located in the dental pulp. Periodontal ligaments stem cells are located in the periodontal ligament. Apical papilla consists of stem cells from the apical papilla (SCAP)
Dental pulp stem cell (DPSC)
These were the first dental stem cells isolated from the human dental pulp, which were [ 125 ] located inside dental pulp (Table 2 ). They have osteogenic and chondrogenic potential. Mesenchymal stem cells (MSCs) of the dental pulp, when isolated, appear highly clonogenic; they can be isolated from adult tissue (e.g. bone marrow, adipose tissue) and foetal (e.g. umbilical cord) [ 126 ] tissue, and they are able to differentiate densely [ 127 ]. MSCs differentiate into odontoblast-like cells and osteoblasts to form dentin and bone. Their best source locations are the third molars [ 125 ]. DPSCs are the most useful dental source of tissue engineering due to their easy surgical accessibility, cryopreservation possibility, increased production of dentin tissues compared to non-dental stem cells, and their anti-inflammatory abilities. These cells have the potential to be a source for maxillofacial and orthopaedic reconstructions or reconstructions even beyond the oral cavity. DPSCs are able to generate all structures of the developed tooth [ 128 ]. In particular, beneficial results in the use of DPSCs may be achieved when combined with other new therapies, such as periodontal tissue photobiomodulation (laser stimulation), which is an efficient technique in the stimulation of proliferation and differentiation into distinct cell types [ 129 ]. DPSCs can be induced to form neural cells to help treat neurological deficits.
Stem cells of human exfoliated deciduous teeth (SHED) have a faster rate of proliferation than DPSCs and differentiate into an even greater number of cells, e.g. other mesenchymal and non-mesenchymal stem cell derivatives, such as neural cells [ 130 ]. These cells possess one major disadvantage: they form a non-complete dentin/pulp-like complex in vivo. SHED do not undergo the same ethical concerns as embryonic stem cells. Both DPSCs and SHED are able to form bone-like tissues in vivo [ 131 ] and can be used for periodontal, dentin, or pulp regeneration. DPSCs and SHED can be used in treating, for example, neural deficits [ 132 ]. DPSCs alone were tested and successfully applied for alveolar bone and mandible reconstruction [ 133 ].
Periodontal ligament stem cells (PDLSCs)
These cells are used in periodontal ligament or cementum tissue regeneration. They can differentiate into mesenchymal cell lineages to produce collagen-forming cells, adipocytes, cementum tissue, Sharpey’s fibres, and osteoblast-like cells in vitro. PDLSCs exist both on the root and alveolar bone surfaces; however, on the latter, these cells have better differentiation abilities than on the former [ 134 ]. PDLSCs have become the first treatment for periodontal regeneration therapy because of their safety and efficiency [ 135 , 136 ].
Stem cells from apical papilla (SCAP)
These cells are mesenchymal structures located within immature roots. They are isolated from human immature permanent apical papilla. SCAP are the source of odontoblasts and cause apexogenesis. These stem cells can be induced in vitro to form odontoblast-like cells, neuron-like cells, or adipocytes. SCAP have a higher capacity of proliferation than DPSCs, which makes them a better choice for tissue regeneration [ 137 , 138 ].
Dental follicle stem cells (DFCs)
These cells are loose connective tissues surrounding the developing tooth germ. DFCs contain cells that can differentiate into cementoblasts, osteoblasts, and periodontal ligament cells [ 139 , 140 ]. Additionally, these cells proliferate after even more than 30 passages [ 141 ]. DFCs are most commonly extracted from the sac of a third molar. When DFCs are combined with a treated dentin matrix, they can form a root-like tissue with a pulp-dentin complex and eventually form tooth roots [ 141 ]. When DFC sheets are induced by Hertwig’s epithelial root sheath cells, they can produce periodontal tissue; thus, DFCs represent a very promising material for tooth regeneration [ 142 ].
Pulp regeneration in endodontics
Dental pulp stem cells can differentiate into odontoblasts. There are few methods that enable the regeneration of the pulp.
The first is an ex vivo method. Proper stem cells are grown on a scaffold before they are implanted into the root channel [ 143 ].
The second is an in vivo method. This method focuses on injecting stem cells into disinfected root channels after the opening of the in vivo apex. Additionally, the use of a scaffold is necessary to prevent the movement of cells towards other tissues. For now, only pulp-like structures have been created successfully.
Methods of placing stem cells into the root channel constitute are either soft scaffolding [ 144 ] or the application of stem cells in apexogenesis or apexification. Immature teeth are the best source [ 145 ]. Nerve and blood vessel network regeneration are extremely vital to keep pulp tissue healthy.
The potential of dental stem cells is mainly regarding the regeneration of damaged dentin and pulp or the repair of any perforations; in the future, it appears to be even possible to generate the whole tooth. Such an immense success would lead to the gradual replacement of implant treatments. Mandibulary and maxillary defects can be one of the most complicated dental problems for stem cells to address.
Acquiring non-dental tissue cells by dental stem cell differentiation
In 2013, it was reported that it is possible to grow teeth from stem cells obtained extra-orally, e.g. from urine [ 146 ]. Pluripotent stem cells derived from human urine were induced and generated tooth-like structures. The physical properties of the structures were similar to natural ones except for hardness [ 127 ]. Nonetheless, it appears to be a very promising technique because it is non-invasive and relatively low-cost, and somatic cells can be used instead of embryonic cells. More importantly, stem cells derived from urine did not form any tumours, and the use of autologous cells reduces the chances of rejection [ 147 ].
Use of graphene in stem cell therapy
Over recent years, graphene and its derivatives have been increasingly used as scaffold materials to mediate stem cell growth and differentiation [ 148 ]. Both graphene and graphene oxide (GO) represent high in-plane stiffness [ 149 ]. Because graphene has carbon and aromatic network, it works either covalently or non-covalently with biomolecules; in addition to its superior mechanical properties, graphene offers versatile chemistry. Graphene exhibits biocompatibility with cells and their proper adhesion. It also tested positively for enhancing the proliferation or differentiation of stem cells [ 148 ]. After positive experiments, graphene revealed great potential as a scaffold and guide for specific lineages of stem cell differentiation [ 150 ]. Graphene has been successfully used in the transplantation of hMSCs and their guided differentiation to specific cells. The acceleration skills of graphene differentiation and division were also investigated. It was discovered that graphene can serve as a platform with increased adhesion for both growth factors and differentiation chemicals. It was also discovered that π-π binding was responsible for increased adhesion and played a crucial role in inducing hMSC differentiation [ 150 ].
Therapeutic potential of extracellular vesicle-based therapies
Extracellular vesicles (EVs) can be released by virtually every cell of an organism, including stem cells [ 151 ], and are involved in intercellular communication through the delivery of their mRNAs, lipids, and proteins. As Oh et al. [ 152 ] prove, stem cells, together with their paracrine factors—exosomes—can become potential therapeutics in the treatment of, e.g. skin ageing. Exosomes are small membrane vesicles secreted by most cells (30–120 nm in diameter) [ 153 ]. When endosomes fuse with the plasma membrane, they become exosomes that have messenger RNAs (mRNAs) and microRNAs (miRNAs), some classes of non-coding RNAs (IncRNAs) and several proteins that originate from the host cell [ 154 ]. IncRNAs can bind to specific loci and create epigenetic regulators, which leads to the formation of epigenetic modifications in recipient cells. Because of this feature, exosomes are believed to be implicated in cell-to-cell communication and the progression of diseases such as cancer [ 155 ]. Recently, many studies have also shown the therapeutic use of exosomes derived from stem cells, e.g. skin damage and renal or lung injuries [ 156 ].
In skin ageing, the most important factor is exposure to UV light, called “photoageing” [ 157 ], which causes extrinsic skin damage, characterized by dryness, roughness, irregular pigmentation, lesions, and skin cancers. In intrinsic skin ageing, on the other hand, the loss of elasticity is a characteristic feature. The skin dermis consists of fibroblasts, which are responsible for the synthesis of crucial skin elements, such as procollagen or elastic fibres. These elements form either basic framework extracellular matrix constituents of the skin dermis or play a major role in tissue elasticity. Fibroblast efficiency and abundance decrease with ageing [ 158 ]. Stem cells can promote the proliferation of dermal fibroblasts by secreting cytokines such as platelet-derived growth factor (PDGF), transforming growth factor β (TGF-β), and basic fibroblast growth factor. Huh et al. [ 159 ] mentioned that a medium of human amniotic fluid-derived stem cells (hAFSC) positively affected skin regeneration after longwave UV-induced (UVA, 315–400 nm) photoageing by increasing the proliferation and migration of dermal fibroblasts. It was discovered that, in addition to the induction of fibroblast physiology, hAFSC transplantation also improved diseases in cases of renal pathology, various cancers, or stroke [ 160 , 161 ].
Oh [ 162 ] also presented another option for the treatment of skin wounds, either caused by physical damage or due to diabetic ulcers. Induced pluripotent stem cell-conditioned medium (iPSC-CM) without any animal-derived components induced dermal fibroblast proliferation and migration.
Natural cutaneous wound healing is divided into three steps: haemostasis/inflammation, proliferation, and remodelling. During the crucial step of proliferation, fibroblasts migrate and increase in number, indicating that it is a critical step in skin repair, and factors such as iPSC-CM that impact it can improve the whole cutaneous wound healing process. Paracrine actions performed by iPSCs are also important for this therapeutic effect [ 163 ]. These actions result in the secretion of cytokines such as TGF-β, interleukin (IL)-6, IL-8, monocyte chemotactic protein-1 (MCP-1), vascular endothelial growth factor (VEGF), platelet-derived growth factor-AA (PDGF-AA), and basic fibroblast growth factor (bFGF). Bae et al. [ 164 ] mentioned that TGF-β induced the migration of keratinocytes. It was also demonstrated that iPSC factors can enhance skin wound healing in vivo and in vitro when Zhou et al. [ 165 ] enhanced wound healing, even after carbon dioxide laser resurfacing in an in vivo study.
Peng et al. [ 166 ] investigated the effects of EVs derived from hESCs on in vitro cultured retinal glial, progenitor Müller cells, which are known to differentiate into retinal neurons. EVs appear heterogeneous in size and can be internalized by cultured Müller cells, and their proteins are involved in the induction and maintenance of stem cell pluripotency. These stem cell-derived vesicles were responsible for the neuronal trans-differentiation of cultured Müller cells exposed to them. However, the research article points out that the procedure was accomplished only on in vitro acquired retina.
Challenges concerning stem cell therapy
Although stem cells appear to be an ideal solution for medicine, there are still many obstacles that need to be overcome in the future. One of the first problems is ethical concern.
The most common pluripotent stem cells are ESCs. Therapies concerning their use at the beginning were, and still are, the source of ethical conflicts. The reason behind it started when, in 1998, scientists discovered the possibility of removing ESCs from human embryos. Stem cell therapy appeared to be very effective in treating many, even previously incurable, diseases. The problem was that when scientists isolated ESCs in the lab, the embryo, which had potential for becoming a human, was destroyed (Fig. 8 ). Because of this, scientists, seeing a large potential in this treatment method, focused their efforts on making it possible to isolate stem cells without endangering their source—the embryo.
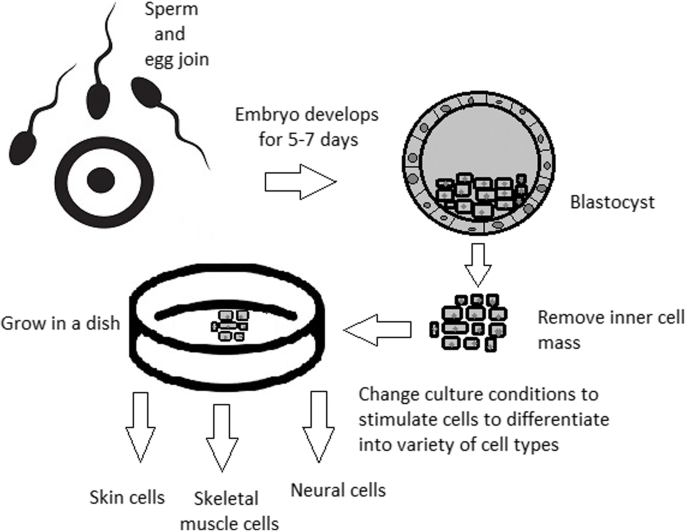
Use of inner cell mass pluripotent stem cells and their stimulation to differentiate into desired cell types
For now, while hESCs still remain an ethically debatable source of cells, they are potentially powerful tools to be used for therapeutic applications of tissue regeneration. Because of the complexity of stem cell control systems, there is still much to be learned through observations in vitro. For stem cells to become a popular and widely accessible procedure, tumour risk must be assessed. The second problem is to achieve successful immunological tolerance between stem cells and the patient’s body. For now, one of the best ideas is to use the patient’s own cells and devolve them into their pluripotent stage of development.
New cells need to have the ability to fully replace lost or malfunctioning natural cells. Additionally, there is a concern about the possibility of obtaining stem cells without the risk of morbidity or pain for either the patient or the donor. Uncontrolled proliferation and differentiation of cells after implementation must also be assessed before its use in a wide variety of regenerative procedures on living patients [ 167 ].
One of the arguments that limit the use of iPSCs is their infamous role in tumourigenicity. There is a risk that the expression of oncogenes may increase when cells are being reprogrammed. In 2008, a technique was discovered that allowed scientists to remove oncogenes after a cell achieved pluripotency, although it is not efficient yet and takes a longer amount of time. The process of reprogramming may be enhanced by deletion of the tumour suppressor gene p53, but this gene also acts as a key regulator of cancer, which makes it impossible to remove in order to avoid more mutations in the reprogrammed cell. The low efficiency of the process is another problem, which is progressively becoming reduced with each year. At first, the rate of somatic cell reprogramming in Yamanaka’s study was up to 0.1%. The use of transcription factors creates a risk of genomic insertion and further mutation of the target cell genome. For now, the only ethically acceptable operation is an injection of hESCs into mouse embryos in the case of pluripotency evaluation [ 168 ].
Stem cell obstacles in the future
Pioneering scientific and medical advances always have to be carefully policed in order to make sure they are both ethical and safe. Because stem cell therapy already has a large impact on many aspects of life, it should not be treated differently.
Currently, there are several challenges concerning stem cells. First, the most important one is about fully understanding the mechanism by which stem cells function first in animal models. This step cannot be avoided. For the widespread, global acceptance of the procedure, fear of the unknown is the greatest challenge to overcome.
The efficiency of stem cell-directed differentiation must be improved to make stem cells more reliable and trustworthy for a regular patient. The scale of the procedure is another challenge. Future stem cell therapies may be a significant obstacle. Transplanting new, fully functional organs made by stem cell therapy would require the creation of millions of working and biologically accurate cooperating cells. Bringing such complicated procedures into general, widespread regenerative medicine will require interdisciplinary and international collaboration.
The identification and proper isolation of stem cells from a patient’s tissues is another challenge. Immunological rejection is a major barrier to successful stem cell transplantation. With certain types of stem cells and procedures, the immune system may recognize transplanted cells as foreign bodies, triggering an immune reaction resulting in transplant or cell rejection.
One of the ideas that can make stem cells a “failsafe” is about implementing a self-destruct option if they become dangerous. Further development and versatility of stem cells may cause reduction of treatment costs for people suffering from currently incurable diseases. When facing certain organ failure, instead of undergoing extraordinarily expensive drug treatment, the patient would be able to utilize stem cell therapy. The effect of a successful operation would be immediate, and the patient would avoid chronic pharmacological treatment and its inevitable side effects.
Although these challenges facing stem cell science can be overwhelming, the field is making great advances each day. Stem cell therapy is already available for treating several diseases and conditions. Their impact on future medicine appears to be significant.
After several decades of experiments, stem cell therapy is becoming a magnificent game changer for medicine. With each experiment, the capabilities of stem cells are growing, although there are still many obstacles to overcome. Regardless, the influence of stem cells in regenerative medicine and transplantology is immense. Currently, untreatable neurodegenerative diseases have the possibility of becoming treatable with stem cell therapy. Induced pluripotency enables the use of a patient’s own cells. Tissue banks are becoming increasingly popular, as they gather cells that are the source of regenerative medicine in a struggle against present and future diseases. With stem cell therapy and all its regenerative benefits, we are better able to prolong human life than at any time in history.
Abbreviations
Basic fibroblast growth factor
Bone morphogenic proteins
Dental follicle stem cells
Dental pulp stem cells
Embryonic bodies
Embryonic stem cells
Fibroblast growth factors
Good Manufacturing Practice
Graphene oxide
Human amniotic fluid-derived stem cells
Human embryonic stem cells
Human foreskin fibroblasts
Inner cell mass
Non-coding RNA
Induced pluripotent stem cells
In vitro fertilization
Knockout serum replacement
Leukaemia inhibitory factor
Monocyte chemotactic protein-1
Fibroblasts
Messenger RNA
Mesenchymal stem cells of dental pulp
Myogenic differentiation
Osteoarthritis
Octamer-binding transcription factor 3 and 4
Platelet-derived growth factor
Platelet-derived growth factor-AA
Periodontal ligament stem cells
Rho-associated protein kinase
Stem cells from apical papilla
Stem cells of human exfoliated deciduous teeth
Synthetic Serum Substitute
Trophectoderm
Vascular endothelial growth factor
Transforming growth factors
Sukoyan MA, Vatolin SY, et al. Embryonic stem cells derived from morulae, inner cell mass, and blastocysts of mink: comparisons of their pluripotencies. Embryo Dev. 1993;36(2):148–58
Larijani B, Esfahani EN, Amini P, Nikbin B, Alimoghaddam K, Amiri S, Malekzadeh R, Yazdi NM, Ghodsi M, Dowlati Y, Sahraian MA, Ghavamzadeh A. Stem cell therapy in treatment of different diseases. Acta Medica Iranica. 2012:79–96 https://www.ncbi.nlm.nih.gov/pubmed/22359076 .
Sullivan S, Stacey GN, Akazawa C, et al. Quality guidelines for clinical-grade human induced pluripotent stem cell lines. Regenerative Med. 2018; https://doi.org/10.2217/rme-2018-0095 .
Amps K, Andrews PW, et al. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat. Biotechnol. 2011; 29 (12):1121–44.
Google Scholar
Amit M, Itskovitz-Eldor J. Atlas of human pluripotent stem cells: derivation and culturing. New York: Humana Press; 2012.
Ludwig TE, Bergendahl V, Levenstein ME, Yu J, Probasco MD, Thomson JA. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006;3:637–46.
CAS PubMed Google Scholar
Kang MI. Transitional CpG methylation between promoters and retroelements of tissue-specific genes during human mesenchymal cell differentiation. J. Cell Biochem. 2007;102:224–39.
Vaes B, Craeye D, Pinxteren J. Quality control during manufacture of a stem cell therapeutic. BioProcess Int. 2012;10:50–5.
Bloushtain-Qimron N. Epigenetic patterns of embryonic and adult stem cells. Cell Cycle. 2009;8:809–17.
Brindley DA. Peak serum: implications of serum supply for cell therapy manufacturing. Regenerative Medicine. 2012;7:809–17.
Solter D, Knowles BB. Immunosurgery of mouse blastocyst. Proc Natl Acad Sci U S A. 1975;72:5099–102.
CAS PubMed PubMed Central Google Scholar
Hoepfl G, Gassmann M, Desbaillets I. Differentiating embryonic stem cells into embryoid bodies. Methods Mole Biol. 2004;254:79–98 https://doi.org/10.1385/1-59259-741-6:079 .
Lim WF, Inoue-Yokoo T, Tan KS, Lai MI, Sugiyama D. Hematopoietic cell differentiation from embryonic and induced pluripotent stem cells. Stem Cell Res Ther. 2013;4(3):71. https://doi.org/10.1186/scrt222 .
Article CAS PubMed PubMed Central Google Scholar
Mohr JC, de Pablo JJ, Palecek SP. 3-D microwell culture of human embryonic stem cells. Biomaterials. 2006;27(36):6032–42. https://doi.org/10.1016/j.biomaterials.2006.07.012 .
Article CAS PubMed Google Scholar
Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of the visceral yolk sac, blood islands, and myocardium. J Embryol Exp Morphol. 1985;87:27–45.
Kurosawa HY. Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J Biosci Bioeng. 2007;103:389–98.
Heins N, Englund MC, Sjoblom C, Dahl U, Tonning A, Bergh C, Lindahl A, Hanson C, Semb H. Derivation, characterization, and differentiation of human embryonic stem cells. Stem Cells. 2004;22:367–76.
Rosowski KA, Mertz AF, Norcross S, Dufresne ER, Horsley V. Edges of human embryonic stem cell colonies display distinct mechanical properties and differentiation potential. Sci Rep. 2015;5:Article number:14218.
PubMed Google Scholar
Chung Y, Klimanskaya I, Becker S, Li T, Maserati M, Lu SJ, Zdravkovic T, Ilic D, Genbacev O, Fisher S, Krtolica A, Lanza R. Human embryonic stem cell lines generated without embryo destruction. Cell Stem Cell. 2008;2:113–7.
Zhang X, Stojkovic P, Przyborski S, Cooke M, Armstrong L, Lako M, Stojkovic M. Derivation of human embryonic stem cells from developing and arrested embryos. Stem Cells. 2006;24:2669–76.
Beers J, Gulbranson DR, George N, Siniscalchi LI, Jones J, Thomson JA, Chen G. Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions. Nat Protoc. 2012;7:2029–40.
Ellerström C, Hyllner J, Strehl R. single cell enzymatic dissociation of human embryonic stem cells: a straightforward, robust, and standardized culture method. In: Turksen K, editor. Human embryonic stem cell protocols. Methods in molecular biology: Humana Press; 2009. p. 584.
Heng BC, Liu H, Ge Z, Cao T. Mechanical dissociation of human embryonic stem cell colonies by manual scraping after collagenase treatment is much more detrimental to cellular viability than is trypsinization with gentle pipetting. Biotechnol Appl Biochem. 2010;47(1):33–7.
Ellerstrom C, Strehl R, Noaksson K, Hyllner J, Semb H. Facilitated expansion of human embryonic stem cells by single-cell enzymatic dissociation. Stem Cells. 2007;25:1690–6.
Brimble SN, Zeng X, Weiler DA, Luo Y, Liu Y, Lyons IG, Freed WJ, Robins AJ, Rao MS, Schulz TC. Karyotypic stability, genotyping, differentiation, feeder-free maintenance, and gene expression sampling in three human embryonic stem cell lines deri. Stem Cells Dev. 2004;13:585–97.
Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–6.
Nie Y, Walsh P, Clarke DL, Rowley JA, Fellner T. Scalable passaging of adherent human pluripotent stem cells. 2014. https://doi.org/10.1371/journal.pone.0088012 .
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7.
Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cellsexpress an immunogenic nonhuman sialic acid. Nat. Med. 2005;11:228–32.
Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, Rogers D. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336(6200):688–90. https://doi.org/10.1038/336688a0 .
Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nature Biotechnol. 2001;19:971–4. https://doi.org/10.1038/nbt1001-971 .
Article CAS Google Scholar
Weathersbee PS, Pool TB, Ord T. Synthetic serum substitute (SSS): a globulin-enriched protein supplement for human embryo culture. J. Assist Reprod Genet. 1995;12:354–60.
Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, Wagner R, Lee GO, Antosiewicz-Bourget J, Teng JM, Thomson JA. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods. 2011;8:424–9.
Sommer CA, Mostoslavsky G. Experimental approaches for the generation of induced pluripotent stem cells. Stem Cell Res Ther. 2010;1:26.
PubMed PubMed Central Google Scholar
Takahashi K, Yamanaka S. Induced pluripotent stem cells in medicine and biology. Development. 2013;140(12):2457–61 https://doi.org/10.1242/dev.092551 .
Shi D, Lu F, Wei Y, et al. Buffalos ( Bubalus bubalis ) cloned by nuclear transfer of somatic cells. Biol. Reprod. 2007;77:285–91. https://doi.org/10.1095/biolreprod.107.060210 .
Gurdon JB. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. Development. 1962;10:622–40 http://dev.biologists.org/content/10/4/622 .
CAS Google Scholar
Kain K. The birth of cloning: an interview with John Gurdon. Dis Model Mech. 2009;2(1–2):9–10. https://doi.org/10.1242/dmm.002014 .
Article PubMed Central Google Scholar
Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;24(51(6)):987–1000.
Quinlan AR, Boland MJ, Leibowitz ML, et al. Genome sequencing of mouse induced pluripotent stem cells reveals retroelement stability and infrequent DNA rearrangement during reprogramming. Cell Stem Cell. 2011;9(4):366–73.
Maherali N, Sridharan R, Xie W, Utika LJ, Eminli S, Arnold K, Stadtfeld M, Yachechko R, Tchieu J, Jaenisch R, Plath K, Hochedlinger K. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70.
Ohi Y, Qin H, Hong C, Blouin L, Polo JM, Guo T, Qi Z, Downey SL, Manos PD, Rossi DJ, Yu J, Hebrok M, Hochedlinger K, Costello JF, Song JS, Ramalho-Santos M. Incomplete DNA methylation underlines a transcriptional memory of somatic cells in human IPS cells. Nat Cell Biol. 2011;13:541–9.
Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–32 https://doi.org/10.1038/nature07314 .
Hilfiker A, Kasper C, Hass R, Haverich A. Mesenchymal stem cells and progenitor cells in connective tissue engineering and regenerative medicine: is there a future for transplantation? Langenbecks Arch Surg. 2011;396:489–97.
Zhang Wendy, Y., de Almeida Patricia, E., and Wu Joseph, C. Teratoma formation: a tool for monitoring pluripotency in stem cell research. StemBook, ed. The Stem Cell Research Community . June 12, 2012. https://doi.org/10.3824/stembook.1.53.1 .
Narsinh KH, Sun N, Sanchez-Freire V, et al. Single cell transcriptional profiling reveals heterogeneity of human induced pluripotent stem cells. J Clin Invest. 2011;121(3):1217–21.
Gertow K, Przyborski S, Loring JF, Auerbach JM, Epifano O, Otonkoski T, Damjanov I, AhrlundRichter L. Isolation of human embryonic stem cell-derived teratomas for the assessment of pluripotency. Curr Protoc Stem Cell Biol . 2007, Chapter 1, Unit 1B 4. 3: 1B.4.1-1B.4.29.
Cooke MJ, Stojkovic M, Przyborski SA. Growth of teratomas derived from human pluripotent stem cells is influenced by the graft site. Stem Cells Dev. 2006;15(2):254–9.
Przyborski SA. Differentiation of human embryonic stem cells after transplantation in immune-deficient mice. Stem Cells. 2005;23:1242–50.
Tannenbaum SE, Turetsky TT, Singer O, Aizenman E, Kirshberg S, Ilouz N, Gil Y, Berman-Zaken Y, Perlman TS, Geva N, Levy O, Arbell D, Simon A, Ben-Meir A, Shufaro Y, Laufer N, Reubinoff BE. Derivation of xeno-free and GMP-grade human embryonic stem cells- platforms for future clinical applications. PLoS One. 2012;7:e35325.
Cohen DE, Melton D. Turning straw into gold: directing cell fate for regenerative medicine. Nat Rev Genet. 2011;12:243–52.
Hwang NS, Varghese S, Elisseeff J. Controlled differentiation of stem cells. Adv Drug Deliv Rev. 2007;60(2):199–214. https://doi.org/10.1016/j.addr.2007.08.036 .
Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–29.
Rao TP, Kuhl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res. 2010;106:1798–806.
Moustakas A, Heldin CH. The regulation of TGFbeta signal transduction. Development. 2009;136:3699–714.
Efthymiou AG, Chen G, Rao M, Chen G, Boehm M. Self-renewal and cell lineage differentiation strategies in human embryonic stem cells and induced pluripotent stem cells. Expert Opin Biol Ther. 2014;14:1333–44.
Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human cardiovascular progenitor cells develop from a KDRþembryonic-stem-cell-derived population. Nature. 2008;453:524–8.
Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D’amour KA, Carpenter MK, Baetge EE. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–52. https://doi.org/10.1038/nbt1393 .
Vallier L, Reynolds D, Pedersen RA. Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev Biol. 2004;275:403–21.
Burridge PW, Zambidis ET. Highly efficient directed differentiation of human induced pluripotent stem cells into cardiomyocytes. Methods Mol Biol. 2013;997:149–61.
Cai J, Zhao Y, Liu Y, Ye F, Song Z, Qin H, Meng S, Chen Y, Zhou R, Song X, Guo Y, Ding M, Deng H. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229–39.
Takasato M, Er PX, Becroft M, Vanslambrouck JM, Stanley EG, Elefanty AG, Little MH. Directing human embryonic stem cell differentiation towards a renal lineage generates a selforganizing kidney. Nat Cell Biol. 2014;16:118–26.
Huang SX, Islam MN, O’Neill J, Hu Z, Yang YG, Chen YW, Mumau M, Green MD, VunjakNovakovic G, Bhattacharya J, Snoeck HW. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol. 2014;32:84–91.
Kadzik RS, Morrisey EE. Directing lung endoderm differentiation in pluripotent stem cells. Cell Stem Cell. 2012;10:355–61.
Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–97.
Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–9.
Oldershaw RA, Baxter MA, Lowe ET, Bates N, Grady LM, Soncin F, Brison DR, Hardingham TE, Kimber SJ. Directed differentiation of human embryonic stem cells toward chondrocytes. Nat Biotechnol. 2010;28:1187–94.
Jun Cai, Ann DeLaForest, Joseph Fisher, Amanda Urick, Thomas Wagner, Kirk Twaroski, Max Cayo, Masato Nagaoka, Stephen A Duncan. Protocol for directed differentiation of human pluripotent stem cells toward a hepatocyte fate. 2012. DOI: https://doi.org/10.3824/stembook.1.52.1 .
Frank-Kamenetsky M, Zhang XM, Bottega S, Guicherit O, Wichterle H, Dudek H, Bumcrot D, Wang FY, Jones S, Shulok J, Rubin LL, Porter JA. Small-molecule modulators of hedgehog signaling: identification and characterization of smoothened agonists and antagonists. J Biol. 2002;1:10.
Oshima K, Shin K, Diensthuber M, Peng AW, Ricci AJ, Heller S. Mechanosensitive hair celllike cells from embryonic and induced pluripotent stem cells. Cell. 2010;141:704–16.
Osakada F, Jin ZB, Hirami Y, Ikeda H, Danjyo T, Watanabe K, Sasai Y, Takahashi M. In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J Cell Sci. 2009;122:3169–79.
Kshitiz PJ, Kim P, Helen W, Engler AJ, Levchenko A, Kim DH. Control of stem cell fate and function by engineering physical microenvironments. Intergr Biol (Camb). 2012;4:1008–18.
Amps K, Andrews PW, Anyfantis G, Armstrong L, Avery S, Baharvand H, Baker J, Baker D, Munoz MB, Beil S, Benvenisty N, Ben-Yosef D, Biancotti JC, Bosman A, Brena RM, Brison D, Caisander G, Camarasa MV, Chen J, ChiaoE CYM, Choo AB, Collins D, et al. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat Biotechnol. 2011;29:1132–44.
Nukaya D, Minami K, Hoshikawa R, Yokoi N, Seino S. Preferential gene expression and epigenetic memory of induced pluripotent stem cells derived from mouse pancreas. Genes Cells. 2015;20:367–81.
Murdoch A, Braude P, Courtney A, Brison D, Hunt C, Lawford-Davies J, Moore H, Stacey G, Sethe S, Procurement Working Group Of National Clinical H, E. S. C. F, National Clinical H, E. S. C. F. The procurement of cells for the derivation of human embryonic stem cell lines for therapeutic use: recommendations for good practice. Stem Cell Rev. 2012;8:91–9.
Hewitson H, Wood V, Kadeva N, Cornwell G, Codognotto S, Stephenson E, Ilic D. Generation of KCL035 research grade human embryonic stem cell line carrying a mutation in HBB gene. Stem Cell Res. 2016;16:210–2.
Daley GQ, Hyun I, Apperley JF, Barker RA, Benvenisty N, Bredenoord AL, Breuer CK, Caulfield T, Cedars MI, Frey-Vasconcells J, Heslop HE, Jin Y, Lee RT, Mccabe C, Munsie M, Murry CE, Piantadosi S, Rao M, Rooke HM, Sipp D, Studer L, Sugarman J, et al. Setting global standards for stem cell research and clinical translation: the 2016 ISSCR guidelines. Stem Cell Rep. 2016;6:787–97.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. https://doi.org/10.1016/j.cell.2006.07.024 .
Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–40.
Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Cacciapuoti I, Parouchev A, Benhamouda N, Tachdjian G, Tosca L, Trouvin JH, Fabreguettes JR, Bellamy V, Guillemain R, SuberbielleBoissel C, Tartour E, Desnos M, Larghero J. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J. 2015;36:2011–7.
Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M, Maguire J, Gay R, Bateman J, Ostrick RM, Morris D, Vincent M, Anglade E, Del Priore LV, Lanza R. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–16.
Ilic D, Ogilvie C. Concise review: human embryonic stem cells-what have we done? What are we doing? Where are we going? Stem Cells. 2017;35:17–25.
Rocha V, et al. Clinical use of umbilical cord blood hematopoietic stem cells. Biol Blood Marrow Transplant. 2006;12(1):34–4.
Longo UG, Ronga M, Maffulli N. Sports Med Arthrosc 17:112–126. Achilles tendinopathy. Sports Med Arthrosc. 2009;17:112–26.
Tempfer H, Lehner C, Grütz M, Gehwolf R, Traweger A. Biological augmentation for tendon repair: lessons to be learned from development, disease, and tendon stem cell research. In: Gimble J, Marolt D, Oreffo R, Redl H, Wolbank S, editors. Cell engineering and regeneration. Reference Series in Biomedical Engineering. Cham: Springer; 2017.
Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–34.
Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14:177–82.
Li R, Lin Q-X, Liang X-Z, Liu G-B, et al. Stem cell therapy for treating osteonecrosis of the femoral head: from clinical applications to related basic research. Stem Cell Res Therapy. 2018;9:291 https://doi.org/10.1186/s13287-018-1018-7 .
Gangji V, De Maertelaer V, Hauzeur JP. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: five year follow-up of a prospective controlled study. Bone. 2011;49(5):1005–9.
Zhao D, Cui D, Wang B, Tian F, Guo L, Yang L, et al. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone. 2012;50(1):325–30.
Sen RK, Tripathy SK, Aggarwal S, Marwaha N, Sharma RR, Khandelwal N. Early results of core decompression and autologous bone marrow mononuclear cells instillation in femoral head osteonecrosis: a randomized control study. J Arthroplast. 2012;27(5):679–86.
Lapasset L, Milhavet O, Prieur A, Besnard E, Babled A, Aït-Hamou N, Leschik J, Pellestor F, Ramirez JM, De Vos J, Lehmann S, Lemaitre JM. Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev. 2011;25:2248–53.
Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–8.
Petkovich DA, Podolskiy DI, Lobanov AV, Lee SG, Miller RA, Gladyshev VN. Using DNA methylation profiling to evaluate biological age and longevity interventions. Cell Metab. 2017;25:954–60 https://doi.org/10.1016/j.cmet.2017.03.016 .
Gerontology, Rejuvenation by cell reprogramming: a new horizon in. Rodolfo G. Goya, Marianne Lehmann, Priscila Chiavellini, Martina Canatelli-Mallat, Claudia B. Hereñú and Oscar A. Brown. Stem Cell Res Therapy . 2018, 9:349. https://doi.org/10.1186/s13287-018-1075-y .
Ocampo A, Reddy P, Martinez-Redondo P, Platero-Luengo A, Hatanaka F, Hishida T, Li M, Lam D, Kurita M, Beyret E, Araoka T, Vazquez-Ferrer E, Donoso D, Roman JLXJ, Rodriguez-Esteban C, Nuñez G, Nuñez Delicado E, Campistol JM, Guillen I, Guillen P, Izpisua. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell. 2016;167:1719–33.
de Lázaro I, Cossu G, Kostarelos K. Transient transcription factor (OSKM) expression is key towards clinical translation of in vivo cell reprogramming. EMBO Mol Med. 2017;9:733–6.
Sun S, Li ZQ, Glencer P, Cai BC, Zhang XM, Yang J, Li XR. Bringing the age-related macular degeneration high-risk allele age-related maculopathy susceptibility 2 into focus with stem cell technology. Stem Cell Res Ther. 2017;8:135 https://doi.org/10.1186/s13287-017-0584-4 .
Liu J. Induced pluripotent stem cell-derived neural stem cells: new hope for stroke? Stem Cell Res Ther. 2013;4:115 https://doi.org/10.1186/scrt326 .
Shahjalal HM, Dayem AA, Lim KM, Jeon TI, Cho SG. Generation of pancreatic β cells for treatment of diabetes: advances and challenges. Stem Cell ResTher. 2018;9:355 https://doi.org/10.1186/s13287-018-1099-3 .
Kroon E, Martinson LA, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26;443–52.
Arora V, Pooja A, Munshi AK. Banking stem cells from human exfoliated deciduous teeth. J Clin Pediatr Dent. 2009;33(4):289–94.
Mao JJ. Stem cells and the future of dental care. New York State Dental J. 2008;74(2):21–4.
Reznick, Jay B. Continuing education: stem cells: emerging medical and dental therapies for the dental Professional. Dentaltown Magazine . 2008, pp. 42–53.
Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26(7):1787–95.
Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith A. Dental pulp tissue engineering with stem cells from exfoliated. J Endod. 2008;34(8):962–9.
Hayashi K, Ohta H, Kurimoto K, Aramaki S, Saitou M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell. 2011;146(4):519–32. https://doi.org/10.1016/j.cell.2011.06.052 .
Sadri-Ardekani H, Atala A. Testicular tissue cryopreservation and spermatogonial stem cell transplantation to restore fertility: from bench to bedside. Stem Cell ResTher. 2014;5:68 https://doi.org/10.1186/scrt457 .
Zhang Q, Xu M, Yao X, Li T, Wang Q, Lai D. Human amniotic epithelial cells inhibit granulosa cell apoptosis induced by chemotherapy and restore the fertility. Stem Cell Res Ther. 2015;6:152 https://doi.org/10.1186/s13287-015-0148-4 .
Ma DK, Bonaguidi MA, Ming GL, Song H. Adult neural stem cells in the mammalian central nervous system. Cell Res. 2009;19:672–82. https://doi.org/10.1038/cr.2009.56 .
Dantuma E, Merchant S, Sugaya K. Stem cells for the treatment of neurodegenerative diseases. Stem Cell ResTher. 2010;1:37 https://doi.org/10.1186/scrt37 .
Wang Q, Matsumoto Y, Shindo T, Miyake K, Shindo A, Kawanishi M, Kawai N, Tamiya T, Nagao S. Neural stem cells transplantation in cortex in a mouse model of Alzheimer’s disease. J Med Invest. 2006;53:61–9. https://doi.org/10.2152/jmi.53.61 .
Article PubMed Google Scholar
Moghadam FH, Alaie H, Karbalaie K, Tanhaei S, Nasr Esfahani MH, Baharvand H. Transplantation of primed or unprimed mouse embryonic stem cell-derived neural precursor cells improves cognitive function in Alzheimerian rats. Differentiation. 2009;78:59–68. https://doi.org/10.1016/j.diff.2009.06.005 .
Byrne JA. Developing neural stem cell-based treatments for neurodegenerative diseases. Stem Cell ResTher. 2014;5:72. https://doi.org/10.1186/scrt461 .
Awe JP, Lee PC, Ramathal C, Vega-Crespo A, Durruthy-Durruthy J, Cooper A, Karumbayaram S, Lowry WE, Clark AT, Zack JA, Sebastiano V, Kohn DB, Pyle AD, Martin MG, Lipshutz GS, Phelps PE, Pera RA, Byrne JA. Generation and characterization of transgene-free human induced pluripotent stem cells and conversion to putative clinical-grade status. Stem Cell Res Ther. 2013;4:87. https://doi.org/10.1186/scrt246 .
Peng J, Zeng X. The role of induced pluripotent stem cells in regenerative medicine: neurodegenerative diseases. Stem Cell ResTher. 2011;2:32. https://doi.org/10.1186/scrt73 .
Wright BL, Barker RA. Established and emerging therapies for Huntington’s disease. 2007;7(6):579–87 https://www.ncbi.nlm.nih.gov/pubmed/17896994/579-87 .
Lin NH, Gronthos S, Bartold PM. Stem cells and periodontal regeneration. Aust Dent J. 2008;53:108–21.
Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55.
Ramseier CA, Abramson ZR, Jin Q, Giannobile WV. Gene therapeutics for periodontal regenerative medicine. Dent Clin North Am. 2006;50:245–63.
Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. OrthodCraniofac Res. 2005;8:191–9.
Iohara K, Nakashima M, Ito M, Ishikawa M, Nakasima A, Akamine A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein. J Dent Res. 2004;83:590–5.
Hu B, Unda F, Bopp-Kuchler S, Jimenez L, Wang XJ, Haikel Y, et al. Bone marrow cells can give rise to ameloblast-like cells. J Dent Res. 2006;85:416–21.
Liu Y, Liu W, Hu C, Xue Z, Wang G, Ding B, Luo H, Tang L, Kong X, Chen X, Liu N, Ding Y, Jin Y. MiR-17 modulates osteogenic differentiation through a coherent feed-forward loop in mesenchymal stem cells isolated from periodontal ligaments of patients with periodontitis. Stem Cells. 2011;29(11):1804–16. https://doi.org/10.1002/stem.728 .
Raspini G, Wolff J, Helminen M, Raspini G, Raspini M, Sándor GK. Dental stem cells harvested from third molars combined with bioactive glass can induce signs of bone formation in vitro. J Oral Maxillofac Res. 2018;9(1):e2. Published 2018 Mar 31. https://doi.org/10.5037/jomr.2018.9102 .
Christodoulou I, Goulielmaki M, Devetzi M, Panagiotidis M, Koliakos G, Zoumpourlis V. Mesenchymal stem cells in preclinical cancer cytotherapy: a systematic review. Stem Cell Res Ther. 2018;9(1;336). https://doi.org/10.1186/s13287-018-1078-8 .
Bansal R, Jain A. Current overview on dental stem cells applications in regenerative dentistry. J Nat Sci Biol Med. 2015;6(1):29–34. https://doi.org/10.4103/0976-9668.149074 .
Article PubMed PubMed Central Google Scholar
Edgar Ledesma-Martínez, Víctor Manuel Mendoza-Núñez, Edelmiro Santiago-Osorio. Mesenchymal stem cells derived from dental pulp: a review. Stem Cells Int . 2016, 4,709,572, p. doi: https://doi.org/10.1155/2016/4709572 ].
Grzech-Leśniak K. Making use of lasers in periodontal treatment: a new gold standard? Photomed Laser Surg. 2017;35(10):513–4.
Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100(10):5807–12. https://doi.org/10.1073/pnas.0937635100 .
Yasui T, Mabuchi Y, Toriumi H, Ebine T, Niibe K, Houlihan DD, Morikawa S, Onizawa K, Kawana H, Akazawa C, Suzuki N, Nakagawa T, Okano H, Matsuzaki Y. Purified human dental pulp stem cells promote osteogenic regeneration. J Dent Res. 2016;95(2):206–14. https://doi.org/10.1177/0022034515610748 .
Yamamoto A, Sakai K, Matsubara K, Kano F, Ueda M. Multifaceted neuro-regenerative activities of human dental pulp stem cells for functional recovery after spinal cord injury. Neurosci Res. 2014;78:16–20. https://doi.org/10.1016/j.neures.2013.10.010 .
d’Aquino R, De Rosa A, Lanza V, Tirino V, Laino L, Graziano A, Desiderio V, Laino G, Papaccio G. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater. 2009;12, PMID: 19908196:75–83.
Wang L, Shen H, Zheng W, Tang L, Yang Z, Gao Y, Yang Q, Wang C, Duan Y, Jin Y. Characterization of stem cells from alveolar periodontal ligament. Tissue Eng. Part A. 2011;17(7–8):1015–26. https://doi.org/10.1089/ten.tea.2010.0140 .
Iwata T, Yamato M, Zhang Z, Mukobata S, Washio K, Ando T, Feijen J, Okano T, Ishikawa I. Validation of human periodontal ligament-derived cells as a reliable source for cytotherapeutic use. J Clin Periodontol. 2010;37(12):1088–99. https://doi.org/10.1111/j.1600-051X.2010.01597.x .
Chen F-M, Gao L-N, Tian B-M, Zhang X-Y, Zhang Y-J, Dong G-Y, Lu H, et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: a randomized clinical trial. Stem Cell Res Ther. 2016;7:33. https://doi.org/10.1186/s13287-016-0288-1 .
Bakopoulou A, Leyhausen G, Volk J, Tsiftsoglou A, Garefis P, Koidis P, Geurtsen W. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch Oral Biol. 2011;56(7):709–21. https://doi.org/10.1016/j.archoralbio.2010.12.008 .
Han C, Yang Z, Zhou W, Jin F, Song Y, Wang Y, Huo N, Chen L, Qian H, Hou R, Duan Y, Jin Y. Periapical follicle stem cell: a promising candidate for cementum/periodontal ligament regeneration and bio-root engineering. Stem Cells Dev. 2010;19(9):1405–15. https://doi.org/10.1089/scd.2009.0277 .
Luan X, Ito Y, Dangaria S, Diekwisch TG. Dental follicle progenitor cell heterogeneity in the developing mouse periodontium. Stem Cells Dev. 2006;15(4):595–608. https://doi.org/10.1089/scd.2006.15.595 .
Handa K, Saito M, Tsunoda A, Yamauchi M, Hattori S, Sato S, Toyoda M, Teranaka T, Narayanan AS. Progenitor cells from dental follicle are able to form cementum matrix in vivo. Connect Tissue Res. 2002;43(2–3):406–8 PMID: 12489190.
Guo W, Chen L, Gong K, Ding B, Duan Y, Jin Y. Heterogeneous dental follicle cells and the regeneration of complex periodontal tissues. Tissue Engineering. Part A. 2012;18(5–6):459–70 https://doi.org/10.1089/ten.tea.2011.0261 .
Bai, Yudi et al. Cementum- and periodontal ligament-like tissue formation by dental follicle cell sheets co-cultured with Hertwig’s epithelial root sheath cells. Bone. 2011, 48, Issue 6, pp. 1417–1426, https://doi.org/10.1016/j.bone.2011.02.016 .
Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. 2008, 34, pp. 962–969.
Dobie K, Smith G, Sloan AJ, Smith AJ. Effects of alginate, hydrogels and TGF-beta 1 on human dental pulp repair in vitro. Connect Tissue Res 2. 2002;43:387–90.
Friedlander LT, Cullinan MP, Love RM. Dental stem cells and their potential role in apexogenesis and apexification. Int Endod J. 2009;42:955–62.
Cai J, Zhang Y, Liu P, Chen S, Wu X, Sun Y, Li A, Huang K, Luo R, Wang L, Liu Y, Zhou T, Wei S, Pan G, Pei D, Generation of tooth-like structures from integration-free human urine induced pluripotent stem cells. Cell Regen (Lond). July 30, 2013, 2(1), pp. 6, doi: https://doi.org/10.1186/2045-9769-2-6 .
Craig J. Taylor, Eleanor M. Bolton, and J. Andrew Bradley 2011 Aug 12 and https://doi.org/10.1098/rstb.2011.0030 ], 366(1575): 2312–2322. [doi:. Immunological considerations for embryonic and induced pluripotent stem cell banking,. Philos Trans R SocLond B Biol Sci. 2011, 366(1575), pp. 2312–2322, doi: https://doi.org/10.1098/rstb.2011.0030 .
T.R. Nayak, H. Andersen, V.S. Makam, C. Khaw, S. Bae, X.F. Xu, P.L.R. Ee, J.H. Ahn, B.H. Hong, G. Pastorin, B. Ozyilmaz, ACS Nano, 5 (6) (2011), pp. 4. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells,. ACS Nano. 2011, pp. 4670–4678.
Lee WC, Lim C, Shi H, Tang LAL, Wang Y, Lim CT, Loh KP. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano. 2011;5(9):7334–41.
Kenry LWC, Loh KP, Lim CT. When stem cells meet graphene: opportunities and challenges in regenerative medicine. Biomaterials. 2018;155:236–50.
Yuan A, Farber EL, Rapoport AL, Tejada D, Deniskin R, Akhmedov NB, et al. Transfer of microRNAs by embryonic stem cell microvesicles. 2009. 2009, 4(3), p. https://doi.org/10.1371/journal.pone . 0004722.
Oh, Myeongsik, et al. Exosomes derived from human induced pluripotent stem cells ameliorate the aging of skin fibroblasts. Int. J. Mol. Sci. 2018, 19(6), p. 1715.
Ramirez MI. et al. Technical challenges of working with extracellular vesicles. Nanoscale. 2018;10:881–906.
Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–9.
Mateescu B, et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA—an ISEV position paper. J. Extracell. Vesicles. 2017;6(1). https://doi.org/10.1080/20013078.2017.1286095 .
Nawaz M, et al. Extracellular vesicles: evolving factors in stem cell biology. Stem Cells Int. 2016;2016:17. Article ID 1073140.
Helfrich, Y.R., Sachs, D.L. and Voorhees, J.J. Overview of skin aging and photoaging. Dermatol. Nurs. 20, pp. 177–183, https://www.ncbi.nlm.nih.gov/pubmed/18649702 .
Julia Tigges, Jean Krutmann, Ellen Fritsche, Judith Haendeler, Heiner Schaal, Jens W. Fischer, Faiza Kalfalah, Hans Reinke, Guido Reifenberger, Kai Stühler, Natascia Ventura, Sabrina Gundermann, Petra Boukamp, Fritz Boege. The hallmarks of fibroblast ageing, mechanisms of ageing and development, 138, 2014, Pages 26–44. 2014, 138, pp. 26–44, ISSN 0047–6374, https://doi.org/10.1016/j.mad.2014.03.004 .
Huh MI, Kim MS, Kim HK, et al. Effect of conditioned media collected from human amniotic fluid-derived stem cells (hAFSCs) on skin regeneration and photo-aging. Tissue Eng Regen Med. 2014;11:171 https://doi.org/10.1007/s13770-014-0412-1 .
Togel F, Hu Z, Weiss K, et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31.
Liu J, Han G, Liu H, et al. Suppression of cholangiocarcinoma cell growth by human umbilical cord mesenchymal stem cells: a possible role of Wnt and Akt signaling. PLoS One. 2013;8:e62844.
Oh M, et al. Promotive effects of human induced pluripotent stem cell-conditioned medium on the proliferation and migration of dermal fibroblasts. Biotechnol. Bioprocess Eng. 2017;22:561–8.
Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PloS One. 2008;3:e1886.
Bae J-S, Lee S-H, Kim J-E, Choi J-Y, Park R-W, Park JY, Park H-S, Sohn Y-S, Lee D-S, Lee EB. βig-h3 supports keratinocyte adhesion, migration, and proliferation through α3β1 integrin. Biochem. Biophys. Res. Commun. 2002;294:940–8.
Zhou B-R, Xu Y, Guo S-L, Xu Y, Wang Y, Zhu F, Permatasari F, Wu D, Yin Z-Q, Luo D. The effect of conditioned media of adipose-derived stem cells on wound healing after ablative fractional carbon dioxide laser resurfacing. BioMed Res. Int. 2013;519:126.
Peng Y, Baulier E, Ke Y, Young A, Ahmedli NB, Schwartz SD, et al. Human embryonic stem cells extracellular vesicles and their effects on immortalized human retinal Müller cells. PLoS ONE. 2018, 13(3), p. https://doi.org/10.1371/journal.pone.019400 .
Harris MT, Butler DL, Boivin GP, Florer JB, Schantz EJ, Wenstrup RJ. Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. J Orthop Res. 2004;22:998–1003.
Mascetti VL, Pedersen RA. Human-mouse chimerism validates human stem cell pluripotency. Cell Stem Cell. 2016;18:67–72.
Gandia C, Armiñan A, García-Verdugo JM, Lledó E, Ruiz A, Miñana MD, Sanchez-Torrijos J, Payá R, Mirabet V, Carbonell-Uberos F, Llop M, Montero JA, Sepúlveda P. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells. 2007;26(3):638–45.
Perry BC, Zhou D, Wu X, Yang FC, Byers MA, Chu TM, Hockema JJ, Woods EJ, Goebel WS. Collection, cryopreservation, and characterization of human dental pulp-derived mesenchymal stem cells for banking and clinical use. Tissue Eng Part C Methods. 2008;14(2):149–56.
Garcia-Olmo D, Garcia-Arranz M, Herreros D, et al. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416–23.
de Mendonça CA, Bueno DF, Martins MT, Kerkis I, Kerkis A, Fanganiello RD, Cerruti H, Alonso N, Passos-Bueno MR. Reconstruction of large cranial defects in nonimmunosuppressed experimental design with human dental pulp stem cells. J Craniofac Surg. 2008;19(1):204–10.
Seo BM, Sonoyama W, Yamaza T, Coppe C, Kikuiri T, Akiyama K, Lee JS, Shi S. SHED repair critical-size calvarial defects in mice. Oral Dis. 2008;14(5):428–34.
Abbas, Diakonov I., Sharpe P. Neural crest origin of dental stem cells. Pan European Federation of the International Association for Dental Research (PEF IADR). 2008, Vols. Seq #96 - Oral Stem Cells.
Kerkis I, Ambrosio CE, Kerkis A, Martins DS, Gaiad TP, Morini AC, Vieira NM, Marina P, et al. Early transplantation of human immature dental pulp stem cells from baby teeth to golden retriever muscular dystrophy (GRMD) dogs. J Transl Med. 2008;6:35.
Xianrui Yang, Li Li, Li Xiao, Donghui Zhang. Recycle the dental fairy’s package: overview of dental pulp stem cells. Stem Cell Res Ther . 2018, 9, 1, 1. https://doi.org/10.1186/s13287-018-1094-8 .
Wang J, Wang X, Sun Z, Wang X, Yang H, Shi S, Wang S. Stem cells from human-exfoliated deciduous teeth can differentiate into dopaminergic neuron-like cells. Stem Cells Dev. 2010;19:1375–83.
Wang J, et al. The odontogenic differentiation of human dental pulp stem cells on nanofibrous poly (L-lactic acid) scaffolds in vitro and in vivo. Acta Biomater. 2010;6(10):3856–63.
Davies OG, Cooper PR, Shelton RM, Smith AJ, Scheven BA. A comparison of the in vitro mineralisation and dentinogenic potential of mesenchymal stem cells derived from adipose tissue, bone marrow and dental pulp. J Bone Miner Metab. 2015;33:371–82.
Huang GT-J, Shagramanova K, Chan SW. Formation of odontoblast-like cells from cultured human dental pulp cells on dentin in vitro. J Endod. 2006;32:1066–73.
Shi S, Robey PG, Gronthos S. Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone. 2001;29(6):532–9.
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–30.
Nuti N, Corallo C, Chan BMF, Ferrari M, Gerami-Naini B. Multipotent differentiation of human dental pulp stem cells: a literature review. Stem Cell Rev Rep. 2016;12:511–23.
Ferro F, et al. Dental pulp stem cells differentiation reveals new insights in Oct4A dynamics. PloS One. 2012;7(7):e41774.
Conde MCM, Chisini LA, Grazioli G, Francia A, Carvalho RVd, Alcázar JCB, Tarquinio SBC, Demarco FF. Does cryopreservation affect the biological properties of stem cells from dental tissues? A systematic review. Braz Dent J. 2016;1210(6):633-40. https://doi.org/10.1590/0103-6440201600980 .
Papaccio G, Graziano A, d’Aquino R, Graziano MF, Pirozzi G, Menditti D, De Rosa A, Carinci F, Laino G. Long-term cryopreservation of dental pulp stem cells (SBP-DPSCs) and their differentiated osteoblasts: a cell source for tissue repair. J Cell Physiol. 2006;208:319–25.
Alge DL, Zhou D, Adams LL, et. al. Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J Tissue Eng Regen Med. 2010;4(1):73–81.
Jo Y-Y, Lee H-J, Kook S-Y, Choung H-W, Park J-Y, Chung J-H, Choung Y-H, Kim E-S, Yang H-C, Choung P-H. Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng. 2007;13:767–73.
Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–5.
Laino G, d’Aquino R, Graziano A, Lanza V, Carinci F, Naro F, Pirozzi G, Papaccio G. A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB). J Bone Miner Res. 2005;20:1394–402.
Zainal A, Shahrul H, et al. In vitro chondrogenesis transformation study of mouse dental pulp stem cells. Sci World J. 2012;2012:827149.
Wei X, et al. Expression of mineralization markers in dental pulp cells. J Endod. 2007;33(6):703–8.
Dai J, et al. The effect of co-culturing costal chondrocytes and dental pulp stem cells combined with exogenous FGF9 protein on chondrogenesis and ossification in engineered cartilage. Biomaterials. 2012;33(31):7699–711.
Vasandan AB, et al. Functional differences in mesenchymal stromal cells from human dental pulp and periodontal ligament. J Cell Mol Med. 2014;18(2):344–54.
Werle SB, et al. Carious deciduous teeth are a potential source for dental pulp stem cells. Clin Oral Investig. 2015;20:75–81.
Nemeth CL, et al. Enhanced chondrogenic differentiation of dental pulp stem cells using nanopatterned PEG-GelMA-HA hydrogels. Tissue Eng A. 2014;20(21–22):2817–29.
Paino F, Ricci G, De Rosa A, D’Aquino R, Laino L, Pirozzi G, et al. Ecto-mesenchymal stem cells from dental pulp are committed to differentiate into active melanocytes. Eur. Cell Mater. 2010;20:295–305.
Ferro F, Spelat R, Baheney CS. Dental pulp stem cell (DPSC) isolation, characterization, and differentiation. In: Kioussi C, editor. Stem cells and tissue repair. Methods in molecular biology (methods and protocols): Humana Press. 2014;1210.
Ishkitiev N, Yaegaki K, Imai T, Tanaka T, Nakahara T, Ishikawa H, Mitev V, Haapasalo M. High-purity hepatic lineage differentiated from dental pulp stem cells in serum-free medium. J Endod. 2012;38:475–80.
Download references
Acknowledgements
Not applicable.
This work is supported by Wrocław Medical University in Poland.
Availability of data and materials
Please contact author for data requests.
Author information
Authors and affiliations.
Department of Experimental Surgery and Biomaterials Research, Wroclaw Medical University, Bujwida 44, Wrocław, 50-345, Poland
Wojciech Zakrzewski, Maria Szymonowicz & Zbigniew Rybak
Department of Conservative Dentistry and Pedodontics, Krakowska 26, Wrocław, 50-425, Poland
Maciej Dobrzyński
You can also search for this author in PubMed Google Scholar
Contributions
WZ is the principal author and was responsible for the first draft of the manuscript. WZ and ZR were responsible for the concept of the review. MS, MD, and ZR were responsible for revising the article and for data acquisition. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Wojciech Zakrzewski .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Zakrzewski, W., Dobrzyński, M., Szymonowicz, M. et al. Stem cells: past, present, and future. Stem Cell Res Ther 10 , 68 (2019). https://doi.org/10.1186/s13287-019-1165-5
Download citation
Published : 26 February 2019
DOI : https://doi.org/10.1186/s13287-019-1165-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Differentiation
- Pluripotency
- Induced pluripotent stem cell (iPSC)
- Stem cell derivation
- Growth media
- Tissue banks
- Tissue transplantation
Stem Cell Research & Therapy
ISSN: 1757-6512
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Open access
- Published: 16 May 2024
Human lung cancer-derived mesenchymal stem cells promote tumor growth and immunosuppression
- Xiaoyan Gao 1 ,
- Zhengrong Zhang 1 , 2 ,
- Shuai Cao 3 ,
- Bo Zhang 1 , 4 ,
- Qiang Sun 4 ,
- Gerry Melino 2 , 5 &
- Hongyan Huang 1
Biology Direct volume 19 , Article number: 39 ( 2024 ) Cite this article
1 Altmetric
Metrics details
The presence of mesenchymal stem cells has been confirmed in some solid tumors where they serve as important components of the tumor microenvironment; however, their role in cancer has not been fully elucidated. The aim of this study was to investigate the functions of mesenchymal stem cells isolated from tumor tissues of patients with non-small cell lung cancer.
Human lung cancer-derived mesenchymal stem cells displayed the typical morphology and immunophenotype of mesenchymal stem cells; they were nontumorigenic and capable of undergoing multipotent differentiation. These isolated cells remarkably enhanced tumor growth when incorporated into systems alongside tumor cells in vivo. Importantly, in the presence of mesenchymal stem cells, the ability of peripheral blood mononuclear cell-derived natural killer and activated T cells to mediate tumor cell destruction was significantly compromised.
Collectively, these data support the notion that human lung cancer-derived mesenchymal stem cells protect tumor cells from immune-mediated destruction by inhibiting the antitumor activities of natural killer and T cells.
Mesenchymal stem cells (MSCs) are fibroblast-like, multipotent progenitor cells that were initially discovered in bone marrow; however, over time, their presence has been confirmed in almost all tissue types, and these cells exhibit the potential for multidirectional differentiation (such as into bone, adipose, cartilage, and muscle cells) as well as the capacity for self-renewal [ 1 , 2 , 3 ]. MSCs can be recruited to the site of tissue injuries where they participate in processes associated with wound repair. Tumors are considered to be "wounds that never heal," and MSCs tend to migrate toward sites of inflammation and tumor microenvironments [ 3 , 4 ]; therefore, many studies have recommended the use of MSCs as therapeutic vectors to target tumors [ 5 , 6 , 7 ]. However, the function of MSCs in cancer remains controversial, and it is essential to clarify their effects within the tumor microenvironment. Several studies have demonstrated that MSCs exert their antitumor effects through several mechanisms, including the inhibition of angiogenesis, the promotion of antitumor immune responses, and the induction of apoptosis in cancer cells [ 8 , 9 , 10 ]. In contrast, several other studies have shown that MSCs can promote tumor growth and metastasis by enhancing tumor cell proliferation, angiogenesis, and metastatic capacity, thereby inducing immunosuppression or inhibiting tumor cell apoptosis [ 11 , 12 , 13 , 14 , 15 , 16 , 17 ]. Possible explanations for the discrepancies between studies could be the variability of MSCs in terms of the tissue and microenvironment from which they were isolated or the fact that some studies have used murine rather than human MSCs. Notably, previous studies have mainly focused on MSC lines or healthy donor-derived MSCs isolated from bone marrow. Only recently has there been growing interest in the effects of tumor-derived MSCs on cancer progression. However, the interactions between tumor-associated MSCs and cancer cells remain obscure and require further investigation.
The aims of this study were to isolate human lung cancer-derived MSCs (hLC-MSCs) to characterize their phenotypes, assess their effects on tumor growth both in vivo and in vitro, and elucidate the mechanisms underlying their tumor-promoting effects.
Materials and methods
Dissociation of tumor-associated mesenchymal cells.
Tumor tissue samples were obtained from two patients with non-small cell lung cancer (NSCLC) who had not received any treatment prior to undergoing surgical resection at Xuanwu Hospital. Written informed consent was obtained according to the guidelines of the Ethics Committee of Capital Medical University. Lung tumor tissues were minced and added to a solution containing a mixture of dispase and collagenase 1A (STEMCELL Technologies Inc, Vancouver, BC, Canada) for digestion. A cell strainer (70 μm; BD Biosciences, Bedford, MA) was used for single-cell isolation and the removal of adipose and other tissues.
Cells and culture conditions
hLC-MSC cell expansion was performed as previously described by Liu et al. [ 18 ]. Briefly, isolated epithelial cells were co-cultivated with irradiated (3,000 rad) Swiss 3T3 fibroblasts (J2 strain) in F medium [3:1 (v/v) Ham’s Nutrient Mixture F-12: Dulbecco's Modified Eagle's Medium (Invitrogen), with 5% fetal bovine serum (FBS), 10 ng/mL epidermal growth factor (Invitrogen, Waltham, MA), 5 μg/mL insulin, 0.4 μg/mL hydrocortisone, 24 μg/mL adenine (Sigma-Aldrich, St. Louis, MO), and 8.4 ng/mL cholera toxin], to which 10 μmol/L Y-27632 (ROCK inhibitor, Tocris Bioscience, Bristol, UK) was added. All cells were cultured in a humidified atmosphere of 5% CO 2 at a temperature of 37 °C and passaged at a ratio of 1:4 after reaching 80%–90% confluence.
Differential trypsinization was performed to separate feeder and epithelial cells during passaging. Briefly, feeder/epithelial co-cultures were rinsed with phosphate-buffered saline (PBS) and incubated with 0.05% trypsin solution at room temperature for 30 s to 1 min, with close monitoring under phase-contrast microscopy. When the feeder cells became rounded and began to detach from the substrate, the cultures were gently tapped to facilitate their detachment and subsequent removal by aspiration, while the epithelial cell colonies remained tightly adherent. The epithelial cells were again rinsed with PBS and trypsinized at 37 °C for 3–5 min. The cells were transferred to a solution of PBS containing 10% serum to neutralize the trypsin and subjected to centrifugation at 500× g . The cell pellets were subsequently resuspended in F medium for passaging. To minimize any potential changes in cellular behavior caused by prolonged culture times, cells at passages P3–P5 were used in this study.
Cell line MC38/CT-26/NIH3T3/A549/HepG2 and its derivatives were routinely maintained in Dulbecco’s Modified Eagle’s Medium (MACGENE Technology Ltd., Beijing, China) supplemented with 10% FBS (Kangyuan Biology, China), and 100 units/mL penicillin plus 100 µg/mL streptomycin (Invitrogen). NK92MI cell line was maintained in RPMI 1640 medium (MACGENE Technology Ltd.) supplemented with 12.5% FBS and 12.5% horse serum (Kangyuan Biology). T cells isolated from peripheral blood and activated were further cultured in RPMI 1640 medium supplemented with 10% FBS supplemented with 100 IU/mL human interleukin 2 (hIL-2). All cells were cultured in a humidified incubator with 5% CO2 at 37 °C.
Constructs and stable cell lines
The luciferase gene was subcloned into a pQCXIP retroviral vector to generate a pQCXIP-luciferase-puro vector. pQCXIP and retroviral helper plasmids vesicular stomatitis virus G (VSV-G) and Gag-Pol-Rev were purchased from Addgene. All constructs were verified using DNA sequencing. More detailed information is provided below.
Stable cell lines were established via viral infection. Retroviruses were packaged into human embryonic kidney 293 T (HEK293T) cells using Lipofectamine 2000 reagent (Invitrogen), as previously described [ 19 , 20 ]. For infection, 1 mL of viral supernatants mixed with 10 μg of Polybrene (Sigma) was added to the target cells in 6-well plates for a period of 12 h, after which the cells were fed with regular media. Virus-infected tumor cells were selected through a 7–14-day exposure to puromycin (2 μg/mL).
Flow cytometric analysis
Cells were detached from the culture plate using TrypLE Express (Invitrogen) before being resuspended in PBS supplemented with 1% bovine serum albumin. The cells were incubated with fluorophore-conjugated antibodies targeting various cluster of differentiation (CD) and human leukocyte antigens (HLA) for 30 min in the dark at 4 °C, including CD14, CD90, CD166, CD144, CD73, CD105, CD45, CD31, CD29, and the HLA-DR isotype. Cell suspensions with isotype-matched immunoglobulins were used as controls. After three washes with PBS, the labeled samples were analyzed using a FACSAria II flow cytometer (BD Biosciences, San Jose, CA).
In vitro assessment of osteogenic differentiation
Cells were cultured in osteo-inductive medium (alpha-Minimum Essential Medium [α-MEM] containing 0.1 μM dexamethasone, 10 mM β-sodium glycerophosphate, 50 μM ascorbic acid, and 10% FBS) for 21 days, with a half-volume change performed every 3 days. Von Kossa staining was performed to detect calcified matrix precipitation. Cells were fixed in neutral formaldehyde solution for 1 h. After washing with deionized water, 2% silver nitrate solution was added for a 10-min reaction at 37 °C in the dark, a 15-min exposure time, and washing in deionized water. The presence of calcified matrix precipitation was assessed using an inverted phase-contrast microscope. Cells in the control group were cultured in α-MEM plus 10% FBS for 3 weeks.
In vitro assessment of adipogenic differentiation
Cells were cultured in adipo-inductive medium (α-MEM containing 1 μM dexamethasone, 200 μM indomethacin, 10 μM insulin, 0.5 mM isobutyl methylxanthine, and 10% FBS), with a half-volume change performed every 3 days. After 3 weeks, the cells were fixed with neutral formaldehyde for 10 min at room temperature; this was followed by staining with Oil Red O and counterstaining of cell nuclei with alum hematoxylin to assess the degree of adipogenesis. Fat droplets were observed using an inverted phase-contrast microscope. The control cells were MSCs cultured in the aforementioned cell expansion medium.
Preparation of cytotoxic T lymphocytes (CTLs)
First, peripheral blood mononuclear cells (PBMCs) were isolated from heparinized venous blood samples collected from healthy adults using Ficoll Histopaque (Sigma Chemical Co., St. Louis, MO) density gradient centrifugation. Subsequently, an EasySep™ Human T Cell Isolation Kit (STEMCELL Technologies Inc.) was used to isolate T cells from the PBMCs. Viable human T cells were seeded in Roswell Park Memorial Institute (RPMI)-1640 complete medium at a density of 1 × 10 6 cells/mL. To activate the T cells, 25 µL/mL of ImmunoCult™ Human CD3/CD28/CD2 T Cell Activator and 100 U/mL of hIL-2 were added to the cell suspension. The cells were subsequently incubated at 37 °C with 5% CO 2 for up to 3 days. To induce T cell expansion after 3 days of activation, the T cells were cultured in RPMI-1640 complete medium containing 100 U/mL of hIL-2 and subcultured once every 2–3 days to maintain a cell density of 1 × 10 6 cells/mL. After 12 days of culture, the CTLs were collected for subsequent experiments.
In vitro assessment of immune cell-induced tumor cell destruction
Tumor cells were plated at a density of 2,000 or 4,000 cells/well in either 96-well flat-bottomed plates or the lower chamber of a transwell system (Corning, NY, USA) in DMEM supplemented with 10% FBS. MSCs were plated at a density of 2,000 or 40,000 cells/well in either 96-well flat-bottomed plates or the upper chamber of a transwell system (Corning, NY, USA) in DMEM supplemented with 10% FBS. The following day, natural killer (NK) or T cells were added to the cultures on top of the tumor cell layer at different effect-to-target ratios in each well in RPMI 1640 medium containing 10% FBS supplemented with hIL-2 to reach a final concentration of 100 IU/mL. Luciferase activity was measured after 24 h and 48 h.
Tumor formation assay
All protocols involving animals were approved by the Animal Care and Use Committee of the Beijing Institute of Biotechnology and performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. To assess the tumor formation capacity, 3 × 10 6 MC38 murine colon adenocarcinoma cells, with or without 3 × 10 6 hLC-MSCs, were suspended in a 100-μL volume at a 1:1 ratio in either standard RPMI 1640 medium alone or medium with Matrigel matrix (BD Biosciences), and transplanted subcutaneously into 4- to 5-week-old C57BL/6N mice. Tumor formation was monitored weekly.
Statistical analysis
All data are presented as the mean with standard deviations (SD) unless stated otherwise. Statistical analyses were performed using SPSS software (version 22.0; IBM Corp., Armonk, NY). For all quantitative measurements, a normal distribution was assumed, and differences between two groups were determined using unpaired, two-tailed Student’s t -tests. Each measurement was based on at least three independent replicates.
Isolation of hLC-MSCs
After 8 days of culture in conditions conducive to the growth of MSCs, the cells that remained adhered to the Petri dish formed several cell colonies (Fig. 1 A-a). Cobblestone-like cell colonies, determined to be primary lung cancer cells, were observed (Fig. 1 A-b), in addition to MSC-like cells that exhibited a long, spindle-shaped morphology and were abundant in all cultures (Fig. 1 A-c). Single-cell suspensions were re-plated to increase the purity of the hLC-MSC clones, and several assays were conducted to confirm the low degree of contamination with other cell types, especially cancer and endothelial cells, to allow for cellular phenotyping (Fig. 1 A-d). Immunophenotyping of the hLC-MSCs was performed based on the immunofluorescent labeling of various molecules, including E-cadherin (a marker of epithelial cells), N-cadherin and vimentin (markers of mesenchymal cells), α-smooth muscle actin (α-SMA) (a fibroblast marker), and cytokeratin 18 (CK18) (a malignant tumor marker). The hLC-MSCs isolated in the present study did not express E-cadherin, whereas they did express N-cadherin, vimentin, α-SMA, and CK18 (Fig. 1 B).
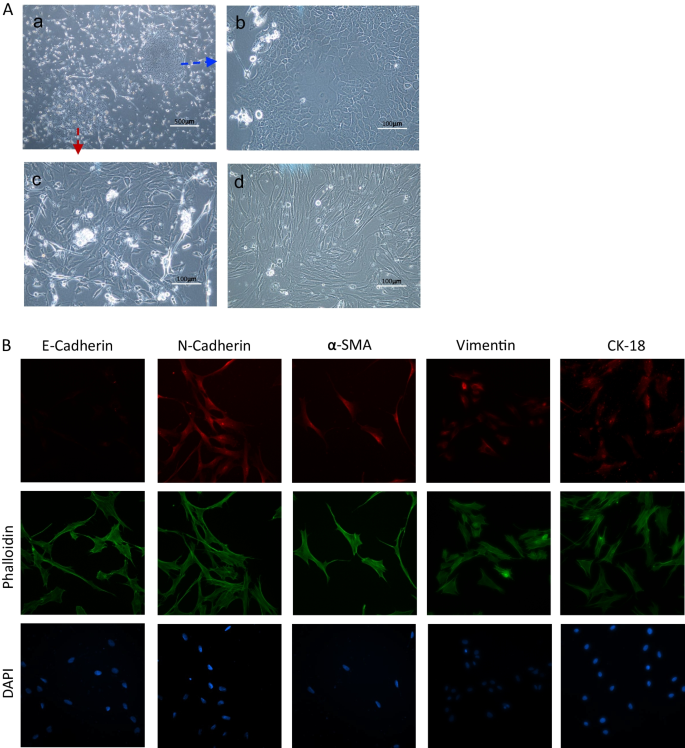
Morphology and immunophenotyping of hLC-MSCs. A (a) Representative images of primary cells arising from NSCLC tissues. Magnification, ×40. (b–d) The resultant distinct, purified colonies of epithelial tumors cells (b) or hLC-MSCs (c–d). Magnification ×200. B Representative images of hLC-MSC clones that were subjected to immunocytochemical staining. The hLC-MSCs were negative for E-cadherin and positive for N-cadherin, vimentin, α-SMA, and CK18 expression. Cytoskeletal proteins were stained using phalloidin, and cell nuclei were stained with DAPI. hLC-MSCs human lung cancer-derived mesenchymal stem cells; NSCLC non-small cell lung cancer; α-SMA alpha smooth muscle actin; CK18 cytokeratin 18; DAPI 4’,6-diamidino-2-phenylindole
Surface markers expressed by hLC-MSCs
To determine whether the colony-forming cells (hLC-MSCs) expressed the same characteristic surface antigens as MSCs, flow cytometric analysis was performed, which confirmed that these cells did in fact express a set of MSC markers, including CD90, CD166, CD73, CD29, and CD105 (Fig. 2 ). Antibodies that target several of these antigens are routinely used to characterize expanded mesenchymal cell populations. In contrast, the cells did not express the lipopolysaccharide receptor CD14, the leukocyte common antigen CD45, the endothelial cell marker CD31, or the epithelial cell marker CD144. Moreover, the cells did not express HLA-DR. Collectively, these data confirmed that the isolated MSC-like cells exhibited surface marker expression patterns typically observed in MSCs, which was in accordance with the accepted phenotypic markers for hMSCs described previously [ 21 ].
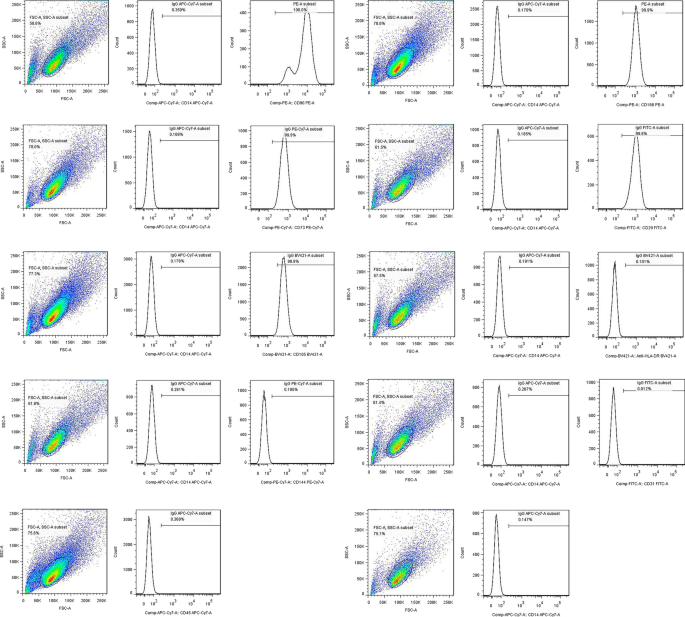
Surface antigens expressed by hLC-MSCs. Surface antigens expressed on hLC-MSCs were analyzed using flow cytometry. The hLC-MSCs were positive for CD90, CD166, CD73, CD29 and CD105, and negative for CD14, CD45, CD31, CD144, and HLA-DR. hLC-MSCs human lung cancer-derived mesenchymal stem cells; CD cluster of differentiation; HLA-DR human leukocyte antigen DR isotype
Multilineage differentiation capacity of hLC-MSCs
True MSCs have the capacity to undergo multipotent differentiation into cells of the adipogenic, osteogenic, and chondrogenic lineages when cultured in specific media [ 1 , 22 , 23 ]. Therefore, the cells were first cultured in a medium containing 1-methyl-3-isobutylxanthine, dexamethasone, insulin, and indomethacin to induce adipogenic differentiation [ 1 ]. After a two-week incubation, the hLC-MSCs had begun to undergo differentiation. In the third week, the hLC-MSCs had clearly differentiated into adipocytes (Fig. 3 A), with lipid droplets with positive Oil Red O staining.
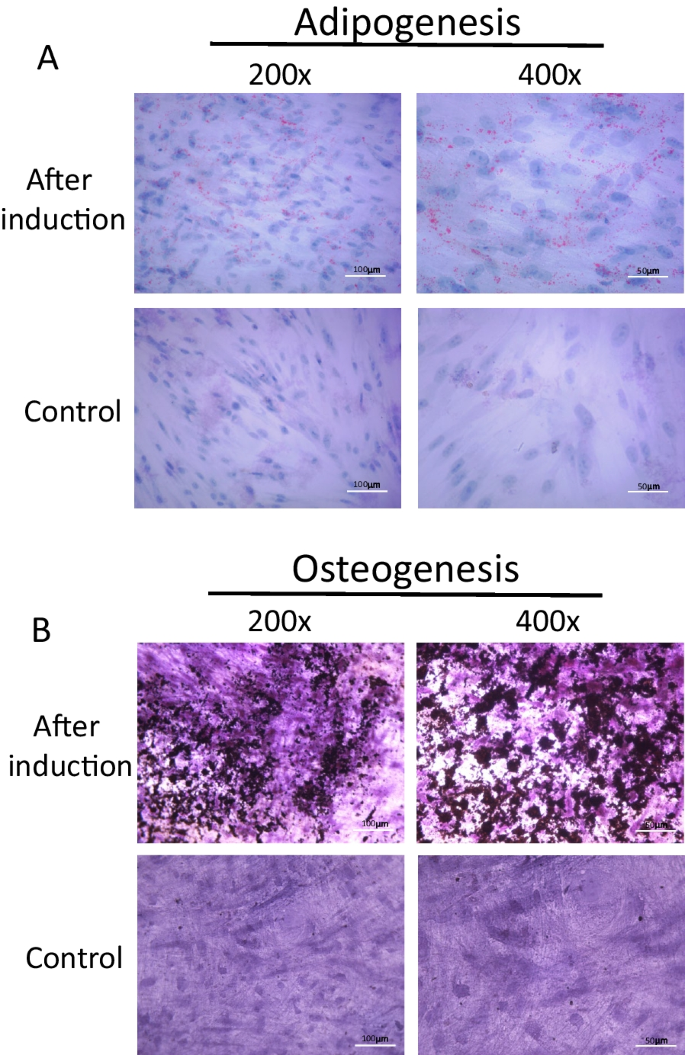
Differentiation potential of hLC-MSCs. A Representative images of hLC-MSCs that had differentiated into adipocytes, with positive Oil-Red-O staining of triglycerides. Most hLC-MSCs exhibited positive Oil-Red-O staining after being induced to differentiate into adipocytes for 21 days, whereas the control cells, which were not exposed to conditions conducive to adipocyte development, failed to undergo such differentiation (magnifications: ×200, ×400). B Representative images of hLC-MSCs that had differentiated into osteoblasts based on positive von Kossa staining of calcium deposition after three weeks of exposure to conditions specifically conducive to osteoblast development. The control cells failed to undergo such differentiation, as evidenced by the negative von Kossa staining (magnification: ×200, ×400). hLC-MSCs human lung cancer-derived mesenchymal stem cells
To promote osteogenic differentiation, the hLC-MSCs were cultured in a medium supplemented with dexamethasone, β-glycerophosphate, and ascorbate. Three weeks later, osteogenic differentiation products were detected using von Kossa staining, whereas the cells that had been cultured in normal media did not undergo such differentiation after culturing for 3–4 weeks or longer. Collectively, these data indicated that the isolated hLC-MSCs possessed the capacity to undergo multipotent differentiation under different culture conditions.
hLC-MSCs enhance cancer cell growth in vivo
Some studies have reported that MSCs promote tumor growth, whereas other have shown they suppress it. To evaluate the effect of hLC-MSCs on tumor formation in vivo, MC38 cells, a line of murine colorectal adenocarcinoma cells, were injected into the flanks of immune-competent C57BL/6N mice, either alone or in combination with hLC-MSCs. Tumors formed by the co-injection of MC38 cells and hLC-MSCs were significantly larger than those formed by the injection of MC38 cells alone. To examine the effect of hLC-MSCs on tumor growth in vivo, hLC-MSCs were also injected alone into C57BL/6N mice; this failed to induce tumor formation under the same conditions. After 6 weeks, the mice were euthanized and the tumors were dissected and weighed (Fig. 4 A). The tumor weights in the MC38 + hLC-MSC group were significantly higher than those in the control group injected with tumor cells alone (Fig. 4 B, C). These data demonstrate that the tumor-promoting abilities in mice with normal immunity were a specific property of the admixed MSCs or their derivatives.
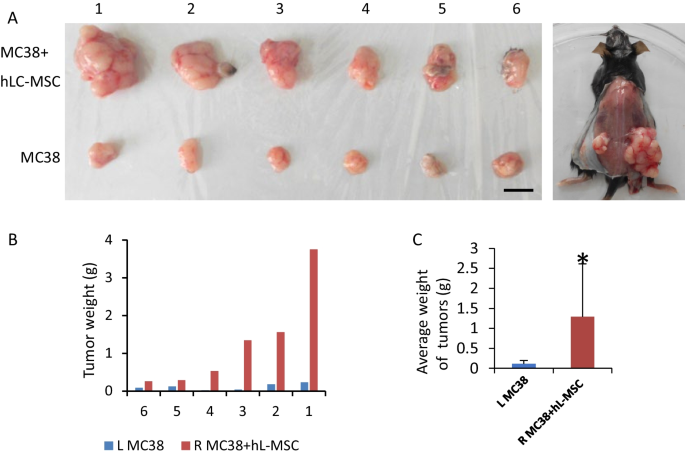
Human lung cancer-derived mesenchymal stem cells enhance tumor cell growth in vivo. A Representative images showing the formation of tumors in C57BL/N6 mice who were subcutaneously injected with either MC38 cells alone (left) or MC38 cells in combination with hLC-MSCs (right) (n = 6). B , C Tumor weights at the end of the experiment. The data are shown as the mean ± SD. * P < 0.05; ** P < 0.01; *** P < 0.001. MC38 murine colorectal adenocarcinoma cells; hLC-MSC human lung cancer-derived mesenchymal stem cells; SD standard deviation
hLC-MSCs inhibit NK cell-mediated tumor destruction in contact and non-contact systems
Tumor cells were co-cultured with hLC-MSCs (at a 1:1 ratio) or without hLC-MSCs at tumor cell:NK cell ratios of 1:0, 1:1, 1:2, 1:4, and 1:5 in a combined medium containing DMEM with 10% FBS plus MSC-conditioned medium. In the contact systems, as presented in Fig. 5 A, B, hLC-MSCs inhibited the killing efficiency of NK cells in a dose-dependent manner; similar results were observed for the transwell system experiments, as presented in Fig. 8 A, suggesting that soluble factors are involved in this process. To rule out the possibility that the effect of mass was mediating this outcome, the same experiments were repeated with NIH3T3 fibroblast or MRC-5 fetal lung fibroblast cell lines rather than hLC-MSCs at a ratio of 1:1. Little inhibitory effect was observed in either the contact cultures or the transwell systems (data not shown). To confirm that these effects were the result of the inhibition of NK cell-mediated destruction and not due to changes in cellular proliferation, luciferase values were compared with those of the tumor cells in the control group that had not been exposed to NK cells; no significant differences were observed between the tumor cell–MSC co-culture group, the tumor cell–control cell co-culture group, and the tumor cell only group (Figs. 6 A, B and 8 C). Collectively, these results suggest that the attenuation of the ability of NK cells to destroy tumor cells can be attributed to the hLC-MSC co-culture.
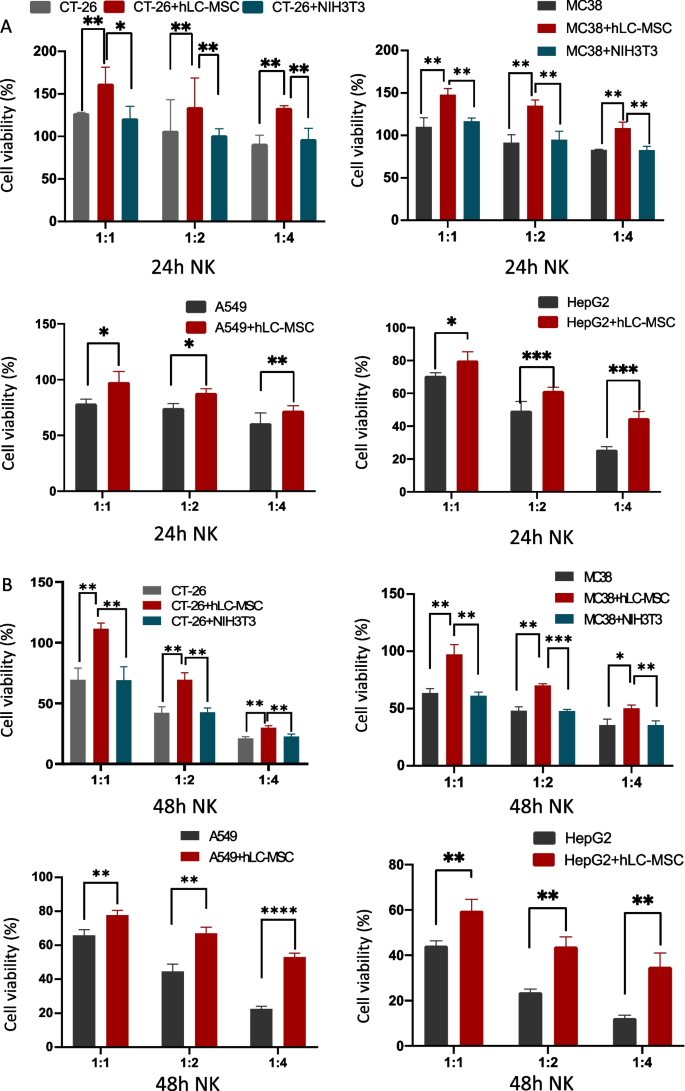
Human lung cancer-derived mesenchymal stem cells inhibit NK cell-mediated tumor destruction in vitro. Tumor cells were cultured in normal medium for 24 h ( A ) or 48 h ( B ) in the presence or absence of hLC-MSCs at hLC-MSC:NK cell ratios of 1:1, 1:2, and 1:4 in the contact system. The data are expressed as the mean ± SD based on data from three independent replicates. *Statistically significant ( P < 0.05) difference compared with that of the cultures performed without hLC-MSCs. NK natural killer; hLC-MSCs human lung cancer-derived mesenchymal stem cells

hLC-MSCs effects on tumor cell growth in an in vitro contact system. hLC-MSCs did not affect the kinetics of CT-26, MC-38, A549, or HepG2 tumor cells in vitro after 24 h ( A ) or 48 h ( B ) of exposure in the contact system. The histograms show the luciferase activity of the indicated cells cultured for 24 h or 48 h in vitro in the presence or absence of hLC-MSCs (n = 5). * P ≤ 0.05, ** P ≤ 0.001, n.s. > 0.05 by Student’s t -test. The error bars represent the SD of three independent experiments. hLC-MSC human lung cancer-derived mesenchymal stem cells; CT-26 an undifferentiated colon carcinoma cell line; MC-38 a murine colon adenocarcinoma cell line; A549 lung carcinoma epithelial cell line; HepG2 a human liver cancer cell line; n.s. not significant; SD standard deviation
hLC-MSCs inhibit T cell-mediated tumor destruction in contact and no-contact systems
Tumor cells were co-cultured with hLC-MSCs (at a 1:1 ratio) or without hLC-MSCs at tumor cell:T cell ratios of 1:0, 1:5, and 1:10 in a combined medium consisting of DMEM with 10% FBS plus MSC-conditioned medium. As presented in Fig. 7 A, hLC-MSCs inhibited the killing efficiency of T cells in a dose-dependent manner. This inhibitory effect was conspicuous when MSCs and cancer cells were in physical contact, and this influence remained significant even when they were cultured separately (Fig. 8 B). In addition, to rule out the possibility that the observed effect was related to mass in both the contact culture and transwell system, hLC-MSCs were replaced with NIH3T3 (or MRC-5) cells, which resulted in the absence of an inhibitory effect. The comparison of luciferase signals confirmed that the observed effects were not a result of changes in tumor cell proliferation (Figs. 7 B, 8 D). Collectively, these results suggest that the compromised T cell-mediated destruction of tumor cells can be attributed to the hLC-MSC co-culture.
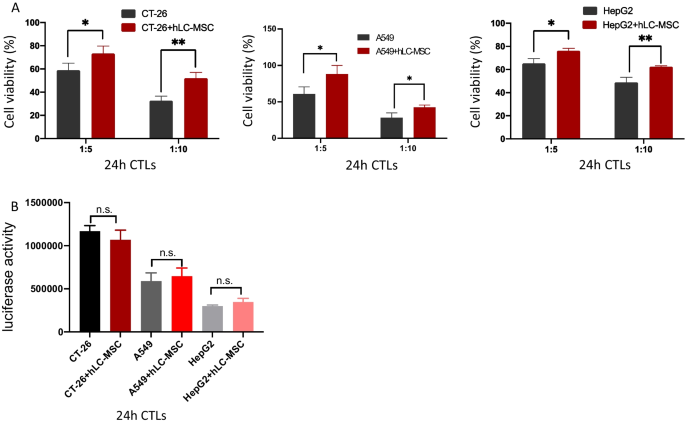
Human lung cancer-derived mesenchymal stem cells inhibit CTL-mediated tumor cell destruction in vitro. A Tumor cells were cultured in ordinary medium for 24 h in the presence or absence of hLC-MSCs, at hLC-MSC:CTLs ratios of 1:5 and 1:10 in a contact system. The in vitro cell viability ratios of the indicated cells are shown (n = 5). B hLC-MSCs did not affect the kinetics of CT-26, A549 or HepG2 tumor cells in vitro following 24 h of exposure in a contact system. The histograms show the luciferase activity of the indicated cells cultured in the presence or absence of hLC-MSCs (n = 5). The data are expressed as the mean ± SD from three independent replicates. * P ≤ 0.05, ** P ≤ 0.001, n.s. > 0.05 by Student’s t -test. The error bars represent SD of three independent experiments. CTL cytotoxic T lymphocytes; hLC-MSCs human lung cancer-derived mesenchymal stem cells; CT-26 an undifferentiated colon carcinoma cell line; A549 lung carcinoma epithelial cell line; HepG2 a human liver cancer cell line; SD standard deviation; n.s. not significant
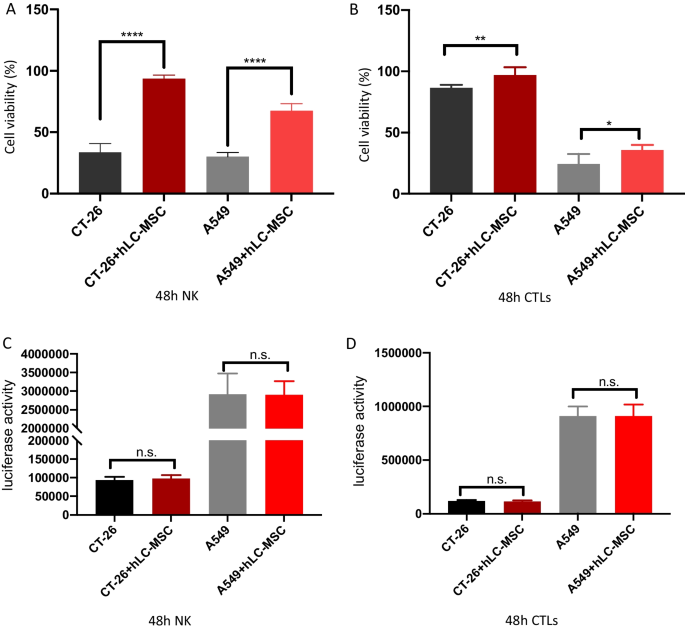
Human lung cancer-derived mesenchymal stem cells inhibit NK-/CTL-mediated tumor cell destructions in vitro. Tumor cells were cultured in ordinary medium for 48 h in the presence or absence of hLC-MSCs at hLC-MSC:CTL ( A ) or hLC-MSC:NK cell ( B ) ratios of 1:5 in a transwell system. The histograms show the viability of the indicated cells cultured for 48 h in vitro in the presence or absence of hLC-MSCs (n = 5). C , D hLC-MSCs did not affect the kinetics of the indicated cells when cultured in a transwell system containing tumor cells for 48 h. The histograms show the luciferase activity of the indicated cells cultured for 48 h in vitro in the presence or absence of hLC-MSCs (n = 5). * P ≤ 0.05, ** P ≤ 0.001, n.s. > 0.05 by Student’s t -test. Error bars represent the SD of three independent experiments. NK natural killer; CTL cytotoxic T lymphocyte; hLC-MSCs human lung cancer-derived mesenchymal stem cells; n.s. not significant; SD standard deviation
MSCs have become a focal point of research in regenerative medicine and immunology because of their capacity for self-renewal, their ability to differentiate into multiple cell types, their tendency to be recruited to sites of inflammatory injury, and their immunosuppressive capabilities. During tumorigenesis, non-cancerous tissue-derived MSCs (such as bone marrow-derived MSCs [BM-MSCs]) are recruited to tumor sites where they become integrated into the tumor stroma where they are instructed to adapt to novel features and become tumor-resident MSCs. Therefore, the properties of tissue-resident MSCs are primarily determined by the tissue in which they reside and their physical location within those tissues [ 24 ].
Although numerous studies have investigated the correlation between noncancerous tissue-derived MSCs and tumor cells, transformed tumor-resident MSCs have not been adequately characterized to date in terms of their properties and their roles in modulating tumor growth and progression. The results of the present study indicate that MSCs are commonly present in the tumors of human patients with lung cancer, which is consistent with previous reports that MSCs are present in those with many other types of cancer [ 25 , 26 ]. The study provides clear experimental evidence that indicates the isolated cells were, in fact, MSCs, and not cancer-associated fibroblasts. First, the hLC-MSCs demonstrated the capacity for multipotent differentiation by transforming into adipose and bone tissues when exposed to certain conditions in vitro. The hLC-MSCs also expressed cell surface markers that are commonly expressed on MSCs, such as CD29, CD73, CD90, CD166, and CD105, whereas hematopoietic markers such as CD14, CD31, and CD45 were absent. Additionally, unlike fibroblasts, these cells could be stably maintained in MSC-specific medium for several months without losing their capacity for multipotent differentiation.
Most previous studies that have conducted in vivo tumorigenic experiments in immunodeficient mice involved models or conditions that failed to objectively and accurately reflect the immunosuppressive effects of tumor-associated MSCs on cancer cell growth and proliferation. Thus, in the present study, C57BL/6N immunocompetent mice were used to more adequately reflect real-world conditions. The data strongly support the fact that MSCs exert tumor-promoting activity within the tumor microenvironment, an effect that appears to be at least partially due to their immunosuppressive capabilities. These results are consistent with those of previous studies; for example, in 2012, Ren et al. reported that murine lymphoma-derived MSCs (L-MSCs) exerted a much more pronounced effect on the promotion of tumor growth compared to that of matched BM-MSCs [ 27 ]. Tumor-associated MSCs differ from BM-MSCs in several ways; for instance, an in-depth in vivo analysis revealed that, unlike their BM-MSC counterparts, these L-MSCs produced high levels of the C–C motif chemokine receptor 2 (CCR2) ligands CCL2, CCL7, and CCL12, which promoted the recruitment of macrophages to tumor sites where they underwent a phenotypic shift to the tumor-promoting M2-like phenotype [ 27 ].
It has long been assumed that the primary mechanism through which the immune system achieves tumor cell destruction involves NK cells and major histocompatibility complex class I (MHC-I)-restricted CTLs [ 7 ]. Tumor cells that downregulate MHC-I molecules are protected from CTL-mediated destruction, although they are still susceptible to NK-mediated killing. Recently, human MSCs have been shown to exhibit immunosuppressive properties that affect NK and T-lymphocyte proliferation in an MHC-independent manner, bypassing the species barrier. MSCs may also be capable of inhibiting several functions of naïve and memory T cells, and they express negligible levels of MHC-II molecules, low levels of MHC-I molecules, and no co-stimulatory molecules [ 28 , 29 , 30 , 31 ]. Since MSCs themselves are not inherently immunogenic, they are incapable of eliciting allogeneic T cell responses [ 32 ]; this phenomenon has been reported to be mediated by the production of certain cytokines, such as transforming growth factor beta 1 (TGF-β1) and hepatocyte growth factor (HGF), rather than by the induction of apoptosis [ 17 , 29 , 33 , 34 , 35 ].
To test this assumption, several in vitro experiments were conducted. First, to test whether this effect was caused by direct contact or if it occurred via soluble mediators, tumor cells were co-cultured with hLC-MSCs in direct or transwell systems. Compared with the abundance seen in the blank control or the group involving mixed fibroblast populations, tumor cells in the hLC-MSC group were significantly more abundant, regardless of which co-culture system was used. To test whether this phenomenon was caused by tumor cell proliferation or the suppression of immune cell-mediated tumor destructions, the number of tumor cells was quantified in each group in which the immune cells were absent from the culture; those experiments revealed that the presence of hLC-MSCs had little impact on the proliferation of tumor cells among the different groups and confirmed that hLC-MSCs promoted tumor growth, at least in part, by inhibiting immune cell-mediated tumor cell destruction.
It is important to acknowledge that the present study was a preliminary exploration of the effects of MSCs on tumor cells, and further studies are required to investigate the underlying mechanisms. Both in vivo and in vitro studies have shown that murine BM-MSCs and human placental MSCs can induce tolerance in monocytes and a phenotypic shift in macrophages from an inflammatory phenotype to an immunosuppressive one that is characterized by increased IL-10 production and the expression of co-inhibitory molecules such as B7-H4 [ 36 , 37 ]. Interleukin 6 (IL-6) is significantly enriched in the supernatant of cultured MSCs and exerts an immunosuppressive effect; however, other studies have shown that IL-6 can promote the expression of programmed death-ligand 1 to inhibit anti-tumor immunity [ 38 , 39 ]. Collectively the findings of the present study and those previously conducted suggest that MSCs, tumor cells, and immune cells affect tumor growth and evolution through intricate regulatory mechanisms, which must be systematically investigated to gain a better understanding of the regulatory processes that control tumor growth and metastasis.
Conclusions
The presence of mesenchymal stem cells has been confirmed in some solid tumors, where they serve as important components of the tumor microenvironment; however, their role in cancer has not been fully elucidated, and there have been contradictory findings reported in terms of whether they suppress or promote tumor growth or survival. This study investigated the functions of mesenchymal stem cells isolated from tumor tissues of a patient with non-small cell lung cancer. In vitro and in vivo experiments were performed to determine the characteristics of these isolated cells and their effects on immune cell-mediated destruction of tumor cells. The isolated human lung cancer-derived mesenchymal stem cells displayed the typical morphology and immunophenotype of MSCs, and the results confirmed that they were nontumorigenic and capable of undergoing multipotent differentiation. These isolated cells remarkably enhanced tumor growth when incorporated into systems alongside tumor cells in vivo. Importantly, in the presence of MSCs, the ability of peripheral blood mononuclear cell-derived natural killer cells and activated T cells to mediate tumor cell destruction was significantly compromised. These data support the notion that hLC-MSCs protect tumor cells from immune-mediated destruction by inhibiting the antitumor activities of NK and T cells, which could contribute to poorer outcomes. This study provides a good basis for further exploration of the mechanisms that regulate the interactions and effects between these cell types in various cancers.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Alpha-Minimum Essential Medium
Alpha-smooth muscle actin
Bone marrow-derived mesenchymal stem cells
Chemokine ligand
C-C motif chemokine receptor 2
Cluster of differentiation
Cytokeratin 18
Cytotoxic T lymphocytes
Fetal bovine serum
4’,6-Diamidino-2-phenylindole
Human interleukin 2
Hepatocyte growth factor
Human leukocyte antigen
Human lung cancer-derived mesenchymal stem cells
Major histocompatibility complex class 1
Lymphoma-derived mesenchymal stem cells
Mesenchymal stem cells
Natural killer
Peripheral blood mononuclear cells
Phosphate-buffered saline
Roswell Park Memorial Institute
Standard deviation
Standard error of the mean
Transforming growth factor beta one
Vesicular stomatitis virus G
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science (New York, NY). 1999;284(5411):143–7.
Article CAS Google Scholar
Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells (Dayton, Ohio). 2010;28(3):585–96.
Article CAS PubMed Google Scholar
Shi Y, Du L, Lin L, Wang Y. Tumour-associated mesenchymal stem/stromal cells: emerging therapeutic targets. Nat Rev Drug Discov. 2017;16(1):35–52.
Goldstein RH, Reagan MR, Anderson K, Kaplan DL, Rosenblatt M. Human bone marrow-derived MSCs can home to orthotopic breast cancer tumors and promote bone metastasis. Cancer Res. 2010;70(24):10044–50.
Article CAS PubMed PubMed Central Google Scholar
Prockop DJ, Gregory CA, Spees JL. One strategy for cell and gene therapy: harnessing the power of adult stem cells to repair tissues. Proc Natl Acad Sci USA. 2003;100(Suppl 1):11917–23.
Dvorak HF. Tumors: wounds that do not heal-redux. Cancer Immunol Res. 2015;3(1):1–11.
Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE, Andreeff M. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96(21):1593–603.
Zhu Y, Sun Z, Han Q, Liao L, Wang J, Bian C, Li J, Yan X, Liu Y, Shao C, et al. Human mesenchymal stem cells inhibit cancer cell proliferation by secreting DKK-1. Leukemia. 2009;23(5):925–33.
Qiao L, Xu Z, Zhao T, Zhao Z, Shi M, Zhao RC, Ye L, Zhang X. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res. 2008;18(4):500–7.
Otsu K, Das S, Houser SD, Quadri SK, Bhattacharya S, Bhattacharya J. Concentration-dependent inhibition of angiogenesis by mesenchymal stem cells. Blood. 2009;113(18):4197–205.
Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–63.
Muehlberg FL, Song YH, Krohn A, Pinilla SP, Droll LH, Leng X, Seidensticker M, Ricke J, Altman AM, Devarajan E, et al. Tissue-resident stem cells promote breast cancer growth and metastasis. Carcinogenesis. 2009;30(4):589–97.
Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19(2):257–72.
Fregni G, Quinodoz M, Möller E, Vuille J, Galland S, Fusco C, Martin P, Letovanec I, Provero P, Rivolta C, et al. Reciprocal modulation of mesenchymal stem cells and tumor cells promotes lung cancer metastasis. EBioMedicine. 2018;29:128–45.
Article PubMed PubMed Central Google Scholar
Zhang X, Hu F, Li G, Li G, Yang X, Liu L, Zhang R, Zhang B, Feng Y. Human colorectal cancer-derived mesenchymal stem cells promote colorectal cancer progression through IL-6/JAK2/STAT3 signaling. Cell Death Dis. 2018;9(2):25.
Suzuki K, Sun R, Origuchi M, Kanehira M, Takahata T, Itoh J, Umezawa A, Kijima H, Fukuda S, Saijo Y. Mesenchymal stromal cells promote tumor growth through the enhancement of neovascularization. Mol Med. 2011;17(7–8):579–87.
Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, Noël D, Jorgensen C. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102(10):3837–44.
Liu X, Ory V, Chapman S, Yuan H, Albanese C, Kallakury B, Timofeeva OA, Nealon C, Dakic A, Simic V, et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 2012;180(2):599–607.
Sun Q, Luo T, Ren Y, Florey O, Shirasawa S, Sasazuki T, Robinson DN, Overholtzer M. Competition between human cells by entosis. Cell Res. 2014;24(11):1299–310.
Overholtzer M, Mailleux AA, Mouneimne G, Normand G, Schnitt SJ, King RW, Cibas ES, Brugge JS. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007;131(5):966–79.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7.
Kögler G, Sensken S, Airey JA, Trapp T, Müschen M, Feldhahn N, Liedtke S, Sorg RV, Fischer J, Rosenbaum C, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200(2):123–35.
Hauner H, Entenmann G, Wabitsch M, Gaillard D, Ailhaud G, Negrel R, Pfeiffer EF. Promoting effect of glucocorticoids on the differentiation of human adipocyte precursor cells cultured in a chemically defined medium. J Clin Invest. 1989;84(5):1663–70.
Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33(11):1402–16.
Cao H, Xu W, Qian H, Zhu W, Yan Y, Zhou H, Zhang X, Xu X, Li J, Chen Z, et al. Mesenchymal stem cell-like cells derived from human gastric cancer tissues. Cancer Lett. 2009;274(1):61–71.
McLean K, Gong Y, Choi Y, Deng N, Yang K, Bai S, Cabrera L, Keller E, McCauley L, Cho KR, et al. Human ovarian carcinoma–associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J Clin Invest. 2011;121(8):3206–19.
Ren G, Zhao X, Wang Y, Zhang X, Chen X, Xu C, Yuan ZR, Roberts AI, Zhang L, Zheng B, et al. CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFα. Cell Stem Cell. 2012;11(6):812–24.
Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5(6):485–9.
Article PubMed Google Scholar
Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–43.
Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101(9):3722–9.
Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103(12):4619–21.
Rasmusson I, Ringdén O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76(8):1208–13.
Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30(1):42–8.
Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24(1):74–85.
Moloudizargari M, Govahi A, Fallah M, Rezvanfar MA, Asghari MH, Abdollahi M. The mechanisms of cellular crosstalk between mesenchymal stem cells and natural killer cells: Therapeutic implications. J Cell Physiol. 2021;236(4):2413–29.
Abumaree MH, Al Jumah MA, Kalionis B, Jawdat D, Al Khaldi A, Abomaray FM, Fatani AS, Chamley LW, Knawy BA. Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell Rev Rep. 2013;9(5):620–41.
Hof-Nahor I, Leshansky L, Shivtiel S, Eldor L, Aberdam D, Itskovitz-Eldor J, Berrih-Aknin S. Human mesenchymal stem cells shift CD8+ T cells towards a suppressive phenotype by inducing tolerogenic monocytes. J Cell Sci. 2012;125(Pt 19):4640–50.
CAS PubMed Google Scholar
Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, Su X, Wang Y, Gonzalez-Angulo AM, Akcakanat A, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2(4):361–70.
Nagasaki T, Hara M, Nakanishi H, Takahashi H, Sato M, Takeyama H. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction. Br J Cancer. 2014;110(2):469–78.
Download references
Acknowledgements
Not applicable.
This work was supported by Beijing Municipal Science and Technology Commission, Adminitrative Commission of Zhongguancun Science Park (Z211100002921033).
Author information
Authors and affiliations.
Department of Oncology, Beijing Shijitan Hospital of Capital Medical University, 10 Tieyi Road, Beijing, 100038, China
Xiaoyan Gao, He Ren, Zhengrong Zhang, Bo Zhang & Hongyan Huang
Department of Experimental Medicine, TOR, University of Rome “Tor Vergata”, Rome, Italy
Zhengrong Zhang & Gerry Melino
Department of Orthopedics, Civil Aviation General Hospital, No.1 Gaojing Street, Chaoyang District, Beijing, 100123, China
Laboratory of Cell Engineering, Institute of Biotechnology, Beijing, China
Bo Zhang & Qiang Sun
DZNE German Center for Neurodegenerative Diseases, Bonn, Germany
Gerry Melino
You can also search for this author in PubMed Google Scholar
Contributions
HH and XG designed the experiments; HH, XG, HR and ZZ performed the experiments; XG, SC, GM, QS and HH analyzed the data. XG and BZ wrote the original draft with the help of HH, GM and QS; XG and HH writing–review & editing; HH supervised the study and obtained funding to support the study. All authors have read and approved the final manuscript.
Corresponding author
Correspondence to Hongyan Huang .
Ethics declarations
Ethics approval and consent to participate.
For components of the study involving humans, written informed consent was obtained according to the guidelines of the Ethics Committee of Capital Medical University. All protocols involving animals were approved by the Animal Care and Use Committee of Beijing Institute of Biotechnology and performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Consent for publication
Competing interests.
The authors declare that they have no conflicts of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Gao, X., Ren, H., Zhang, Z. et al. Human lung cancer-derived mesenchymal stem cells promote tumor growth and immunosuppression. Biol Direct 19 , 39 (2024). https://doi.org/10.1186/s13062-024-00479-w
Download citation
Received : 01 March 2024
Accepted : 30 April 2024
Published : 16 May 2024
DOI : https://doi.org/10.1186/s13062-024-00479-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Mesenchymal stem cell
- Non-small cell lung cancer
- Immunophenotype
- Tumorigenic
- Multipotent differentiation
- Natural killer cell
Biology Direct
ISSN: 1745-6150
- General enquiries: [email protected]

IMAGES
COMMENTS
Mesenchymal stem cells are frequently studied for research and clinical use as heterogeneous cell populations, giving rise to the term mesenchymal stromal cells (MSCs). MSCs have wide-ranging therapeutic applications but aspects of MSC biology require further work in order to maximise their potential.
Abstract. The terms MSC and MSCs have become the preferred acronym to describe a cell and a cell population of multipotential stem/progenitor cells commonly referred to as mesenchymal stem cells ...
Cellular & Molecular Immunology - Mesenchymal stem/stromal cells (MSCs): origin, immune regulation, and clinical applications ... Research articles Reviews & Analysis News & Comment ...
Mesenchymal stem cells articles from across Nature Portfolio. Mesenchymal stem cells are multipotent adult stem cells that are present in multiple tissues, including umbilical cord, bone marrow ...
In the last decade, stem cells are increasingly applied as a therapeutic method for numerous disorders. Stem cell therapy, traditionally applied for hematopoietic disorders, nonetheless, is now established for the treatment of non-hematologic disorders [1, 2].Accumulating evidence has shown that mesenchymal stem cells (MSCs) offer an encouraging option for cell treatment and reconstruction of ...
The selected key terms were Mesenchymal Stem Cells. This review used the following inclusion and exclusion criteria for the selection of the articles and divided them into two stages: Stage 1: a) Human CT using MSCs. b) CT at phase 1-phase 4: 1.- location, 2.- field of application, 3.- phase, and 4.- status. Stage 2:
Mesenchymal stem cells (MSCs), coming from a wide range of sources, have multi-directional differentiation ability. MSCs play vital roles in immunomodulation, hematopoiesis and tissue repair. The microenvironment of cells often refers to the intercellular matrix, other cells, cytokines and humoral components. It is also the place for cells' interaction. The stability of the microenvironment ...
STEM CELLS, a peer reviewed journal published monthly, provides a forum for prompt publication of original investigative papers and concise reviews. STEM CELLS is read and written by clinical and basic scientists whose expertise encompasses the rapidly expanding fields of stem and progenitor cell biology. STEM CELLS welcomes original articles and concise reviews describing basic laboratory ...
With the continuous improvement of human technology, the medical field has gradually moved from molecular therapy to cellular therapy. As a safe and effective therapeutic tool, cell therapy has successfully created a research boom in the modern medical field. Mesenchymal stem cells (MSCs) are derived from early mesoderm and have high self-renewal and multidirectional differentiation ability ...
Mesenchymal stem cells (MSCs) have emerged as promising candidates for regenerative medicine. Priming MSCs, a novel strategy, enhances their therapeutic efficacy for optimal in vivo survival and function. This bibliometric analysis provides a comprehensive overview of research activity in the field of priming MSCs.
Mesenchymal stem/stromal cells (MSCs), originating from the mesoderm, represent a multifunctional stem cell population capable of differentiating into diverse cell types and exhibiting a wide range of biological functions. Despite more than half a century of research, MSCs continue to be among the most extensively studied cell types in clinical research projects globally. However, their ...
Herein, a production of human amnion-derived MSCs (hMSCs) at a scale of over 1 × 10 9 cells per batch was reported using a three-dimensional (3D) culture technology based on microcarriers coupled with a spinner bioreactor system. The present study revealed that this large-scale production technology improved the inflammation-guided migration ...
Special Issue Information. Dear Colleagues, Mesenchymal stem cells (MSCs) are multipotent cells that can be isolated from various tissue sources, including bone marrow, adipose tissue, the umbilical cord, and dental pulp. In recent years, stem-cell-based therapies have been at the forefront of treating various diseases and ailments.
Mesenchymal stem cells (MSCs) are a prototypical adult stem cell with capacity for self-renewal and differentiation with a broad tissue distribution. Initially described in bone marrow, MSCs have the capacity to differentiate into mesoderm- and nonmesoderm-derived tissues. The endogenous role for MSCs is maintenance of stem cell niches ...
The labeled mesenchymal stem cells are injected using a 10-microliter Hamilton syringe. A volume of 2 µl containing about 300,000 cells is injected into the right lateral ventricle within 4 min. In the MSC-CM group, mice received 2 µl CM by stereotaxic device and with the same coordinates and conditions as the cell transplantation mentioned ...
The successful approval of cancer immunotherapies in the US and mesenchymal stem cell (MSC)-based therapies in Europe have turned the wheel of regenerative medicine to become prominent treatment ...
Systemic sclerosis (SSc) refers to an autoimmune disease characterized by immune dysfunction, vascular endothelial damage, and multi-organ fibrosis. Thus far, this disease is incurable, and its high mortality rate is significantly correlated with fibrotic events. Fibrosis has been confirmed as a difficult clinical treatment area that should be urgently treated in clinical medicine. Mesenchymal ...
The mitochondrial donation from mesenchymal stromal/stem cells (MSCs) has gained increasing attention in recent years [], suggesting its potential in stem cell therapy [].MSCs-mediated MT is emerging as a critical regulatory mechanism for cell and tissue regeneration, and damage repair, where a remarkable restoration of cellular bioenergetics and a reduction in oxidative stress have been ...
In recent years, stem cell therapy has become a very promising and advanced scientific research topic. The development of treatment methods has evoked great expectations. This paper is a review focused on the discovery of different stem cells and the potential therapies based on these cells. The genesis of stem cells is followed by laboratory steps of controlled stem cell culturing and derivation.
The presence of mesenchymal stem cells has been confirmed in some solid tumors where they serve as important components of the tumor microenvironment; however, their role in cancer has not been fully elucidated. The aim of this study was to investigate the functions of mesenchymal stem cells isolated from tumor tissues of patients with non-small cell lung cancer.
Mesenchymal stem cells (MSCs), pivotal for tissue repair, utilize collagen to restore structural integrity in damaged tissue, preserving its organization through concomitant remodeling. The non-enzymatic glycation of collagen potentially compromises MSC communication, particularly upon advancing the process, underlying various pathologies such as late-stage diabetic complications and aging.
Recently, mesenchymal stem cells (MSCs)-derived exosomes have raised many hopes for treating neurodegenerative sequela of brain disorders. This study aimed to determine the therapeutic potential of MSCs-derived exosomes on cognitive function and neurogenesis of METH-addicted rodents.
In fact, the neuroprotective effect of EVs derived from mesenchymal stem cells has been extensively reported in the literature in several models of neurodegeneration [31,32,33,34,35]. In particular, our group demonstrated that MSC-EVs protect motor neurons from degeneration in ALS models, both in vitro and in vivo [ 36 ], and are able to rescue ...