An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


Genetics of Obesity in Humans: A Clinical Review
Ranim mahmoud, virginia kimonis, merlin g butler.
- Author information
- Article notes
- Copyright and License information
Correspondence: [email protected]
Received 2022 Jul 31; Accepted 2022 Sep 10; Collection date 2022 Oct.
Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( https://creativecommons.org/licenses/by/4.0/ ).
Obesity is a complex multifactorial disorder with genetic and environmental factors. There is an increase in the worldwide prevalence of obesity in both developed and developing countries. The development of genome-wide association studies (GWAS) and next-generation sequencing (NGS) has increased the discovery of genetic associations and awareness of monogenic and polygenic causes of obesity. The genetics of obesity could be classified into syndromic and non-syndromic obesity. Prader–Willi, fragile X, Bardet–Biedl, Cohen, and Albright Hereditary Osteodystrophy (AHO) syndromes are examples of syndromic obesity, which are associated with developmental delay and early onset obesity. Non-syndromic obesity could be monogenic, polygenic, or chromosomal in origin. Monogenic obesity is caused by variants of single genes while polygenic obesity includes several genes with the involvement of members of gene families. New advances in genetic testing have led to the identification of obesity-related genes. Leptin ( LEP ), the leptin receptor ( LEPR ), proopiomelanocortin ( POMC ), prohormone convertase 1 ( PCSK1 ), the melanocortin 4 receptor ( MC4R ), single-minded homolog 1 ( SIM1 ), brain-derived neurotrophic factor ( BDNF ), and the neurotrophic tyrosine kinase receptor type 2 gene ( NTRK2 ) have been reported as causative genes for obesity. NGS is now in use and emerging as a useful tool to search for candidate genes for obesity in clinical settings.
Keywords: obesity, genetics, monogenic, polygenic, Prader-Willi, syndrome
1. Introduction
Obesity is a major health problem worldwide. It is more common in established countries but is on the increase in developing countries. The worldwide prevalence of obesity [body mass index (BMI) ≥ 30 kg m 2 ] has doubled between 1980 and 2008, with a prevalence of 13% in the adult population reported in 2014. In 2013, there were 42 million obese children below the age of five years [ 1 ]. BMI, according to the World Health Organization (WHO), is classified in adults as overweight at 25–29.9 kg/m 2 , obese at 30–39 kg/m 2 , and morbidly obese at 40 kg/m 2 and above [ 2 ].
Obesity is a complex multifactorial disorder with genetic and environmental factors. The increased prevalence of obesity is impacted by the environment as high caloric food sources with a sedentary lifestyle has decreased energy expenditure. Twin and family studies have documented the role of genetic factors in obesity, with the risk of childhood obesity increasing with a positive family history of obesity. There is a high concordance rate for obesity in monozygotic twins vs dizygotic twins and an estimated heritability for obesity at between 40% and 75% in twin studies [ 3 ].
The recognition of obesity and inheritance with associated genes has been impeded by a limited knowledge and understanding of genetics at the human genome level and with the biological pathways involved in obesity. However, the development of genome-wide association studies (GWAS) and next-generation sequencing (NGS) has increased the discovery of genetic associations and awareness of monogenic and polygenic causes of obesity. About 127 informative sites in the human genome have been reported to show linkage with obesity by GWAS [ 4 ] and over 500 obesity-related genes recognized in humans [ 5 ]. There are approximately 30 neuro-endocrine peptides in humans that are known to inhibit eating behavior, but only ghrelin increases eating with an important role in appetite regulation and energy balance [ 6 ]. This balance is in response to changes in peripheral circulating signals from adipose tissue, stomach, and endocrine organs. Regions of the brain and neurons help with energy balance and homeostasis by sensing and processing various metabolic signals with major activity observed in the hypothalamus. Many monogenic neuroendocrine disorders involving the leptin pathway are recognized and associated with early onset obesity in childhood. The genetics of obesity could be classified into syndromic and non-syndromic obesity with or without congenital defects and developmental delay. For example, Prader–Willi, fragile X, Bardet–Biedl, Cohen, and Albright Hereditary Osteodystrophy (AHO) syndromes are associated with developmental delay and early onset obesity [ 7 ]. Non-syndromic obesity could be monogenic, polygenic, or chromosomal in origin. Monogenic obesity is caused by variants of single genes while polygenic obesity includes several genes with the involvement of members of gene families with or without syndromic findings but accompanied with obesity and recognized phenotypes.
New advances in genetic evaluation and analysis have led to the identification of obesity-related genes. For example, eight genes have been reported as causes for obesity, including leptin ( LEP ), the leptin receptor ( LEPR ), proopiomelanocortin ( POMC ), prohormone convertase 1 ( PCSK1 ), the melanocortin 4 receptor ( MC4R ), single-minded homolog 1 ( SIM1 ), brain-derived neurotrophic factor ( BDNF ), and the neurotrophic tyrosine kinase receptor type 2 gene ( NTRK2 ) [ 8 , 9 ], from over 500 obesity-related genes [ 5 ]. One important method is termed GWAS, which incorporates hundreds, or thousands, of polymorphic DNA markers and single nucleotide polymorphisms (SNPs) located throughout the human genome with the ability to search for markers for new genes with no previous evidence of disease involvement. In the past decade, close to 1000 published GWAS results have been reported and 165 traits found in humans with a number of SNPs from obese and nonobese individuals marked gene loci and potential candidate obesity genes [ 10 ]. Hence, genetic factors can be divided into the following three categories: Mendelian (monogenic) syndromic obesity, Mendelian non-syndromic obesity, and polygenic obesity. Meanwhile, NGS is now in use and emerging as a useful tool to search for candidate genes for obesity in clinical settings [ 5 , 11 ]. The results of these recent investigations need to be replicated to warrant further consideration.
2. Obesity-Related Genes and Defects
2.1. leptin.
Leptin is a protein secreted by white adipose tissue and encoded by a gene on chromosome 7 in humans. Leptin crosses the blood–brain barrier to bind to the presynaptic GABAergic neurons of the hypothalamus and decreases appetite and increases energy expenditure [ 12 ]. In the arcuate nucleus of the hypothalamus, leptin binds to its receptor and inhibits the neuropeptide Y (NPY)/agouti-related protein (AgRP) pathway [ 13 ]. The role of leptin and the leptin receptor gene in human obesity is now emerging but not well understood [ 14 ]. Farooqi (2005) reported that inherited human leptin deficiency in patients caused severe early-onset obesity (e.g., 8 years and 86 kg, or 2 years and 29 kg) due to a frame-shift mutation in the homozygous obesity leptin gene (deletion of G133) and a truncated protein [ 7 ]. Other studies reported a high level of leptin in obese patients, but it was associated with a decrease in the level of soluble leptin receptors, which contributed directly to leptin function. Another study on 110 patients including 55 obese and 55 healthy controls showed significantly higher levels of leptin in the obese group than in controls [ 4 ]. This phenomenon is known as leptin resistance. These receptors are not only found in the CNS but are also present in peripheral organs, such as the liver, skeletal muscles, pancreatic beta cells, and even adipose cells, thereby playing an important role in energy regulation.
2.2. Proopiomelanocortin (POMC) Deficiency
POMC is an appetite inhibitory gene found on chromosome 2 in humans. It influences the leptin–melanocortin system as a deficiency of the POMC protein causes an absence of ACTH and alpha-MSH, which are cleaved from the POMC protein [ 15 ]. Hence, a deficiency of POMC leads to hyperphagia, lower resting metabolic rate, and resultant severe obesity with red hair and pale skin [ 16 ]. Errors in the cleavage of master proteins such as POMC require pro-hormone convertase, which cleaves this large protein into smaller functional peptides and as noted, interacts with appetite control, pigment, and obesity [ 17 ].
2.3. Melanocortin-4 Receptor
The melanocortin-4 receptor (MC4R ) gene is now considered the most common associated gene for childhood obesity and found in about 4% of affected cases prior to advanced genetic testing and next generation sequencing (NGS) [ 9 ]. It was first discovered to be related to body weight in 1998 and now multiple studies have investigated its mechanism and the function of different mutations [ 18 ]. The MC4R gene codes for the MC4R protein, which plays an important role in energy homeostasis and food intake behavior [ 19 ]. The central melanocortin pathway regulates energy balance and homeostasis by activating or inhibiting leptin and its receptor is mediated by two subsets of neurons as well as MC3R and MC4R in the arcuate nucleus of the hypothalamus.
2.4. FTO (Fat Mass and Obesity Associated Gene)
FTO was the first obesity-susceptibility gene discovered through GWAS in European patients with type 2 diabetes [ 20 ]. Multiple single nucleotide polymorphisms (SNPs) in the first intron of the gene have shown a significant association with type 2 diabetes. However, after controlling for BMI, there was no association with type 2 diabetes, thereby suggesting that the FTO and type 2 diabetes association was mediated through FTO’s effect on BMI. Another study was conducted in Sardinian patients and confirmed the same results. The rs9939609 and rs9930506 SNPs were identified in FTO with significant association with BMI [ 21 , 22 ]. Other GWAS studies in European populations have reported several other SNPs located in the same chromosomal location. In addition, significant association between FTO SNPs (rs9939609, rs17817449, rs12149832) and BMI was reported in three large studies conducted in Asian populations [ 23 , 24 , 25 ].
Kalantari et al. (2018) reported that the role of FTO gene polymorphisms, a haplotype not a SNP, are close to each other so that they can affect other gene expression through a sequence of the first intron region [ 26 ]. The association between FTO SNPs with food intake and physical activity was investigated in many studies, which revealed the associations between FTO SNPs and increased intake of dietary fat, protein, energy, increased appetite, but decreased satiety. However, FTO SNPs were not associated with the level of physical activity. This finding highlighted the importance of physical activity in the modulation of body weight even in those with genetic susceptibility to obesity [ 27 ].
Additional studies by Castro et al. on four obesity-related genes ( PPARG -rs1801282; PPARGC1A -rs8192678; FTO -rs9939609; MC4R -rs17782313) showed that three of the four genes ( PPARG, FTO, MC4R ) had a combined effect on overweight and obesity at an odds ratio of 1.65 ( p = 0.008) in a large case-control study in the Brazilian population [ 28 ]. The same MC4R variant (rs17782313) and an FTO variant (rs9930506) were significantly associated with obesity in children, reported in multiple separate studies involving thousands of individual subjects, particularly Caucasians and Asians [ 29 ]. Further studies in children and adolescents with the same genes ( FTO and MC4R ) and variants were reported by Resende et al. in a systematic review of the literature with an association with overweight and obesity [ 30 ].
Dastgheib et al. performed a metanalysis involving 13 studies with 9565 cases and 11,956 controls on MC4R rs17782313 and 18 studies with 4789 cases and 15,918 controls on FTO rs9939609. They found that odds ratios showed significant results indicating that these variants were associated with a higher risk of obesity [ 29 ].
Many forms of obesity are thought to be polygenic with variants involved in the same or different genes that act synergistically per individual affecting body weight, composition, and size quantitatively. Polycystic ovary syndrome (PCOS) is a common polygenic metabolic disorder affecting 5–8% of women in the childbearing period. PCOS is defined according to the Rotterdam consensus based on diagnostic criteria to include at least two of the following features: (1) clinical or biochemical hyperandrogenism; (2) oligo-anovulation; and (3) polycystic ovaries (PCO) and excluding similar endocrinopathies. Most women with PCOS are overweight or obese. Many studies investigated the role of genetic contribution for obesity in patients with PCOS [ 31 , 32 ]. Ewens et al. reported five SNPs in FTO and two in MC4R with significant association with BMI in the PCOS families [ 33 ]. Another study by Tu et al. reported association between LEPR Lys109Arg (rs1137100) and PCOS susceptibility in 326 Han Chinese patients with PCOS [ 34 ].
2.5. Chromosomal Defects and Obesity
Syndromic childhood obesity is a rare form of obesity that is part of multiple clinical manifestations. Advanced genetic testing has helped in the detection of structural defects of the chromosome and at the DNA level and has led to the diagnosis of rare and common forms of obesity. The determination of genetic causes of obesity could be helpful for genetic counselling and the selection of appropriate treatment. In addition, Dasouki et al. and Cheon et al. each summarized chromosomal abnormalities with syndromic obesity [ 35 , 36 ]. Kaur et al. reported 79 obesity syndromes described in the literature, with obesity considered to be a cardinal feature in 55 of them, while the prevalence of obesity in the other 24 syndromes was higher than that in the general population. Forty-nine syndromes have been mapped to specific chromosome regions or locations including a causative gene [ 1 ]. Some examples of syndromic obesity due to chromosomal defects will be discussed in this review such as Prader–Willi syndrome (PWS), Down syndrome, Bardet–Biedl syndrome, fragile X syndrome, Alstrom syndrome, and Cornelia de Lange syndrome. Table 1 highlights other common causes of obesity syndromes and their clinical and genetic findings.
Other obesity-related disorders with reported clinical and genetic findings.
3. Obesity-Related Syndromes
3.1. prader–willi syndrome.
Prader–Willi syndrome (PWS) is a complex genetic disorder affecting multiple body systems. It occurs in 1 in 10,000 to 1 in 29,000 people, affecting both males and females equally and in all races [ 6 , 46 ]. PWS is characterized by hypotonia, decreased muscle tone, and extreme floppiness as an infant, which leads to feeding difficulty and poor weight gain in the newborn or in infancy. Then, completely on the other end of the spectrum, it progresses after infancy to hyperphagia or excessive food drive, which can lead to obesity in childhood and beyond [ 47 ]. PWS is a chromosomal disorder with the region associated with PWS located on the chromosome 15q11.2-q13. Typically, people have two different copies of chromosome 15, one inherited from their mother and one from their father. The paternal copy is important for typical development; if a person has not inherited a copy of this region from their father, such as a paternal 15q11-q13 deletion, PWS occurs. Most genes in the 15q11.2-q13 region include imprinted genes and snoRNAs, which are involved in RNA and protein processing of neuroregulators and hormones. When altered, neuronal development and endocrine function are impacted [ 48 ].
There are three different genetic mechanisms by which PWS can occur. The most common genetic etiology of PWS is due to the loss of paternal gene expression in the 15q11.2-q13 region, which accounts for about 70% of all PWS cases, caused by a de novo paternally derived chromosome 15q11.2-q13 deletion [ 48 ]. The less common form of PWS, occurring in about 30% of all PWS cases, is caused when an individual inherits both copies of chromosome 15 from the mother, known as maternal uniparental disomy (UPD) [ 6 ]. A rare form, occurring in about 3% of PWS cases, is a mutation or defect of the imprinting control center in chromosome 15. Therefore, PWS is due to genomic imprinting errors and disturbances of an epigenetic phenomenon resulting in parent-of-origin gene expression, involving methylation and histone modifications and causing monoallelic expression of specific genes [ 49 ].
As PWS is characterized by severe hypotonia in the newborn period causing severe floppiness and difficulty in feeding, it can eventually lead to the placement of a feeding gastric tube (G-tube) directly into the stomach or nasogastric tube for feeding assistance in early infancy. A study in France of 19 infants, who were diagnosed with PWS before two months of age, concluded that hospitalization time and duration of tube feeding were reduced due to very early diagnosis. They also found that multidisciplinary care provided (which included growth hormone treatment given between ages 6 months to just under 2 years old) resulted in only 1 infant becoming obese at age 2.5 years [ 50 ]. A cross-sectional study of 42 children with PWS and 9 controls, aged 7 months–5 years, investigated differences in appetite hormones that may explain the development of abnormal eating behavior. They found no significant relationship between eating behavior in PWS and the level of any hormone or insulin resistance, independent of age [ 51 ]. Oldzej et al. reported that PWS patients with deletion were significantly heavier than those with UPD [ 52 ]. Further, Mahmoud et al. in 2021 concluded from a large cohort of PWS patients that higher BMI scores were present in patients with the deletion subtype compared to UPD [ 53 ].
PWS has been classically described as having two clinical stages: poor feeding, with failure to thrive (FTT) in infancy (Stage 1), followed by hyperphagia leading to obesity in later childhood (Stage 2). The identification of these phases has assisted in the diagnosis of individuals affected with PWS. Additionally, a study identified a total of seven different nutritional phases, with five main phases and sub-phases in phases 1 and 2 and concluded the progression of nutritional phases in PWS is more complex than previously recognized [ 54 ]. An awareness of the various nutritional phases for parents of newly diagnosed infants with PWS may prevent or possibly slow the early onset of obesity. Those affected with PWS are characterized in later infancy or early childhood with hyperphagia or excessive eating with hyperphagia as a difficult symptom to cope with because of the constant desire to eat, even though the individual may have just eaten. (See Figure 1 of an individual with Prader–Willi syndrome as an example of syndromic obesity).

Frontal and profile views of a 16-year-old female with Prader–Willi syndrome due to maternal disomy 15, showing the classical features observed in this obesity-related syndrome.
The source of hyperphagia is believed to be located deep in the brain structure in the hypothalamus, a small gland that has multiple roles. It is both an endocrine gland and a key center for a wide variety of behaviors related to survival. The hypothalamus signals to its close neighbor, the pituitary gland, which acts as a master gland with secretions controlling many other glands to release hormones necessary for growth, metabolism, learning, and memory. The hypothalamus also contains key centers for controlling aggression, body temperature, sexual activity, and food and water intake as well as hunger [ 55 ]. For people with PWS, the hypothalamus does not regulate emotions and appetite normally as the brain does not receive/process signals of feeling “full or satisfied” and drives the individual to consume more food or eat as much as possible [ 56 ]. The brain of an individual with PWS sends signals that the body is starving, lowers the metabolic rate to conserve energy, and drives the individual to find food and eat as much as possible. This excessive food drive, plus the slowed metabolic rate, leads to rapid weight gain and morbid obesity [ 57 ]. Obesity often changes the body structure, causing a shorter torso and larger mid-section appearance. Obesity is a major cause of morbidity due to respiratory disease and non-insulin dependent (type 2) diabetes mellitus with comorbidities [ 57 , 58 ].
3.2. Alstrom Syndrome
Alstrom syndrome is a rare obesity-related single gene disorder inherited in an autosomal recessive pattern. The estimated range is from 1 in 500,000 to 1 in 1,000,000 and is due to mutations in the ALMS1 gene located on chromosome 2p13. The ALMS1 protein has an important role in ciliary function, energy metabolism, and cell cycle control. Li et al. (2007) suggested that the absence of the ALMS1 protein leads to abnormal ciliary formation with Alstrom syndrome classified as one of the ciliopathies due to abnormal ciliary function [ 59 ].
More than one hundred different mutations have been reported in the literature in the ALMS1 gene. The symptoms usually start in infancy and progress during childhood with expanded variability in presentation, which makes the diagnosis challenging. The first clinical manifestations are visual problems, nystagmus, and early blindness due to cone-rod dystrophy. Many endocrine abnormalities are reported to occur in Alstrom syndrome including hypothyroidism, hypogonadotropic hypogonadism in males, hyperandrogenism in females, childhood truncal obesity, hypertriglyceridemia, and insulin resistance with type 2 diabetes mellitus. More than 70% of patients with Alstrom syndrome have congestive heart failure due to cardiomyopathy along with short stature, neurodevelopmental delay, scoliosis and kyphosis, and progressive pulmonary, hepatic, and renal dysfunction with associated complications [ 60 ].
3.3. Fragile X Syndrome (FXS)
FXS is the most common cause of intellectual disability in males. It affects about 1 in 4000 males in the general population and occurs due to the triplet repeat expansion of CGG repeats greater than 200 in size in the 5′ untranslated region of the FMR1 gene at chromosome Xq27.3 [ 61 ]. The carrier state or the premutation form of this gene occurs when the number of CGG repeats is between 50 and 200. Premutation occurs in females and could expand to a full mutation in the subsequent generation. This mutation leads to the loss of fragile X mental retardation protein (FMRP), a protein that plays an important role in protein translation for neuronal synaptic connections [ 62 ].
The common clinical features include intellectual disability, large ears, a narrow head, long face, and prognathism. Joint laxity, mitral valve prolapse, and macroorchidism are also common. Behavioral problems in FXS include anxiety, autistic behavior, self-injury, and compulsive disorders. About 10% of individuals with FXS will have severe obesity, hyperphagia, hypogonadism, or delayed puberty as observed in PWS. This type of FXS patient is termed the Prader–Willi phenotype (PWP) [ 63 ]. A large survey of families with FXS reported that the prevalence of obesity in adults with FXS was similar to the general population [ 64 ]. Another study conducted by the Fragile X Clinical and Research Consortium reported that patients with FXS had higher weights than in the general population [ 65 ]. Choo et al. conducted a longitudinal study on 1223 patients with FXS in different age groups and found an increasing BMI with age and higher BMI Z-scores in adulthood, further supporting obesity as a feature [ 66 ].
3.4. Down Syndrome
Down syndrome (DS) is one of the most common chromosomal disorders in humans [ 67 ]. It occurs in 1:600–700 newborns. The most common cause of DS is the presence of an extra copy of chromosome 21. The other causes are Robertsonian translocations and mosaicism involving chromosome 21. In Robertsonian translocations, the long arm of chromosome 21 is translocated and attached to another acrocentric chromosome. In mosaicism, the meiotic non-division occurs after fertilization and at some point during cell division, a chromosome 21 is lost so that the patient has mosaic DS or now has two cell lineages (one with the normal number of chromosomes, and other one with an extra number 21) [ 68 ].
Many reviews have examined obesity in children with developmental disabilities specifically targeting children with physical disabilities, coordination disorder, and intellectual disability [ 69 , 70 , 71 ]. Many mechanisms have been proposed for the development of obesity in DS including increased serum leptin levels associated with increased appetite as the leptin hormone affects the hunger and satiety centers in the brain, decreases energy expenditure, and decreases physical activity [ 72 , 73 ].
The high risk of obesity in DS could be linked to many factors such as genetic predisposition, hypothyroidism, decreased physical activity, high serum cholesterol and triglycerides, and an abnormal diet. In addition, hypotonia, increased susceptibility to systemic inflammation, decreased metabolic rate, depression, and absence of social and financial support could play a role. Decreased cognitive function could be one of the precipitating factors for obesity as it could affect food choice and level of physical activity. Nordstrom et al. in 2020 compared DS patients with mild and moderate intellectual disability along with their nutritional status and found no significant correlation [ 74 ]. Fructuoso et al. in 2018 reported an increase in the level of obesity-associated inflammatory biomarkers galectin-3 and HSP72 in a mouse model of DS [ 75 ]. This suggested that increased levels in the adipose tissue leading to low-grade inflammation are important risk factors for the development of obesity in DS.
3.5. Bardet–Biedl Syndrome
Bardet–Biedl syndrome is a rare form of syndromic obesity that is inherited in an autosomal recessive pattern. The main clinical manifestations are central obesity, retinal cone-rod dystrophy, postaxial polydactyly, learning difficulties, hearing loss, hypogonadism, and genitourinary abnormalities with renal problems such as polycystic kidney disease.
BBS is associated with high genetic heterogeneity, variable expressivity, and pleiotropy. Twenty-four loci are involved with different types of mutations or variants, which could explain different clinical presentations and findings [ 67 ]. The different types of mutations include missense, nonsense, deletions, and insertions/duplications of genes causing Bardet–Biedl syndrome. Bardet–Biedl syndrome is a multisubunit complex with involvement of eight proteins coded by BBS1 , BBS2 , BBS4 , BBS5 , BBS7 , TTC8 , BBS9 , and BBIP genes. Most of the Bardet–Biedl syndrome cases in Europe and North America present with mutations in either BBS1 or BBS10 genes. Obesity is a common feature as it affects 89% of BBS cases with an early age onset of 2 to 3 years. Obesity occurs in BBS due to gene mutations that lead to a decrease in the number of cilia and altered neuroendocrine signaling from ciliated neurons to fat storage tissues. These disturbances lead to the dysregulation of appetite with changes in leptin resistance and impaired leptin receptor signaling [ 76 ].
3.6. Albright Hereditary Osteodystrophy
Albright hereditary osteodystrophy (AHO) is an autosomal dominant genetic disorder due to mutations in the GNAS1 gene. The clinical manifestations include short stature, brachydactyly, developmental delay, pseudo-hypoparathyroidism, a round face, and early onset obesity [ 77 ]. GNAS is a complex imprinted locus on chromosome 20q13.11. and many transcripts are produced using alternative promoters and splice sites. Alteration in these transcripts can lead to many clinical disorders or presentations. The GNAS1 gene coding Gα s (stimulatory G-protein alpha subunit) mediates signaling by hormones and ligands that bind to G protein–coupled receptors (GPCRs) for generating cyclic AMP. When mutations occur on the maternally inherited alleles expressed in the thyroid or pituitary glands and the renal proximal tubule, a resistance develops to parathyroid hormone (PTH) and other hormones that signal through the Gα s -coupled receptors generating disease (pseudohypoparathyroidism type 1A). When mutations occur on the paternally inherited alleles, the patients develop Albright hereditary osteodystrophy without hormone resistance. The role of genomic imprinting is involved in the development of this genetic disorder [ 78 , 79 ].
The etiology of obesity in AHO is not well known but different theories exist including mutations in MC4R , which is transduced by Gsα, and mediated anorexigenic signals from hormones and other neurotransmitters. The loss of such anorexigenic signals through MC4R could produce hyperphagia, but this hypothesis has not been widely studied in AHO individuals with obesity [ 80 , 81 ].
3.7. WAGR Syndrome
WAGR syndrome occurs due to deletion at chromosome 11p13 (location of the WT1 and PAX6 genes). This syndrome is characterized by predisposition to Wilms tumor aniridia, ambiguous genitalia, and mental retardation (WAGR). Many behavioral and psychiatric disorders have been reported in this syndrome including autism spectrum disorders, attention-deficit disorder, obsessive-compulsive disorder, other anxiety disorders, and depression. WAGR syndrome has been associated with a deletion in the brain-derived neurotrophic factor ( BDNF ) gene in the chromosome 11p13 region, which leads to the obesity phenotype. Although persons with WAGR syndrome typically have low-normal birth weight, marked obesity subsequently develops in a substantial subgroup of patients [ 82 , 83 ]. Many case reports have been described with severe hyperphagia, obesity, and cytogenetic deletions of chromosome 11p BDNF gene locus [ 84 , 85 , 86 ]. Han et al. (2008) conducted a study on 33 patients with WAGR and reported that patients with BDNF haploinsufficiency had significantly higher BMIs during childhood, with a 100% prevalence of childhood-onset obesity [ 87 ].
3.8. Cohen Syndrome
Cohen syndrome is caused by a mutation of the vacuolar protein sorting 13 homolog B ( VPS13B) gene on chromosome 8q22.2. VPS13B is a transmembrane protein that plays an important role in the development and function of the eye, hematological system, and central nervous system via vesicle-mediated transport and the sorting of proteins within the cells [ 88 ]. Cohen syndrome has variable clinical manifestations including progressive retinochoroidal dystrophy and myopia, acquired microcephaly, developmental delay, hypotonia, joint laxity, characteristic facial features with prominent central incisors, truncal obesity, cheerful disposition, and neutropenia. Patients with Cohen syndrome usually suffer from failure to gain weight in infancy and early childhood, but later become significantly overweight in their teenage years with mainly truncal fat accumulation. This change usually occurs very rapidly, with a weight gain of 10–15 kg observed over a short period of time from four to six months [ 89 ]. Functional studies have shown that the increased fat accumulation in patients with Cohen syndrome is due to an increased propensity of pre-adipocytes lacking the VPS13B protein to differentiate into fat-storing cells [ 90 ].
3.9. Smith–Magenis Syndrome
Smith–Magenis syndrome is a genetic condition due to an interstitial deletion of chromosome 17p11.2, which is inherited in an autosomal dominant pattern. Patients with Smith–Magenis syndrome are characterized by mental retardation, developmental delay, renal anomalies, sleep disturbances, dysmorphic features, and behavioral problems including maladaptive/self-injurious, aggressive, and food seeking behaviors like patients with PWS. More than 90% of patients with Smith–Magenis syndrome are overweight or obese after 10 years of age [ 91 ].
3.10. Kallmann Syndrome
Kallmann syndrome is a rare genetic condition of gonadotropin-releasing hormone deficiency and anosmia. Some patients have some additional anomalies including abnormal eye movements, ptosis, hearing loss, unilateral renal agenesis, cleft lip or palate, and obesity. It occurs due to mutations in KAL1 , FGFR1 , FGF8 , PROKR2 , and PROK2 genes and most of the cases are inherited in an X-linked recessive pattern and autosomal recessive or dominant pattern with incomplete penetrance [ 92 ].
4. Management of Genetic Obesity
There are three therapeutic categories to treat obesity: lifestyle modification, medical treatment, and bariatric surgery. The role of genetic factors in obesity is not only a risk factor but also affects the response to therapeutic options for losing weight based on pharmacogenetics and precision medicine with a multidisciplinary approach. Since hyperphagia is a main clinical feature of monogenic obesity, the most effective management is food restriction. This will need adequate training and involvement of the parents and care providers to prevent early onset obesity. Environmental factors such as physical activity, socioeconomic state, and type of diet could modulate the penetrance of obesity associated with pathogenic mutations to avoid unhealthy environments for these patients [ 9 ].
Setmelanotide (Imcivree) is a melanocortin-4 (MC4) receptor agonist used for the treatment of obesity due to proopiomelanocortin (POMC), proprotein convertase subtilisin/keying type 1 (PCSK1), or leptin receptor (LEPR) deficiency. The US Food and Drug Administration approved the drug for chronic weight management in patients 6 years and older with obesity caused by POMC, PCSK1, and LEPR deficiency. Setmelanotide is under consideration for other rare genetic disorders associated with obesity including Bardet–Biedl syndrome, Alstrom syndrome, POMC, and other MC4R pathway heterozygous deficiency obesities. Setmelanotide activates areas in the brain that regulate appetite and fullness, causing patients with specific defects in these areas of the brain not to eat as much and helps to lose weight. It also may increase resting metabolism that can contribute to weight loss. Setmelanotide may lead to weight loss in patients with obesity associated with these conditions but does not treat the genetic defects that cause the condition or other symptoms or signs [ 93 ].
The management of adrenal insufficiency is very important with the maintenance of physiologic hydrocortisone replacement in POMC deficiency. Patients with congenital leptin deficiency could be treated by daily injections of recombinant human leptin, which decreases obesity and associated phenotypic abnormalities. Leptin treatment may reduce food intake, fat mass, hyperinsulinemia, and hyperlipidemia in humans, and restores normal pubertal development, endocrine, and immune function [ 94 ].
Growth hormone treatment is beneficial in the management of PWS. One of the first comprehensive studies to measure the benefits of growth and body composition with the use of growth hormone on individuals with Prader–Willi syndrome was completed in 1997 by Lindgren et al. This study included 27 affected individuals: 15 with growth hormone treatment for 2 years and 12 with growth hormone treatment for 1 year [ 95 ]. They reported that all 27 enrolled individuals showed an increase in height velocity and muscle mass and a decrease in body fat percentage, regardless of time on growth hormone. This study also suggested measurable benefits with growth hormone treatment in regard to a decline in adverse behavioral and psychiatric issues that are associated with PWS [ 95 ].
A follow-up study to the aforementioned study was completed by Lindgren et al. in 1998. The intent of this study was to measure and compare the growth and body composition in affected individuals with Prader–Willi syndrome treated with growth hormone in comparison to those not treated. Lindgren et al. found in this study that those treated with growth hormone had an increase in height and a decrease in fat mass and BMI in comparison to those not treated [ 96 ].
Another comprehensive study to measure the benefits of growth hormone treatment in affected individuals was completed in 1998 by Eiholzer et al. Twelve affected individuals with Prader–Willi syndrome were enrolled in this study and were grouped and compared based on three different groups: (1) overweight and pre-pubertal, (2) underweight and pre-pubertal, and (3) pubertal. After 12 months of growth hormone treatment within all groups, this study showed a marked increase in growth including height, foot and hand length, and arm span and an increase in lean body mass, muscle mass, and physical performance with increased energy expenditure. They also showed a marked decrease in weight for height, BMI, skin fold thickness, and body fat. Finally, individuals in this study reported to be more active and had increased energy [ 97 ].
Whitman et al. also documented similar changes in behavior and physical characteristics with the use of growth hormone treatment in PWS individuals. They noted that the benefits of growth hormone treatment in these patients included having more energy and being more physically fit and demonstrated improvement in memory, sleeping patterns, and social skills [ 98 ].
Goldstone et al. in 2008 determined that the highest level of benefit with the treatment of growth hormone to all patients with PWS is similar to those with isolated growth hormone deficiency, including improvement in growth, body composition, and behavior [ 99 ]. Festen et al. in 2008 also noted improvement in body composition as one of the most appreciable benefits of growth hormone treatment in affected individuals [ 100 ]. Because studies show significant benefits with treatment of growth hormone in individuals with Prader–Willi syndrome, the Food and Drug Administration in 2000 approved injectable somatropin (growth hormone) as a treatment and thus the standard of care for PWS [ 101 ]. Similar positive impacts of GH treatment in previously untreated adults with PWS on weight, fat mass, and physical activity levels were also noted by Butler et al. in 2013 [ 102 ].
Author Contributions
Writing—original draft preparation, R.M.; writing—review and editing, V.K. and M.G.B.; supervision, V.K. and M.G.B. All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
Written informed consent for publication of the photograph was obtained from the patient.
Data Availability Statement
The data supporting reported material can be obtained upon request from the co-authors.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
National Institutes of Health (NIH) grant number U54 HD061222 and RR019478, as well as the Prader–Willi Syndrome Association USA.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- 1. Kaur Y., de Souza R.J., Gibson W.T., Meyre D. A systematic review of genetic syndromes with obesity. Obes. Rev. 2017;18:603–634. doi: 10.1111/obr.12531. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Purnell J.Q. Definitions, Classification, and Epidemiology of Obesity. In: Feingold K.R., Anawalt B., Boyce A., Chrousos G., de Herder W.W., Dhatariya K., Dungan K., Hershman J.M., Hofland J., Kalra S., et al., editors. Endotext. MDText.com, Inc.; South Dartmouth, MA, USA: 2000. [ PubMed ] [ Google Scholar ]
- 3. Wardle J., Carnell S., Haworth C.M., Plomin R. Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment. Am. J. Clin. Nutr. 2008;87:398–404. doi: 10.1093/ajcn/87.2.398. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Singh R.K., Kumar P., Mahalingam K. Molecular genetics of human obesity: A comprehensive review. Comptes Rendus Biol. 2017;340:87–108. doi: 10.1016/j.crvi.2016.11.007. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Duis J., Butler M.G. Syndromic and Nonsyndromic Obesity: Underlying Genetic Causes in Humans. Adv. Biol. 2022:e2101154. doi: 10.1002/adbi.202101154. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Butler M.G. Single Gene and Syndromic Causes of Obesity: Illustrative Examples. Prog. Mol. Biol. Transl. Sci. 2016;140:1–45. doi: 10.1016/bs.pmbts.2015.12.003. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Farooqi I.S. Genetic and hereditary aspects of childhood obesity. Best Pract. Res. Clin. Endocrinol. Metab. 2005;19:359–374. doi: 10.1016/j.beem.2005.04.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Xia Q., Grant S.F. The genetics of human obesity. Ann. N. Y. Acad. Sci. 2013;1281:178–190. doi: 10.1111/nyas.12020. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Choquet H., Meyre D. Genetics of Obesity: What have we Learned? Curr. Genom. 2011;12:169–179. doi: 10.2174/138920211795677895. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Lyon H.N., Hirschhorn J.N. Genetics of common forms of obesity: A brief overview. Am. J. Clin. Nutr. 2005;82((Suppl. S1)):215S–217S. doi: 10.1093/ajcn/82.1.215S. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Dietrich J., Lovell S., Veatch O.J., Butler M.G. PHIP gene variants with protein modeling, interactions, and clinical phenotypes. Am. J. Med. Genet. Part A. 2022;188:579–589. doi: 10.1002/ajmg.a.62557. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Friedman J.M., Halaas J.L. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Vohra M.S., Benchoula K., Serpell C.J., Hwa W.E. AgRP/NPY and POMC neurons in the arcuate nucleus and their potential role in treatment of obesity. Eur. J. Pharmacol. 2022;915:174611. doi: 10.1016/j.ejphar.2021.174611. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Franks P.W., Brage S., Luan J., Ekelund U., Rahman M., Farooqi I.S., Halsall I., O’Rahilly S., Wareham N.J. Leptin predicts a worsening of the features of the metabolic syndrome independently of obesity. Obes. Res. 2005;13:1476–1484. doi: 10.1038/oby.2005.178. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Krude H., Gruters A. Implications of proopiomelanocortin (POMC) mutations in humans: The POMC deficiency syndrome. Trends Endocrinol. Metab. 2000;11:15–22. doi: 10.1016/S1043-2760(99)00213-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Hilado M.A., Randhawa R.S. A novel mutation in the proopiomelanocortin (POMC) gene of a Hispanic child: Metformin treatment shows a beneficial impact on the body mass index. J. Pediatr. Endocrinol. Metab. 2018;31:815–819. doi: 10.1515/jpem-2017-0467. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Gregoric N., Groselj U., Bratina N., Debeljak M., Zerjav Tansek M., Suput Omladic J., Kovac J., Battelino T., Kotnik P., Avbelj Stefanija M. Two Cases With an Early Presented Proopiomelanocortin Deficiency-A Long-Term Follow-Up and Systematic Literature Review. Front. Endocrinol. 2021;12:689387. doi: 10.3389/fendo.2021.689387. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Yeo G.S., Farooqi I.S., Aminian S., Halsall D.J., Stanhope R.G., O’Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat. Genet. 1998;20:111–112. doi: 10.1038/2404. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Tao Y.X. The melanocortin-4 receptor: Physiology, pharmacology, and pathophysiology. Endocr. Rev. 2010;31:506–543. doi: 10.1210/er.2009-0037. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Frayling T.M., Timpson N.J., Weedon M.N., Zeggini E., Freathy R.M., Lindgren C.M., Perry J.R., Elliott K.S., Lango H., Rayner N.W., et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Scuteri A., Sanna S., Chen W.M., Uda M., Albai G., Strait J., Najjar S., Nagaraja R., Orru M., Usala G., et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3:e115. doi: 10.1371/journal.pgen.0030115. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. Dina C., Meyre D., Gallina S., Durand E., Korner A., Jacobson P., Carlsson L.M., Kiess W., Vatin V., Lecoeur C., et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat. Genet. 2007;39:724–726. doi: 10.1038/ng2048. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Cho Y.S., Go M.J., Kim Y.J., Heo J.Y., Oh J.H., Ban H.J., Yoon D., Lee M.H., Kim D.J., Park M., et al. A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nat. Genet. 2009;41:527–534. doi: 10.1038/ng.357. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Wen W., Cho Y.S., Zheng W., Dorajoo R., Kato N., Qi L., Chen C.H., Delahanty R.J., Okada Y., Tabara Y., et al. Meta-analysis identifies common variants associated with body mass index in east Asians. Nat. Genet. 2012;44:307–311. doi: 10.1038/ng.1087. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Okada Y., Kubo M., Ohmiya H., Takahashi A., Kumasaka N., Hosono N., Maeda S., Wen W., Dorajoo R., Go M.J., et al. Common variants at CDKAL1 and KLF9 are associated with body mass index in east Asian populations. Nat. Genet. 2012;44:302–306. doi: 10.1038/ng.1086. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Kalantari N., Keshavarz Mohammadi N., Izadi P., Gholamalizadeh M., Doaei S., Eini-Zinab H., Salonurmi T., Rafieifar S., Janipoor R., Azizi Tabesh G. A complete linkage disequilibrium in a haplotype of three SNPs in Fat Mass and Obesity associated (FTO) gene was strongly associated with anthropometric indices after controlling for calorie intake and physical activity. BMC Med. Genet. 2018;19:146. doi: 10.1186/s12881-018-0664-z. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Loos R.J.F., Yeo G.S.H. The genetics of obesity: From discovery to biology. Nat. Rev. Genet. 2022;23:120–133. doi: 10.1038/s41576-021-00414-z. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Castro G.V., Latorre A.F.S., Korndorfer F.P., de Carlos Back L.K., Lofgren S.E. The Impact of Variants in Four Genes: MC4R, FTO, PPARG and PPARGC1A in Overweight and Obesity in a Large Sample of the Brazilian Population. Biochem. Genet. 2021;59:1666–1679. doi: 10.1007/s10528-021-10079-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Dastgheib S.A., Bahrami R., Setayesh S., Salari S., Mirjalili S.R., Noorishadkam M., Sadeghizadeh-Yazdi J., Akbarian E., Neamatzadeh H. Evidence from a meta-analysis for association of MC4R rs17782313 and FTO rs9939609 polymorphisms with susceptibility to obesity in children. Diabetes Metab. Syndr. 2021;15:102234. doi: 10.1016/j.dsx.2021.102234. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Resende C.M.M., Silva H., Campello C.P., Ferraz L.A.A., de Lima E.L.S., Beserra M.A., Muniz M.T.C., da Silva L.M.P. Polymorphisms on rs9939609 FTO and rs17782313 MC4R genes in children and adolescent obesity: A systematic review. Nutrition. 2021;91–92:111474. doi: 10.1016/j.nut.2021.111474. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Dumesic D.A., Oberfield S.E., Stener-Victorin E., Marshall J.C., Laven J.S., Legro R.S. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr. Rev. 2015;36:487–525. doi: 10.1210/er.2015-1018. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Glueck C.J., Goldenberg N. Characteristics of obesity in polycystic ovary syndrome: Etiology, treatment, and genetics. Metab. Clin. Exp. 2019;92:108–120. doi: 10.1016/j.metabol.2018.11.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Ewens K.G., Jones M.R., Ankener W., Stewart D.R., Urbanek M., Dunaif A., Legro R.S., Chua A., Azziz R., Spielman R.S., et al. FTO and MC4R gene variants are associated with obesity in polycystic ovary syndrome. PLoS ONE. 2011;6:e16390. doi: 10.1371/journal.pone.0016390. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Tu X., Yu C., Gao M., Zhang Y., Zhang Z., He Y., Yao L., Du J., Sun Y., Sun Z. LEPR gene polymorphism and plasma soluble leptin receptor levels are associated with polycystic ovary syndrome in Han Chinese women. Pers. Med. 2017;14:299–307. doi: 10.2217/pme-2017-0016. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Dasouki M.J., Youngs E.L., Hovanes K. Structural Chromosome Abnormalities Associated with Obesity: Report of Four New subjects and Review of Literature. Curr. Genom. 2011;12:190–203. doi: 10.2174/138920211795677930. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Cheon C.K. Genetics of Prader-Willi syndrome and Prader-Will-Like syndrome. Ann. Pediatr. Endocrinol. Metab. 2016;21:126–135. doi: 10.6065/apem.2016.21.3.126. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 37. Bellad A., Bandari A.K., Pandey A., Girimaji S.C., Muthusamy B. A Novel Missense Variant in PHF6 Gene Causing Borjeson-Forssman-Lehman Syndrome. J. Mol. Neurosci. 2020;70:1403–1409. doi: 10.1007/s12031-020-01560-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Hidestrand P., Vasconez H., Cottrill C. Carpenter syndrome. J. Craniofac. Surg. 2009;20:254–256. doi: 10.1097/SCS.0b013e318184357a. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Gupta D., Goyal S. Cornelia de-Lange syndrome. J. Indian Soc. Pedod. Prev. Dent. 2005;23:38–41. doi: 10.4103/0970-4388.16026. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Raible S.E., Mehta D., Bettale C., Fiordaliso S., Kaur M., Medne L., Rio M., Haan E., White S.M., Cusmano-Ozog K., et al. Clinical and molecular spectrum of CHOPS syndrome. Am. J. Med. Genet. Part A. 2019;179:1126–1138. doi: 10.1002/ajmg.a.61174. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Abidi F.E., Cardoso C., Lossi A.M., Lowry R.B., Depetris D., Mattei M.G., Lubs H.A., Stevenson R.E., Fontes M., Chudley A.E., et al. Mutation in the 5’ alternatively spliced region of the XNP/ATR-X gene causes Chudley-Lowry syndrome. Eur. J. Hum. Genet. 2005;13:176–183. doi: 10.1038/sj.ejhg.5201303. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Rogers R.C., Abidi F.E. Coffin-Lowry Syndrome. In: Adam M.P., Mirzaa G.M., Pagon R.A., Wallace S.E., Bean L.J.H., Gripp K.W., Amemiya A., editors. GeneReviews((R)) University of Washington; Seattle, WA, USA: 1993. [ PubMed ] [ Google Scholar ]
- 43. Kleefstra T., de Leeuw N. Kleefstra, T.; de Leeuw, N. Kleefstra Syndrome. In: Adam M.P., Mirzaa G.M., Pagon R.A., Wallace S.E., Bean L.J.H., Gripp K.W., Amemiya A., editors. GeneReviews((R)) University of Washington; Seattle, WA, USA: 1993. [ Google Scholar ]
- 44. Milani D., Manzoni F.M., Pezzani L., Ajmone P., Gervasini C., Menni F., Esposito S. Rubinstein-Taybi syndrome: Clinical features, genetic basis, diagnosis, and management. Ital. J. Pediatr. 2015;41:4. doi: 10.1186/s13052-015-0110-1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Kagami M., Nagasaki K., Kosaki R., Horikawa R., Naiki Y., Saitoh S., Tajima T., Yorifuji T., Numakura C., Mizuno S., et al. Temple syndrome: Comprehensive molecular and clinical findings in 32 Japanese patients. Genet. Med. 2017;19:1356–1366. doi: 10.1038/gim.2017.53. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Yearwood E.L., McCulloch M.R., Tucker M.L., Riley J.B. Care of the patient with Prader-Willi syndrome. Medsurg. Nurs. 2011;20:113–122. [ PubMed ] [ Google Scholar ]
- 47. Cassidy S.B., Driscoll D.J. Prader-Willi syndrome. Eur. J. Hum. Genet. EJHG. 2009;17:3–13. doi: 10.1038/ejhg.2008.165. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Bittel D.C., Butler M.G. Prader-Willi syndrome: Clinical genetics, cytogenetics and molecular biology. Expert Rev. Mol. Med. 2005;7:1–20. doi: 10.1017/S1462399405009531. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Gardner R.M., Sutherland G.R., Shaffer L.G. Chromosome Abnormalities and Genetic Counseling. 4th ed. Oxford University Press; New York, NY, USA: 2012. [ Google Scholar ]
- 50. Bachere N., Diene G., Delagnes V., Molinas C., Moulin P., Tauber M. Early diagnosis and multidisciplinary care reduce the hospitalization time and duration of tube feeding and prevent early obesity in PWS infants. Horm. Res. 2008;69:45–52. doi: 10.1159/000111795. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Butler J.V., Whittington J.E., Holland A.J., McAllister C.J., Goldstone A.P. The transition between the phenotypes of Prader-Willi syndrome during infancy and early childhood. Dev. Med. Child Neurol. 2010;52:e88–e93. doi: 10.1111/j.1469-8749.2009.03530.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Oldzej J., Manazir J., Gold J.A., Mahmoud R., Osann K., Flodman P., Cassidy S.B., Kimonis V.E. Molecular subtype and growth hormone effects on dysmorphology in Prader-Willi syndrome. Am. J. Med. Genet. Part A. 2020;182:169–175. doi: 10.1002/ajmg.a.61408. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Mahmoud R., Leonenko A., Butler M.G., Flodman P., Gold J.A., Miller J.L., Roof E., Dykens E., Driscoll D.J., Kimonis V. Influence of molecular classes and growth hormone treatment on growth and dysmorphology in Prader-Willi syndrome: A multicenter study. Clin. Genet. 2021;100:29–39. doi: 10.1111/cge.13947. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. Miller J.L., Lynn C.H., Driscoll D.C., Goldstone A.P., Gold J.A., Kimonis V., Dykens E., Butler M.G., Shuster J.J., Driscoll D.J. Nutritional phases in Prader-Willi syndrome. Am. J. Med. Genet. Part A. 2011;155:1040–1049. doi: 10.1002/ajmg.a.33951. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. Bereket A., Atay Z. Current status of childhood obesity and its associated morbidities in Turkey. J. Clin. Res. Pediatr. Endocrinol. 2012;4:1–7. doi: 10.4274/jcrpe.506. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 56. Brambilla P., Crino A., Bedogni G., Bosio L., Cappa M., Corrias A., Delvecchio M., Di Candia S., Gargantini L., Grechi E., et al. Metabolic syndrome in children with Prader-Willi syndrome: The effect of obesity. Nutr. Metab. Cardiovasc. Dis. NMCD. 2011;21:269–276. doi: 10.1016/j.numecd.2009.10.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Miller J.L., Goldstone A.P., Couch J.A., Shuster J., He G., Driscoll D.J., Liu Y., Schmalfuss I.M. Pituitary abnormalities in Prader-Willi syndrome and early onset morbid obesity. Am. J. Med. Genet. Part A. 2008;146:570–577. doi: 10.1002/ajmg.a.31677. [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Butler J.V., Whittington J.E., Holland A.J., Boer H., Clarke D., Webb T. Prevalence of, and risk factors for, physical ill-health in people with Prader-Willi syndrome: A population-based study. Dev. Med. Child Neurol. 2002;44:248–255. doi: 10.1017/S001216220100202X. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Li G., Vega R., Nelms K., Gekakis N., Goodnow C., McNamara P., Wu H., Hong N.A., Glynne R. A role for Alstrom syndrome protein, alms1, in kidney ciliogenesis and cellular quiescence. PLoS Genet. 2007;3:e8. doi: 10.1371/journal.pgen.0030008. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 60. Choudhury A.R., Munonye I., Sanu K.P., Islam N., Gadaga C. A review of Alstrom syndrome: A rare monogenic ciliopathy. Intractable Rare Dis. Res. 2021;10:257–262. doi: 10.5582/irdr.2021.01113. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Hunter J.E., Berry-Kravis E., Hipp H., Todd P.K. FMR1 Disorders. In: Adam M.P., Mirzaa G.M., Pagon R.A., Wallace S.E., Bean L.J.H., Gripp K.W., Amemiya A., editors. GeneReviews((R)) University of Washington; Seattle, WA, USA: 1993. [ Google Scholar ]
- 62. Gantois I., Popic J., Khoutorsky A., Sonenberg N. Metformin for Treatment of Fragile X Syndrome and Other Neurological Disorders. Annu. Rev. Med. 2019;70:167–181. doi: 10.1146/annurev-med-081117-041238. [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Nowicki S.T., Tassone F., Ono M.Y., Ferranti J., Croquette M.F., Goodlin-Jones B., Hagerman R.J. The Prader-Willi phenotype of fragile X syndrome. J. Dev. Behav. Pediatr. JDBP. 2007;28:133–138. doi: 10.1097/01.DBP.0000267563.18952.c9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 64. Raspa M., Bailey D.B., Bishop E., Holiday D., Olmsted M. Obesity, food selectivity, and physical activity in individuals with fragile X syndrome. Am. J. Intellect. Dev. Disabil. 2010;115:482–495. doi: 10.1352/1944-7558-115.6.482. [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Kidd S.A., Lachiewicz A., Barbouth D., Blitz R.K., Delahunty C., McBrien D., Visootsak J., Berry-Kravis E. Fragile X syndrome: A review of associated medical problems. Pediatrics. 2014;134:995–1005. doi: 10.1542/peds.2013-4301. [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Choo T.H., Xu Q., Budimirovic D., Lozano R., Esler A.N., Frye R.E., Andrews H., Velinov M. Height and BMI in fragile X syndrome: A longitudinal assessment. Obesity. 2022;30:743–750. doi: 10.1002/oby.23368. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 67. Presson A.P., Partyka G., Jensen K.M., Devine O.J., Rasmussen S.A., McCabe L.L., McCabe E.R. Current estimate of Down Syndrome population prevalence in the United States. J. Pediatr. 2013;163:1163–1168. doi: 10.1016/j.jpeds.2013.06.013. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 68. Asim A., Kumar A., Muthuswamy S., Jain S., Agarwal S. Down syndrome: An insight of the disease. J. Biomed. Sci. 2015;22:41. doi: 10.1186/s12929-015-0138-y. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 69. Hendrix C.G., Prins M.R., Dekkers H. Developmental coordination disorder and overweight and obesity in children: A systematic review. Obes. Rev. 2014;15:408–423. doi: 10.1111/obr.12137. [ DOI ] [ PubMed ] [ Google Scholar ]
- 70. Liou T.H., Pi-Sunyer F.X., Laferrere B. Physical disability and obesity. Nutr. Rev. 2005;63:321–331. doi: 10.1111/j.1753-4887.2005.tb00110.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 71. Maiano C., Normand C.L., Aime A., Begarie J. Lifestyle interventions targeting changes in body weight and composition among youth with an intellectual disability: A systematic review. Res. Dev. Disabil. 2014;35:1914–1926. doi: 10.1016/j.ridd.2014.04.014. [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Magge S.N., O’Neill K.L., Shults J., Stallings V.A., Stettler N. Leptin levels among prepubertal children with Down syndrome compared with their siblings. J. Pediatr. 2008;152:321–326. doi: 10.1016/j.jpeds.2007.08.008. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 73. Hill D.L., Parks E.P., Zemel B.S., Shults J., Stallings V.A., Stettler N. Resting energy expenditure and adiposity accretion among children with Down syndrome: A 3-year prospective study. Eur. J. Clin. Nutr. 2013;67:1087–1091. doi: 10.1038/ejcn.2013.137. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 74. Nordstrom M., Retterstol K., Hope S., Kolset S.O. Nutritional challenges in children and adolescents with Down syndrome. Lancet Child Adolesc. Health. 2020;4:455–464. doi: 10.1016/S2352-4642(19)30400-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 75. Fructuoso M., Rachdi L., Philippe E., Denis R.G., Magnan C., Le Stunff H., Janel N., Dierssen M. Increased levels of inflammatory plasma markers and obesity risk in a mouse model of Down syndrome. Free Radic. Biol. Med. 2018;114:122–130. doi: 10.1016/j.freeradbiomed.2017.09.021. [ DOI ] [ PubMed ] [ Google Scholar ]
- 76. Florea L., Caba L., Gorduza E.V. Bardet-Biedl Syndrome-Multiple Kaleidoscope Images: Insight into Mechanisms of Genotype-Phenotype Correlations. Genes. 2021;12:1353. doi: 10.3390/genes12091353. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 77. Mantovani G., Elli F.M. Inactivating PTH/PTHrP Signaling Disorders. Front. Horm. Res. 2019;51:147–159. doi: 10.1159/000491045. [ DOI ] [ PubMed ] [ Google Scholar ]
- 78. Thiele S., de Sanctis L., Werner R., Grotzinger J., Aydin C., Juppner H., Bastepe M., Hiort O. Functional characterization of GNAS mutations found in patients with pseudohypoparathyroidism type Ic defines a new subgroup of pseudohypoparathyroidism affecting selectively Gsalpha-receptor interaction. Hum. Mutat. 2011;32:653–660. doi: 10.1002/humu.21489. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 79. Butler M.G. Imprinting disorders in humans: A review. Curr. Opin. Pediatr. 2020;32:719–729. doi: 10.1097/MOP.0000000000000965. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 80. Ong K.K., Amin R., Dunger D.B. Pseudohypoparathyroidism--another monogenic obesity syndrome. Clin. Endocrinol. 2000;52:389–391. doi: 10.1046/j.1365-2265.2000.00911.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 81. Delaval K., Wagschal A., Feil R. Epigenetic deregulation of imprinting in congenital diseases of aberrant growth. BioEssays. 2006;28:453–459. doi: 10.1002/bies.20407. [ DOI ] [ PubMed ] [ Google Scholar ]
- 82. Fischbach B.V., Trout K.L., Lewis J., Luis C.A., Sika M. WAGR syndrome: A clinical review of 54 cases. Pediatrics. 2005;116:984–988. doi: 10.1542/peds.2004-0467. [ DOI ] [ PubMed ] [ Google Scholar ]
- 83. Breslow N.E., Norris R., Norkool P.A., Kang T., Beckwith J.B., Perlman E.J., Ritchey M.L., Green D.M., Nichols K.E., National Wilms Tumor Study Group Characteristics and outcomes of children with the Wilms tumor-Aniridia syndrome: A report from the National Wilms Tumor Study Group. J. Clin. Oncol. 2003;21:4579–4585. doi: 10.1200/JCO.2003.06.096. [ DOI ] [ PubMed ] [ Google Scholar ]
- 84. Gul D., Ogur G., Tunca Y., Ozcan O. Third case of WAGR syndrome with severe obesity and constitutional deletion of chromosome (11)(p12p14) Am. J. Med. Genet. 2002;107:70–71. doi: 10.1002/ajmg.10013. [ DOI ] [ PubMed ] [ Google Scholar ]
- 85. Marlin S., Couet D., Lacombe D., Cessans C., Bonneau D. Obesity: A new feature of WAGR (del 11p) syndrome. Clin. Dysmorphol. 1994;3:255–257. doi: 10.1097/00019605-199407000-00012. [ DOI ] [ PubMed ] [ Google Scholar ]
- 86. Tiberio G., Digilio M.C., Giannotti A. Obesity and WAGR syndrome. Clin. Dysmorphol. 2000;9:63–64. doi: 10.1097/00019605-200009010-00014. [ DOI ] [ PubMed ] [ Google Scholar ]
- 87. Han J.C., Liu Q.R., Jones M., Levinn R.L., Menzie C.M., Jefferson-George K.S., Adler-Wailes D.C., Sanford E.L., Lacbawan F.L., Uhl G.R., et al. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. N. Engl. J. Med. 2008;359:918–927. doi: 10.1056/NEJMoa0801119. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 88. Rodrigues J.M., Fernandes H.D., Caruthers C., Braddock S.R., Knutsen A.P. Cohen Syndrome: Review of the Literature. Cureus. 2018;10:e3330. doi: 10.7759/cureus.3330. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 89. Wang H., Falk M.J., Wensel C., Traboulsi E.I. Cohen Syndrome. In: Adam M.P., Mirzaa G.M., Pagon R.A., Wallace S.E., Bean L.J.H., Gripp K.W., Amemiya A., editors. GeneReviews((R)) University of Washington; Seattle, WA, USA: 1993. [ PubMed ] [ Google Scholar ]
- 90. Limoge F., Faivre L., Gautier T., Petit J.M., Gautier E., Masson D., Jego G., El Chehadeh-Djebbar S., Marle N., Carmignac V., et al. Insulin response dysregulation explains abnormal fat storage and increased risk of diabetes mellitus type 2 in Cohen Syndrome. Hum. Mol. Genet. 2015;24:6603–6613. doi: 10.1093/hmg/ddv366. [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Smith A.C.M., Boyd K.E., Brennan C., Charles J., Elsea S.H., Finucane B.M., Foster R., Gropman A., Girirajan S., Haas-Givler B. Smith-Magenis Syndrome. In: Adam M.P., Mirzaa G.M., Pagon R.A., Wallace S.E., Bean L.J.H., Gripp K.W., Amemiya A., editors. GeneReviews((R)) University of Washington; Seattle, WA, USA: 1993. [ PubMed ] [ Google Scholar ]
- 92. Stamou M.I., Georgopoulos N.A. Kallmann syndrome: Phenotype and genotype of hypogonadotropic hypogonadism. Metabolism. 2018;86:124–134. doi: 10.1016/j.metabol.2017.10.012. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 93. Markham A. Setmelanotide: First Approval. Drugs. 2021;81:397–403. doi: 10.1007/s40265-021-01470-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 94. Farooqi I.S., Matarese G., Lord G.M., Keogh J.M., Lawrence E., Agwu C., Sanna V., Jebb S.A., Perna F., Fontana S., et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J. Clin. Investig. 2002;110:1093–1103. doi: 10.1172/JCI0215693. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 95. Lindgren A.C., Hagenas L., Muller J., Blichfeldt S., Rosenborg M., Brismar T., Ritzen E.M. Effects of growth hormone treatment on growth and body composition in Prader-Willi syndrome: A preliminary report. The Swedish National Growth Hormone Advisory Group. Acta Paediatr. 1997;423:60–62. doi: 10.1111/j.1651-2227.1997.tb18372.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 96. Lindgren A.C., Hagenas L., Muller J., Blichfeldt S., Rosenborg M., Brismar T., Ritzen E.M. Growth hormone treatment of children with Prader-Willi syndrome affects linear growth and body composition favourably. Acta Paediatr. 1998;87:28–31. doi: 10.1111/j.1651-2227.1998.tb01380.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 97. Eiholzer U., Gisin R., Weinmann C., Kriemler S., Steinert H., Torresani T., Zachmann M., Prader A. Treatment with human growth hormone in patients with Prader-Labhart-Willi syndrome reduces body fat and increases muscle mass and physical performance. Eur. J. Pediatr. 1998;157:368–377. doi: 10.1007/s004310050832. [ DOI ] [ PubMed ] [ Google Scholar ]
- 98. Whitman B.Y., Myers S., Carrel A., Allen D. The behavioral impact of growth hormone treatment for children and adolescents with Prader-Willi syndrome: A 2-year, controlled study. Pediatrics. 2002;109:E35. doi: 10.1542/peds.109.2.e35. [ DOI ] [ PubMed ] [ Google Scholar ]
- 99. Goldstone A.P., Holland A.J., Hauffa B.P., Hokken-Koelega A.C., Tauber M., Speakers Contributors at the Second Expert Meeting of the Comprehensive Care of Patients with PWS Recommendations for the diagnosis and management of Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 2008;93:4183–4197. doi: 10.1210/jc.2008-0649. [ DOI ] [ PubMed ] [ Google Scholar ]
- 100. Festen D.A., de Lind van Wijngaarden R., van Eekelen M., Otten B.J., Wit J.M., Duivenvoorden H.J., Hokken-Koelega A.C. Randomized controlled GH trial: Effects on anthropometry, body composition and body proportions in a large group of children with Prader-Willi syndrome. Clin. Endocrinol. 2008;69:443–451. doi: 10.1111/j.1365-2265.2008.03228.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 101. Medscape FDA Approves First Drug to Treat Children with Prader-Willi Syndrome Medscape Medical News [Online], 2000. [(accessed on 14 May 2010)]. Available online: http://www.medscape.com/viewarticle/411964 .
- 102. Butler M.G., Smith B.K., Lee J., Gibson C., Schmoll C., Moore W.V., Donnelly J.E. Effects of growth hormone treatment in adults with Prader-Willi syndrome. Growth Horm. IGF Res. 2013;23:81–87. doi: 10.1016/j.ghir.2013.01.001. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
- View on publisher site
- PDF (704.7 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
The genetics of obesity: from discovery to biology
Affiliations.
- 1 Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, Copenhagen, Denmark. [email protected].
- 2 Charles Bronfman Institute for Personalized Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, USA. [email protected].
- 3 Mindich Child Health and Development Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA. [email protected].
- 4 Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA. [email protected].
- 5 MRC Metabolic Diseases Unit, University of Cambridge Metabolic Research Laboratories, Wellcome-MRC Institute of Metabolic Science, Addenbrooke's Hospital, Cambridge, UK. [email protected].
- PMID: 34556834
- PMCID: PMC8459824
- DOI: 10.1038/s41576-021-00414-z
The prevalence of obesity has tripled over the past four decades, imposing an enormous burden on people's health. Polygenic (or common) obesity and rare, severe, early-onset monogenic obesity are often polarized as distinct diseases. However, gene discovery studies for both forms of obesity show that they have shared genetic and biological underpinnings, pointing to a key role for the brain in the control of body weight. Genome-wide association studies (GWAS) with increasing sample sizes and advances in sequencing technology are the main drivers behind a recent flurry of new discoveries. However, it is the post-GWAS, cross-disciplinary collaborations, which combine new omics technologies and analytical approaches, that have started to facilitate translation of genetic loci into meaningful biology and new avenues for treatment.
© 2021. Springer Nature Limited.
Publication types
- Research Support, N.I.H., Extramural
- Research Support, Non-U.S. Gov't
- Eating / genetics
- Gene-Environment Interaction
- Genetic Predisposition to Disease / genetics*
- Genetic Variation*
- Genome, Human / genetics*
- Genome-Wide Association Study / methods*
- Multifactorial Inheritance / genetics
- Obesity / genetics*
- Overweight / genetics
- Whole Genome Sequencing / methods*
Grants and funding
- R01 DK107786/DK/NIDDK NIH HHS/United States
- MR/S026193/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_12012/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_00014/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_12012/5/MRC_/Medical Research Council/United Kingdom
- R01 HL142302/HL/NHLBI NIH HHS/United States
- R01 DK110113/DK/NIDDK NIH HHS/United States
- R01 DK124097/DK/NIDDK NIH HHS/United States
- MC_UU_00014/5/MRC_/Medical Research Council/United Kingdom
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 24 August 2023
Deciphering the genetic landscape of obesity: a data-driven approach to identifying plausible causal genes and therapeutic targets
- Mia Yang Ang ORCID: orcid.org/0000-0003-2709-6754 1 , 2 ,
- Fumihiko Takeuchi ORCID: orcid.org/0000-0003-3185-5661 2 &
- Norihiro Kato ORCID: orcid.org/0000-0002-2433-8415 1 , 2
Journal of Human Genetics volume 68 , pages 823–833 ( 2023 ) Cite this article
6550 Accesses
5 Citations
47 Altmetric
Metrics details
- Data mining
- Data processing
- Gene regulatory networks
Genome-wide association studies (GWAS) have successfully revealed numerous susceptibility loci for obesity. However, identifying the causal genes, pathways, and tissues/cell types responsible for these associations remains a challenge, and standardized analysis workflows are lacking. Additionally, due to limited treatment options for obesity, there is a need for the development of new pharmacological therapies. This study aimed to address these issues by performing step-wise utilization of knowledgebase for gene prioritization and assessing the potential relevance of key obesity genes as therapeutic targets.
Methods and results
First, we generated a list of 28,787 obesity-associated SNPs from the publicly available GWAS dataset (approximately 800,000 individuals in the GIANT meta-analysis). Then, we prioritized 1372 genes with significant in silico evidence against genomic and transcriptomic data, including transcriptionally regulated genes in the brain from transcriptome-wide association studies. In further narrowing down the gene list, we selected key genes, which we found to be useful for the discovery of potential drug seeds as demonstrated in lipid GWAS separately. We thus identified 74 key genes for obesity, which are highly interconnected and enriched in several biological processes that contribute to obesity, including energy expenditure and homeostasis. Of 74 key genes, 37 had not been reported for the pathophysiology of obesity. Finally, by drug-gene interaction analysis, we detected 23 (of 74) key genes that are potential targets for 78 approved and marketed drugs.
Conclusions
Our results provide valuable insights into new treatment options for obesity through a data-driven approach that integrates multiple up-to-date knowledgebases.
Similar content being viewed by others
GWA-based pleiotropic analysis identified potential SNPs and genes related to type 2 diabetes and obesity
Integrating untargeted metabolomics, genetically informed causal inference, and pathway enrichment to define the obesity metabolome
Genome-wide discovery of genetic loci that uncouple excess adiposity from its comorbidities
Introduction.
Obesity is a multifaceted condition characterized by excessive fat accumulation in the body, often associated with chronic conditions such as heart disease, diabetes, high blood pressure, and cancers [ 1 ]. Despite concerted efforts, the prevalence of obesity has significantly increased, with the proportion of obese adults in the United States rising from 30.5% to 42.4% in less than two decades [ 2 ]. While lifestyle modifications have limited efficacy in controlling obesity, few available drugs are serving as anti-obesity agents [ 3 ]. Unfortunately, current research methods are insufficient for developing personalized therapies, and the traditional drug discovery process is time-consuming, laborious, expensive, and risky [ 4 ]. Furthermore, concerns exist regarding the long-term effects of FDA and EMA-approved weight-loss drugs [ 5 ]. Therefore, it is imperative to address obesity seriously, necessitates effective strategies to identify and target associated key genes and pathways.
Genome-wide association studies (GWAS) represent significant advancement in sequencing technology for identifying genetic associations with various traits and diseases. Nevertheless, GWAS encounters several inherent limitations [ 6 ], including non-coding variants introducing complexity and necessitating tissue-specific exploration contexts, as well as the proximity of closely situated genes, which complicates the determination of their significance. Furthermore, linkage disequilibrium (LD) can result in false positives, obscuring the identification of true causal variants. Additionally, complex diseases often arise from disruptions in intracellular biological network, rather than single gene abnormalities.
Despite considerable efforts to investigate the functional implications of obesity-related GWAS [ 7 , 8 ], certain gaps persist. Previous research has investigated the genetic regulation of blood pressure regulatory genes using post-GWAS data [ 9 ], but similar investigations for obesity remain limited. Although potential causal SNPs and hub genes [ 10 ] have been identified based on their proximity to GWAS signals, the lack of eQTL data and investigation of relevant tissues has hindered causal inference. Additionally, drug repositioning application in the post-GWAS analysis of obesity have not been addressed. Nonetheless, a recent study employed expression datasets to identify differentially expressed genes and screened potential drugs targeting important obesity hub genes [ 11 ].
Accordingly, we conducted data-driven integrative analysis by leveraging a credible GWAS dataset [ 8 ] with updated bioinformatics tools and knowledgebases [ 12 , 13 , 14 ], prioritizing obesity-associated genes with significant in silico evidence. Expanding upon previous research [ 7 ], our study incorporated a larger study population, allowing us to identify and update the most plausible causal genes and evaluate their clinical relevance as potential therapeutic targets. Protein-protein interaction [ 15 ] and network centrality analysis [ 16 ] pinpointed key genes, while gene-set enrichment analysis [ 17 ] shed light on underlying biological processes and pathways. Drug-gene interactions [ 18 ] analysis, as well as adverse drug reactions [ 19 ], unveiled promising opportunities for drug repurposing. Our study informs future obesity research and guides future experimental assays to investigate mechanisms and targeted therapies. An overview of this study is illustrated in Fig. 1 .
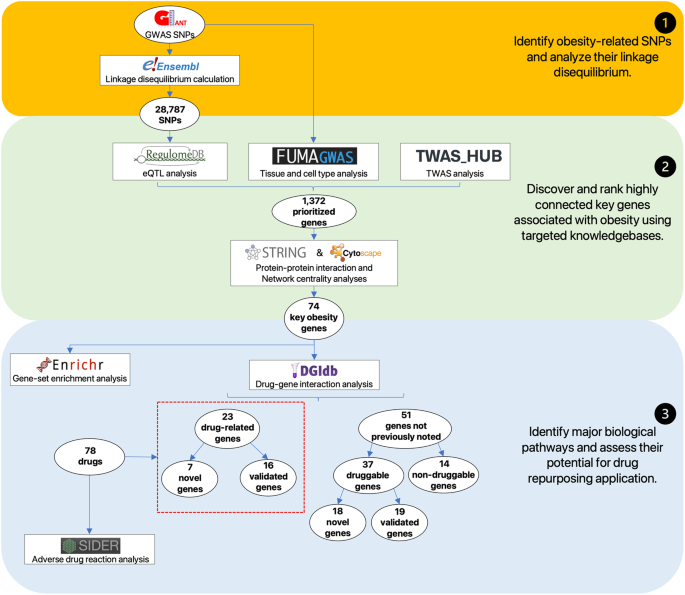
Overview of the data-driven integrative approach. We extract 28,787 obesity-associated SNPs from publicly available GWAS results (top panel) and systematically prioritize 74 plausible key obesity genes, by utilizing a series of bioinformatics tools and genomic and transcriptomic evidence (middle panel). We then explore major biological mechanisms of obesity from the key obesity genes, highlighting 23 potential candidates that are useful for the development of obesity therapeutics (low panel)
Materials and methods
Gwas snps analysis.
Associations identified through GWAS provide a foundation for investigating the biological underpinning of obesity. Given this, we compiled a credible catalog of 941 near-independent genome-wide significant SNPs (COJO P < 1E-08), which captured meaningful association in the GWAS while minimizing potential false positives [ 20 ], identified from the Genetic Investigation of Anthropometric Traits (GIANT) consortium, the largest centralized BMI GWAS dataset derived from ~800,000 individuals [ 8 ]. To identify additional SNPs that equally contribute to obesity, we calculated linkage disequilibrium (LD) using genotype data from the 1000 Genomes Phase 3 Project, focusing on the European ancestry (CEU) population reference panel. Our search criteria included a distance range of ± 500 kb from the query significant SNPs and r 2 > 0.9 from pairwise LD calculation. These LD SNPs were consolidated together with the genome-wide significant SNPs to create a list of obesity-associated SNPs.
eQTL analysis
Integrating expression Quantitative Trait Loci (eQTL) data with GWAS offers insights into the genetic variance associated with changes in gene expression of disease phenotypes [ 21 ]. To prioritize eQTL-genes and explore their potential regulatory roles, we utilized RegulomeDB [ 12 ], a comprehensive knowledgebase that provides functional interpretation of SNPs based on curated references, where these SNPs were scored based on their combinatorial existence of functional categories. We focused specifically on category 1 variants (1a–1f), also known as eQTL-SNPs which demonstrate strong evidence of influencing the expression of eQTL-genes associated with obesity.
Tissue expression analysis
Tissue expression analysis revealed specific tissues where particular genes are expressed, highlighting their potential involvement in disease pathogenesis [ 22 ]. To determine specific tissues associated with obesity, we performed tissue expression analysis using FUMA, a web platform capable of performing functional mapping and annotation of genetic variations identified in GWAS studies. Within FUMA, we utilized Multi-marker Analysis of GenoMic Annotation (MAGMA) [ 23 ] to evaluate the enrichment of genes in specific tissues and prioritized differentially upregulated genes for further functional analysis of their potential roles in the pathophysiology of obesity.
TWAS analysis
Transcriptome-wide association studies (TWAS) integrate genotype and phenotype data from GWAS with reference expression panels, providing insights into potential causal genes in diseases [ 24 ]. TWAS complements GWAS findings by uncovering genes that are missed by GWAS, providing additional regulatory evidence. Using TWAS Hub [ 14 ] with searchable access to TWAS results of complex traits and expression studies, we identified gene expression associated with obesity-related phenotypes, including BMI, fat mass, waist circumference, and weight. Considering the emerging role of the brain in weight regulation [ 25 ], we focused on transcriptionally regulated genes associated with the brain, such as caudate basal ganglia, cerebellar hemisphere, cerebellum, cortex, frontal cortex BA9, hippocampus, hypothalamus, nucleus accumbens basal ganglia and putamen basal ganglia. These genes were prioritized for further investigation.
Protein-protein interactions and network centrality analysis
Protein-protein interactions (PPI) play a crucial role in regulating biological processes and provide valuable insights into the functions and interactions of proteins within the cells [ 26 ]. To better understand the network involved in obesity-associated genes, we used STRING [ 15 ] to reconstruct networks by integrating associations between proteins derived from computational approaches and assigning confidence scores (0-1) to quantify the strength of supporting evidence. We chose the default medium confidence score of 0.4 to interpret the interactions between the obesity-associated genes. Given the significance of network centrality analysis in identifying important hub genes [ 27 ], we employed cytoHubba [ 16 ] to perform a topological analysis of the network structure using centrality algorithms such as betweenness, closeness, and degree to assess the importance of individual proteins within the network. We ranked the top 100 hub genes using these algorithms and identified key genes by finding overlaps in the resulting lists.
Gene set enrichment analysis
Gene Set Enrichment Analysis (GSEA) integrates knowledge about the function of a group of genes, taking into account their involvement in specific biological pathways or co-expression under certain conditions [ 28 ]. Gene ontology (GO) is commonly used for gene functional annotation covering biological processes, molecular functions, and cellular components. Similarly, the Kyoto Encyclopedia of Genes and Genomes (KEGG) increases the explanatory power of specific gene sets to gain insights into underlying biology pathways. To identify significantly enriched GO and KEGG terms of the key obesity genes, we utilized Enrichr [ 17 ], an integrative web-based application. Enriched GO and KEGG pathways terms with adjusted p -value < 0.05 and involving > 3 genes were considered statistically significant.
Drug-gene interaction and adverse drug reaction analysis
Drug-gene interaction (DGI) refers to the interaction between genes and drugs that can potentially influence drug responses [ 29 ]. To identify target genes and potential drugs that interact with key obesity genes, we queried the Drug Gene Interaction Database (DGIdb) [ 18 ], a consolidated resource of DGI interactions and druggable genes. We focused on FDA-approved drugs supported by evidence from two or more databases and PubMed sources, considering them as potential candidates for drug repurposing in obesity. Adverse drug reaction (ADR) refers to unintended and potentially harmful effects arising from the therapeutic use of medications [ 30 ]. To examine the safety profile of our repurposed drug candidates, we conducted a search using the Side Effect Resource (SIDER) database [ 19 ], a resource containing information on potential side effects of approved drugs. Specifically, we focused on side effects classified as “very common” according to the Medical Dictionary for Regulatory Activities (MedDRA) hierarchy, as they have a frequency of ≥ 10% and are likely to occur in a significant proportion of patients using the medication.
Negative control and benchmarking analysis
Negative control and benchmarking analysis are crucial for experimental reliability [ 31 ]. In our workflow, we first prioritized obesity-associated genes by integrating multiple human sources. In the second part, we identified highly interconnected hub genes in the PPI network. To validate the effectiveness of each step, we conducted analyses with negative controls, resulting in different sets of key genes. We then calculated the overlap of these genes with genes from the mouse knockout database [ 32 ] and drug-related information from the drug-gene interaction database [ 18 ]. Additionally, we performed two benchmarking analyses by reanalyzing previous investigations of BMI GWAS study [ 7 ], utilizing GWAS summary statistics and prioritized genes as starting materials, respectively.

Identification of obesity-associated SNPs
To obtain a credible list of genetic associations with obesity, we acquired 941 near-independent genome-wide significant SNPs from BMI GWAS of the GIANT consortium, and subsequently examined representative signals of obesity based on LD. From the significant SNPs, we identified 27,846 LD SNPs applying a threshold of ± 500 kb from the query SNPs and an r 2 > 0.9. After trimming off overlapping SNPs, a non-redundant list of 28,787 obesity-associated SNPs was assembled, which were then clustered into 640 genomic loci based on < 500 kb distances (Supplementary Table 1 ).
Prioritization of obesity eQTL-SNPs and eQTL-genes
To identify SNPs located in regulatory regions of the genome, we employed RegulomeDB to score the obesity-associated SNPs for regulatory functions. Of the 28,787 SNPs examined, 25,776 ( ~ 90%) were assigned with putative regulatory functions. From these, we extracted 867 putative eQTL-SNPs exhibiting high potential for regulatory function (score 1) for downstream analysis. These SNPs were predicted to have an influence on the expression of 243 eQTL-genes, which were prioritized based on their potential to cause obesity through changes in gene expression (Supplementary Table 1 ).
Prioritization of differentially upregulated genes in obesity tissues
To identify the most relevant tissues associated with obesity, we used MAGMA [ 23 ], which was incorporated in FUMA [ 13 ] for tissue expression analysis. Our findings revealed that nearly all brain tissues (10 out of 13) were significant at P < 0.001, with the brain cerebellum having the strongest p-value ( P = 5.45 × 10 − 15 ), demonstrating a strong relationship between the brain and obesity. Additionally, pituitary tissue was also significant with a P = 6.48 × 10 − 5 . Subsequent differential analysis identified a set of differentially upregulated genes found in these brain tissues at adjusted P < 0.05 (Supplementary Table 2 ). Notably, cortex exhibited the strongest upregulation pattern ( P = 3.25 × 10 − 6 ), followed by frontal cortex BA9 ( P = 8.04 × 10 − 6 ), and cerebellum ( P = 1.17 × 10 − 5 ), with slightly weaker but still strongly significant upregulation observed in the cerebellar hemisphere ( P = 1.28 × 10 − 4 ) and anterior cingulate cortex BA24 ( P = 3.05 × 10 − 4 ). A total of 845 differentially upregulated genes from these brain tissues were prioritized for downstream analysis on their potential roles in causing obesity.
Prioritization of TWAS genes associated with obesity
TWAS enabled the identification of genes whose expression is associated with obesity. We analyzed TWAS experiments from TWAS Hub [ 14 ], specifically targeting 4 phenotypes capable of defining obesity. Given the enrichment of brain tissues in our tissue enrichment analysis, we expanded our analysis to include TWAS experiments to uncover additional genes involved in transcriptional activity related to obesity. We prioritized 396 genes exhibiting transcriptional activity in the brain, which are listed in Supplementary Table 3 . A total of 63 genes are shared between both TWAS Hub and FUMA. In addition, 31 genes were found to be common between TWAS Hub and RegulomeDB, with a total of 14 genes identified in all three targeted knowledge resources.
Identification of key obesity genes
We aimed to explore the interconnectedness of genes implicated in obesity, deducing their significance in important biological pathways associated with obesity by being closely integrated within a protein network. To achieve this, we merged the genes prioritized based on in silico evidence from targeted knowledge resources, namely RegulomeDB (243 genes), FUMA (845 genes), and TWAS Hub (396 genes), resulting in a gene set of 1,372 genes associated with obesity.
To identify potential physical and functional associations among these genes, we utilized STRING [ 15 ] and applied a minimum interaction score > 0.4. Subsequently, we employed cytoHubba [ 16 ] to evaluate the nodes in the PPI network using three centrality parameters, namely betweenness, closeness, and degree, and ranked the top 100 hub genes from each algorithm respectively. By selecting genes that overlapped in all three network centrality analyses, we identified 74 key obesity genes highly integrated within a protein network (Fig. 2A ). Through extensive literature review, which included assessing single gene knock-out experiments conducted by the International Mouse Phenotyping Consortium (IMPC), we classified these key genes into predicted novel ( n = 37) and functionally validated ( n = 37). For the functionally validated known genes, we further categorized them to their association with obesity, based on their regulation of appetite, fat, size, lipid, and glucose (Fig. 2B ).
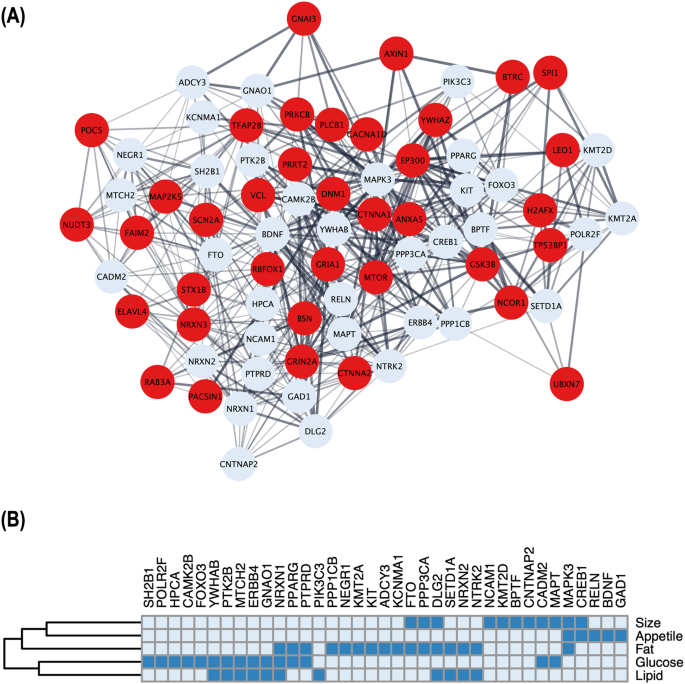
Relationship and classification of key obesity genes identified through network analyses. A Schematic illustration of a protein-protein interaction (PPI) subset involving 74 key obesity genes, where thicker edges indicate stronger data support. Of these, 37 red nodes represent newly reported genes that have not been functionally validated for obesity. B For the 37 functionally validated known genes, the heatmap shows their involvement in five phenotypic groups i.e., appetite, fat, size, lipid, and glucose, reported in the literature; presence by dark blue and absence by light blue
Identification of major biological pathways associated with obesity
To further investigate the biological significance of the key obesity genes, we performed gene set enrichment analysis using Enrichr. To indicate strong enrichment, stringent parameters such as adjusted p -value < 0.05 and the presence of more than 3 genes in a gene set were employed (Fig. 3 ). Our analysis indicated that the key obesity genes were significantly enriched in a total of 119 GO terms, with 91 terms (~76%) associated with biological processes, 12 terms (~11%) associated with molecular functions, and 16 terms (~13%) associated with cellular components (Supplementary Table 5 ). In addition, 104 KEGG pathway terms were significantly enriched (Supplementary Table 6 ).
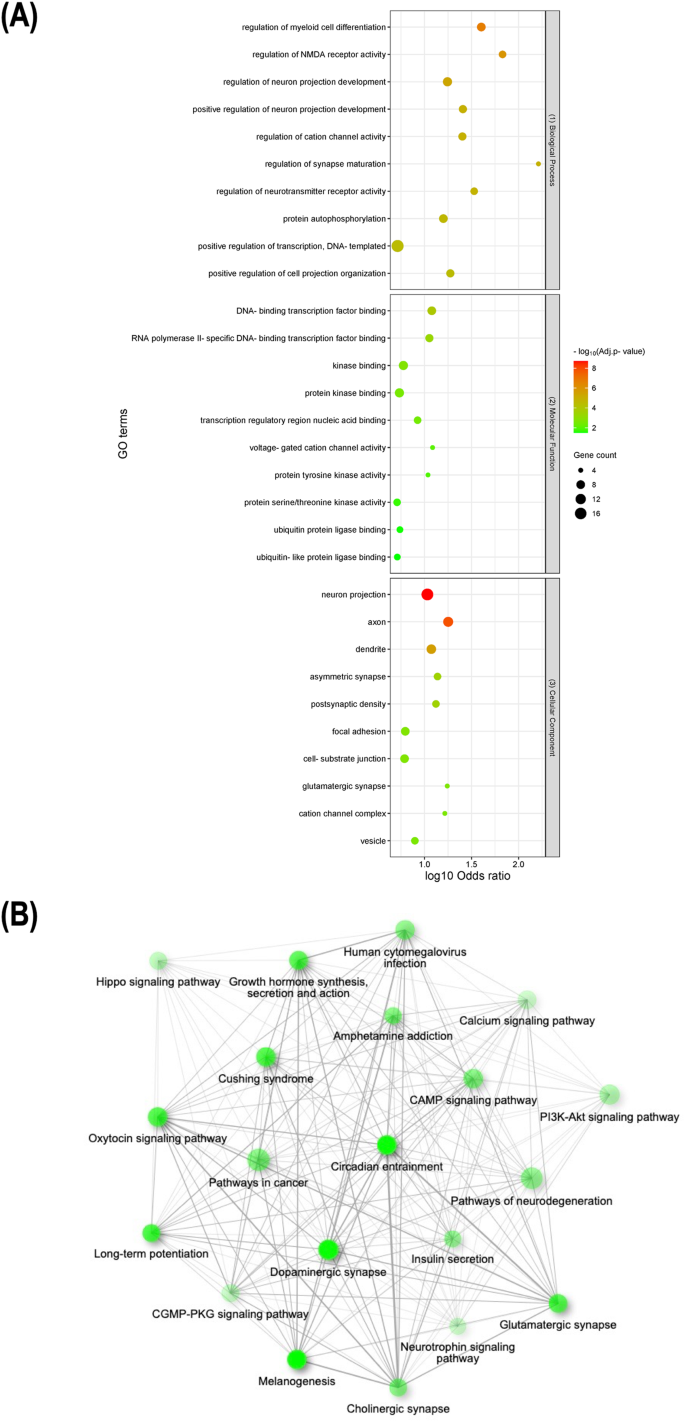
Representative results for enrichment analyses of key obesity genes. A Lists of the top 10 significantly enriched GO terms from biological processes (top), molecular functions (middle), and cellular components (low), respectively. B Schematic illustration of pairwise relationships between top 20 significantly enriched KEGG pathways, where darker and larger nodes indicate more significantly enriched and larger gene sets and thicker edges represent more overlapped genes
Identification of repurposed drug candidates and adverse drug reactions
Key genes are essential in maintaining the structure and function of PPI networks, making them attractive candidates for novel therapeutics. In search of potential therapeutic targets, we utilized DGIdb to analyze drug-gene interactions among the key obesity genes. We focused on reliable interactions supported by at least two resources and PubMed references while excluding cancer-specific resources and limiting our search to FDA-approved drugs or drugs in clinical trials (Fig. 4 ). Among the 74 key obesity genes, we identified 23 drug-related genes (Supplementary Table 7 ), as well as 51 genes not previously noted. Further analysis of the genes not previously noted showed 37 druggable and 14 non-druggable (Supplementary Table 8 ). The 23 drug-related genes were found interacting with 78 drugs, where of these drugs, 47 drugs can lead to weight loss, another 19 were associated with weight gain, while 12 had unknown effects based on prior reports.
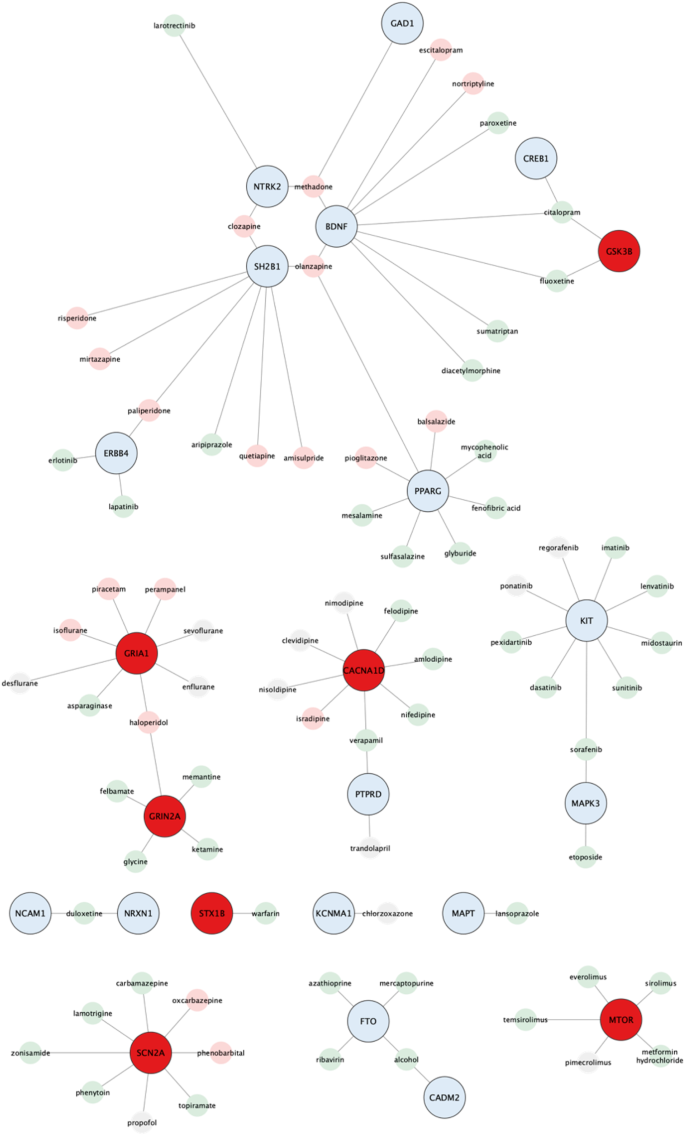
Schematic illustration of 23 key obesity genes and 78 FDA-approved drugs. Genes highlighted in blue are validated, while those in red are not for functional relevance to obesity. These drugs are further classified into two groups based on their experimental evidence; promotion of weight gain (pink) and weight loss (light green) in case of over-expression of the corresponding gene product
To evaluate the safety profile, we searched the SIDER database for side effect data of the 78 drug candidates, focusing on side effects classified as “very common” to identify the most frequent occurrences. Among the 78 drug candidates, 19 had reported side effects (Supplementary Fig. 1 ). These findings could inform future research on the safety and potential use of these drugs for obesity management in clinical settings (Supplementary Table 9 ).
Validation of workflow through negative control and benchmarking analyses
Our workflow underwent rigorous validation tests to ensure its reliability. It involved two steps: (1) prioritizing 1372 obesity-associated genes by integrating multiple human data sources, and (2) narrowing down the list to 74 key hub genes in the PPI network. The mice knockout database [ 32 ] and drug-gene interaction database [ 18 ] both revealed 21 genes (~28%) with reported mice knockout abnormalities and 23 drug-related genes (~31%).
To evaluate the effectiveness of the first step, we randomly selected 1372 genes from a pool of 14,937 well-annotated human genes (obtained from Enrichr’s GO Biological Process library) and performed hub genes identification analysis using our workflow ten times (Fig. 5 ). Our workflow detected a significantly greater percentage of genes with mice knockout abnormalities compared to the negative analyses, indicating effective enrichment of true obesity genes (t-test P < 0.00001). Additionally, the percentage of genes with drug-related information was also significantly greater at P = 0.035517. To evaluate the second step, we randomly selected two groups of 74 genes from the 1372 prioritized obesity-associated gene set to serve as substitutes for hub genes and repeated the analysis ten times. The first set comprised genes with limited connections in the PPI network (node degree < 2), while the second set consisted of genes without considering their interconnectivity information. Our analysis revealed a significantly greater percentage of genes with drug information in our workflow than the negative analyses (t-test P < 0.00001), indicating effective enrichment of true drug target genes. Moreover, genes with lower node degree had a lower likelihood of having drug-related information compared to genes with higher node degree.
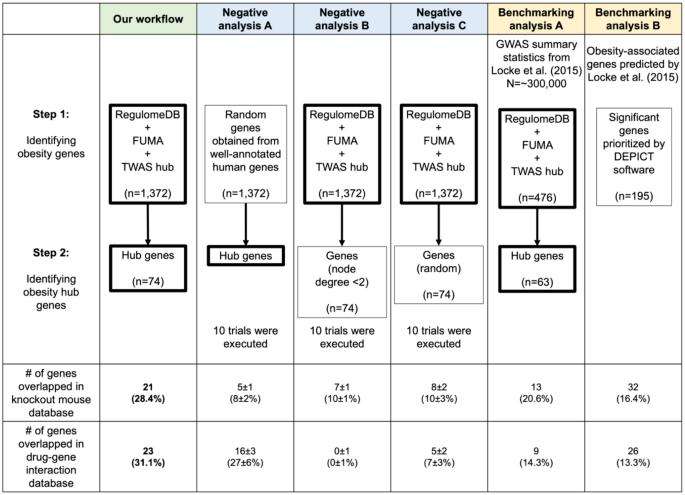
Effectiveness of workflow against negative-control and benchmarking analyses. We evaluate the effectiveness of our workflow by comparison with negative control and benchmarking analyses. Genes overlapping between the mice knockout database and the drug-gene interaction database are counted. The thick border represents our method, with 10 trials conducted for negative control analyses. The number and percentage of genes that overlapped in negative control analyses are shown as median and standard deviation
Lastly, leveraging the GWAS summary statistics and 195 significant genes prioritized from a previous investigation of BMI GWAS [ 7 ], we conducted two benchmarking analyses. Our workflow outperformed both benchmarking analyses in identifying a higher percentage of genes associated with mice knockout abnormalities and drug information. This improvement can be attributed to the utilization of a larger GWAS size (Benchmarking analysis A) and updated bioinformatics tools (Benchmarking analysis B) as demonstrated in Supplementary Table 10 . Our findings showed the importance of incorporating larger sample sizes and employing up-to-date bioinformatics tools are crucial for identifying key genes and druggable targets.
Discussions
In this study, we prioritized obesity genes with potential causal roles utilizing targeted knowledgebases and identified relevant biological processes and pathways for clinical translation in obesity drug applications. Leveraging GWAS with a larger sample size, we conducted a series of integrative analyses using the latest bioinformatics tools and knowledgebases. Exploring the relationship between SNPs and gene expression, we prioritized 243 eQTL-genes. We then examined tissue expression and prioritized 845 differentially upregulated genes from the brain and another 396 transcriptionally regulated genes from TWAS experiments, highlighting expression patterns of the brain in obesity. Establishing a ~1400 prioritized obesity-associated gene set, we performed protein-protein interaction analysis and identified 74 key genes enriched in biological pathways regulating feeding behavior, energy expenditure, metabolic homeostasis, and insulin secretion. Lastly, our drug-gene interaction analysis identified 23 genes targeted by 78 existing drugs in repurposing applications, as well as additional 37 druggable genes with potential for drug development, offering valuable insights for new obesity therapeutics. Our workflow was inspected through negative and benchmarking analyses, where a greater percentage of genes associated with mice knockout abnormalities and drug-related information were identified, indicating its effectiveness.
Our study presents two novel findings that contribute to the current understanding of genetic regulation and therapeutic options for obesity. The first finding addresses the lack of knowledge regarding regulatory genes involved in obesity pathogenesis. While comprehensive integrative analysis with updated bioinformatics tools and knowledgebases on post-GWAS data of blood pressure [ 9 ], the field of obesity has lagged behind in achieving similar advancements, despite recent computational analysis of obesity-associated GWAS SNPs [ 10 ] that focused on genes closest to the GWAS signals. To fill this gap, we implemented a data-driven annotation strategy that employed three-layer evidence from targeted knowledgebases to prioritize regulatory genes associated with obesity. Our analysis prioritized 74 key genes associated with obesity, half of which were predicted novel genes that lacked functional validation in relation to obesity. The second novel finding concerns drug repurposing for obesity treatment. While previous studies [ 11 ] have identified drugs using expression datasets, no attempt has been made using post-GWAS obesity data. Our approach identified 23 drug-related genes and 78 drugs supported by evidence from human and animal experiments suitable for repurposing as novel therapeutic options for obesity. On the remaining 51 genes not previously noted, 37 were found to be druggable, providing opportunities for future drug development. To ensure the validity of our findings, negative analyses were performed at every major step of our analysis and demonstrated the significant improvement of our prediction workflow compared to previous reports, predicting and identifying truly enriched key obesity genes, and potential drug target genes for obesity.
In contrast to a previous study [ 10 ] that focused on the nearest genes to obesity-associated SNPs, our study instead highlights the relevance of studying eQTL-genes in explaining the regulatory mechanisms of obesity. To investigate this, we gathered significant GWAS SNPs from BMI GWAS of the GIANT consortium and calculated LD to find SNPs contributing equally to obesity. We identified 867 eQTL-SNPs with a RegulomeDB score of ≤ 1, capable of regulating a total of 243 eQTL-genes associated with obesity. Among these, 63 of them were genome-wide significant eQTL-SNPs capable of regulating 76 eQTL-genes. Tissue expression analysis enables the identification of genes expressed in particular tissues, providing insights into their contribution to the various cell types and organs. To supplement the prioritized eQTL-genes that lack tissue-specific information, we conducted tissue expression analysis and prioritized 845 differentially upregulated genes present in the brain tissues, consistent with previous studies from other groups proposing that brain tissues play a crucial role in the regulation of body weight and the development of obesity [ 7 , 33 ].
Emerging evidence suggests that vulnerability to obesity extends across multiple brain regions, receiving signals from internal and external sources to collectively regulate feeding habits and energy storage [ 34 , 35 ]. Also, a previous study [ 36 ] reported the enrichment of neuronal cells in the brain, providing valuable evidence of the neurological consequences of obesity. The brain is extensively reviewed as a critical regulator of metabolic traits and physiological processes, including energy metabolism and glucose regulation in the central nervous system [ 37 ]. Moreover, another study showed that the dysregulation of neuronal pathways in the brain disrupts energy balance, resulting in excessive food intake and reduced energy expenditure in mice [ 38 ]. Enrichment in brain tissues prompted us to expand our investigation by integrating TWAS experiments to identify and prioritize an additional 396 transcriptionally regulated genes associated with the brain, 63 of which were previously identified in our tissue expression analysis.
PPI provides valuable insights into the organization and coordination of biological processes underlying diseases. Specifically, our analysis identified 74 key genes, of which 37 were predicted novel and have not been functionally validated in relation to obesity. We further examined the potential association of these novel genes to shed light on their underlying mechanism. ANXA5 is known for anticoagulation and is involved in triglyceride biosynthesis [ 39 ]. Additionally, AXIN1 and BTRC regulate adipose tissue lipogenesis through the Wnt signaling pathway [ 40 ]. CACNA1D affects insulin secretion and has been linked to various conditions [ 41 ]. Other genes, such as CTNNA1 and CTNNA2 are involved in the Hippo signaling pathway, regulating adipogenesis [ 42 ]. Impairment of DNM1 causes insulin secretion failure and hyperglycemia in mice [ 43 ] while inhibiting the expression of EP300 reduces adiposity in larval zebrafish [ 44 ]. FAIM2 is linked to obesity and dyslipidemia in the Chinese population [ 45 ], while GNAI3 is associated with non-alcoholic fatty liver disease (NAFLD) [ 46 ]. Furthermore, GRIA1 influences appetite in T2D patients [ 47 ]. GSK3B regulates inflammation in diabetes patients [ 48 ]. MAP2K5 is known to activate ERK5 , a critical regulator of adipogenesis through the PKA signaling pathway [ 49 ], while inhibiting MTOR signaling resulting in reduced food intake and body weight in mice [ 50 ]. The homolog of NUDT3 in Drosophila, Aps , is involved in insulin signaling [ 51 ], while defects in PACSIN1 have been associated with schizophrenia-like behavior in mice, another condition linked to obesity [ 52 ]. PRKCB deficiency reduces the obesity syndrome of mice [ 53 ], while RAB3A is involved in the regulation of insulin secretion [ 54 ]. Moreover, RBFOX1 regulates BDNF , crucial for neuronal development and energy metabolism in mice [ 55 ]. TFAP2B is linked to insulin resistance and adiposity [ 56 ]. These predicted novel genes are involved in various biological pathways, such as lipid and energy metabolism, insulin secretion, adipogenesis, and neural development. Further investigation is required to evaluate the potential link between BSN, GRIN2A, H2AFX, LEO1, NCOR1, NRXN3, PLCB1, POC5, PRRT2, SCN2A, SPI1, STX1B, UBXN7, VCL, YWHAZ , and obesity.
Subsequently, we examined the 74 key obesity genes to determine their presence in prior analysis by Locke’s GWAS dataset [ 7 ], as reported by DEPICT software [ 57 ]. Among these 74 genes, DEPICT identified 36 genes, while the remaining 38 genes were not reported. Notably, within this group of unreported genes, we observed that 12 genes were situated close to the sentinel SNPs of Yengo’s GWAS dataset [ 8 ] (dataset used in this analysis), with distances ranging from 0 (closest) to 18,724 base pairs (farthest). Interestingly, only one gene among these 12 genes was also found to be situated near the sentinel SNPs of Locke’s GWAS datasets [ 7 ]. Conversely, the remaining 26 unreported genes were not found to be the nearest genes to the GWAS SNPs. These findings highlight the importance of larger GWAS datasets and updated bioinformatics tools in achieving greater precision in research outcomes.
GO terms and KEGG pathways enrichment analysis offers valuable conclusions about gene sets. Our GO analysis indicated that key obesity genes are enriched in biological processes related to the brain and nervous system, such as myeloid cell differentiation, NMDA receptor activity, and neuron projection development. Deficiencies in myeloid cells protect mice from diet-induced obesity and insulin resistance [ 58 ], while NMDA receptor signaling is involved in appetite regulation [ 59 ]. Furthermore, these key obesity genes were involved in regulating cation channel activity, synapse maturation, and neurotransmitter receptor activity, which could impact food intake, energy expenditure, and glucose metabolism [ 60 ]. Meanwhile, KEGG pathway enrichment analysis revealed that key obesity genes were enriched in signaling pathways in the brain, specifically neurotransmitter signaling, involving dopaminergic, glutamatergic, and cholinergic synapses. Brain scans of humans indicated dopamine-regulated brain circuits were involved in obesity [ 61 ], while obese mice on a high-fat diet displayed reduced levels of multiple enzymes involved in dopamine production when switching to the low-fat diet [ 62 ]. Changes in glutamate transmission in obese animals showed increased dopamine transmission and altered synaptic functions [ 63 ]. Basal forebrain cholinergic signaling was reported to regulate feeding behavior in rats [ 64 ], while the frontal cortex and hippocampus displayed functional impairments in cholinergic and synaptic activity, leading to weight gain, hypertension, and dysmetabolism [ 65 ]. Moreover, a decrease in growth hormone secretion has been associated with obesity [ 66 ] and the suppression of insulin secretion led to weight and fat mass reduction [ 67 ]. Our enrichment analysis offers evidence of the complex interplay between key obesity genes and the brain, impacting feeding behavior, energy expenditure, metabolic homeostasis, and insulin secretion.
Hub genes have shown promise as targets for drug development [ 68 ]. To validate this hypothesis, we analyzed a recent lipid GWAS [ 69 ] and identified hub genes associated with LDL cholesterol from the reported genes. Notably, our analysis revealed the inclusion of HMGCR and PCSK9 as hub genes. These genes have been extensively studied and play crucial roles in hypercholesterolemia treatment. HMGCR is a major target of statins, regulating cholesterol levels by inhibiting its expression [ 70 ]. Furthermore, PCSK9 has been linked to blood cholesterol levels, and inhibitors of PCSK9 have proven effective in lowering LDL cholesterol [ 71 ]. These findings further support the potential utilities of hub genes, making them attractive targets for drug development and repurposing.
We examined our key genes with DGI analysis and identified 23 drug-related genes that serve as targets for 78 FDA-approved drugs that showed potential in regulating body weight based on previous studies involving human and animal experiments (Supplementary Table 7 ). Among these genes, four were targeted by five or more drugs, with KIT being the focus of seven weight loss drugs. Among the 47 weight loss-associated drugs, fluoxetine [ 72 ] and citalopram [ 73 ] are commonly prescribed antidepressants for treating binge eating disorder linked to obesity. Conversely, topiramate [ 74 ] and zonisamide [ 75 ] are antiepileptic medications capable of suppressing appetite and increase energy expenditure, leading to weight loss. Additionally, metformin [ 76 ] has shown effectiveness in promoting weight loss by reducing glucose production in the liver and improving insulin sensitivity. Mesalamine [ 77 ] reduces fasting glucose levels and BMI while increasing HDL-cholesterol. Of the 78 candidate drugs listed, SIDER reported 19 drugs with common side effects including asthenia, headache, nausea, fatigue, dermatitis, musculoskeletal discomfort, vomiting, decreased appetite, and diarrhea (Supplementary Fig. 1 ). While not life-threatening, they can considerably affect the health and well-being of patients, leading to discontinuation of treatment or additional medical attention. We proposed that drugs with high reported side effects (e.g., ribavirin, n = 52), may not be suitable for repurposing. Conversely, drugs with low reported side effects, (e.g., duloxetine and paliperidone, n = 1; quetiapine, n = 2; amisulpride, n = 7; oxcarbazepine, n = 8), targeting genes with obesity-related knockout abnormalities in mice could be considered for repurposing. However, it is important to note that drug repurposing is a complex process and requires careful evaluation.
Our study has strengths and weaknesses. We translated biological data into functional knowledge and treatment interventions, suggesting promising key obesity genes as targets for new obesity therapeutics. However, the specificity of computational tools and inadequate specific information on biological processes and pathways remained challenging to establish causality. To address this, the integration of multiple credible biological resources and statistical tools could compensate for the specificity limitation of each resource, further enhancing the prioritization of candidate genes and markers [ 78 ]. Additionally, our approach explored anti-obesity therapy and uncovered novel repurposed applications and adverse drug reaction information for the key obesity genes. However, we lack knowledge of the interactions between these drugs and the genes in the context of obesity. Furthermore, adverse drug reactions may differ among diverse populations [ 79 ]. As a follow-up to our study, we proposed the integration of genome editing techniques, such as CRISPR-Cas9 [ 80 ], to validate our prioritized key obesity genes in animal experiments. Supplementing our findings with empirical experiments would improve our comprehension of regulatory gene interactions and their role in obesity, bringing us closer to effective obesity treatment.
In conclusion, our study provides valuable contributions to the obesity research field by utilizing a systematic data-driven in silico approach to identify and predict novel regulatory genes and potential therapeutic targets for obesity through the translation of GWAS results. Firstly, we prioritized key obesity genes from multiple knowledgebases and identified novel genes which had not been functionally validated in regard to obesity. These genes were involved in various biological pathways, such as lipid and energy metabolism, insulin secretion, adipogenesis, and neural development, adding insights into the underlying mechanism of obesity. Secondly, we identified promising drug-related genes and repurposing drug candidates for novel obesity management. These drugs are capable of regulating energy metabolism and expenditure, appetite control, glucose homeostasis, and insulin sensitivity, offering promising avenues for the development of effective treatments.
Code availability
The codes used in this analysis are available on our GitHub page at https://github.com/angmiayang/integrative_obesity_analysis.git . These codes are freely available, enabling reproducibility and further exploration of our findings.
Keramat SA, Alam K, Rana RH, Chowdhury R, Farjana F, Hashmi R, et al. Obesity and the risk of developing chronic diseases in middle-aged and older adults: Findings from an Australian longitudinal population survey, 2009–2017. PLoS One. 2021;16:e0260158.
Article CAS PubMed PubMed Central Google Scholar
Hales CM, National Center for Health S. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2020.
Dietrich MO, Horvath TL. Limitations in anti-obesity drug development: the critical role of hunger-promoting neurons. Nat Rev Drug Discov. 2012;11:675–91.
Article CAS PubMed Google Scholar
Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019;18:41–58.
Gjermeni E, Kirstein AS, Kolbig F, Kirchhof M, Bundalian L, Katzmann JL, et al. Obesity-An Update on the Basic Pathophysiology and Review of Recent Therapeutic Advances. Biomolecules. 2021;11:1426.
Tam V, Patel N, Turcotte M, Bosse Y, Pare G, Meyre D. Benefits and limitations of genome-wide association studies. Nat Rev Genet. 2019;20:467–84.
Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206.
Yengo L, Sidorenko J, Kemper KE, Zheng Z, Wood AR, Weedon MN, et al. Meta-analysis of genome-wide association studies for height and body mass index in approximately 700000 individuals of European ancestry. Hum Mol Genet. 2018;27:3641–9.
Kamali Z, Keaton JM, Haghjooy Javanmard S, International Consortium Of Blood P, Million Veteran P, e QC. Large-scale multi-omics studies provide new insights into blood pressure regulation. Int J Mol Sci. 2022;23:7557.
Cheng M, Mei B, Zhou Q, Zhang M, Huang H, Han L, et al. Computational analyses of obesity associated loci generated by genome-wide association studies. PLoS One. 2018;13:e0199987.
Article PubMed PubMed Central Google Scholar
Yu Y, Zhang YH, Liu L, Yu LL, Li JP, Rao JA, et al. Bioinformatics analysis of candidate genes and potential therapeutic drugs targeting adipose tissue in obesity. Adipocyte 2022;11:1–10.
Article PubMed Google Scholar
Dong S, Zhao N, Spragins E, Kagda MS, Li M, Assis P, et al. Annotating and prioritizing human non-coding variants with RegulomeDB v.2. Nat Genet. 2023;55:724–6.
Watanabe K, Taskesen E, van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat Commun. 2017;8:1826.
Mancuso N, Shi H, Goddard P, Kichaev G, Gusev A, Pasaniuc B. Integrating Gene Expression with Summary Association Statistics to Identify Genes Associated with 30 Complex Traits. Am J Hum Genet. 2017;100:473–87.
Szklarczyk D, Kirsch R, Koutrouli M, Nastou K, Mehryary F, Hachilif R, et al. The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023;51:D638–46.
Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014;8:S11.
Evangelista JE, Xie Z, Marino GB, Nguyen N, Clarke DJB, Ma’ayan A. Enrichr-KG: bridging enrichment analysis across multiple libraries. Nucleic Acids Res. 2023;51:W168–79.
Freshour SL, Kiwala S, Cotto KC, Coffman AC, McMichael JF, Song JJ, et al. Integration of the Drug-Gene Interaction Database (DGIdb 4.0) with open crowdsource efforts. Nucleic Acids Res 2021;49:D1144–51.
Kuhn M, Letunic I, Jensen LJ, Bork P. The SIDER database of drugs and side effects. Nucleic Acids Res. 2016;44:D1075–9.
Yang J, Ferreira T, Morris AP, Medland SE, Genetic Investigation of ATC, Replication DIG. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat Genet. 2012;44:369–75.
Nica AC, Dermitzakis ET. Expression quantitative trait loci: present and future. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120362.
Consortium GT. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 2020;369:1318–30.
Article Google Scholar
de Leeuw CA, Mooij JM, Heskes T, Posthuma D. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput Biol. 2015;11:e1004219.
Gusev A, Ko A, Shi H, Bhatia G, Chung W, Penninx BW, et al. Integrative approaches for large-scale transcriptome-wide association studies. Nat Genet. 2016;48:245–52.
Loos RJF, Yeo GSH. The genetics of obesity: from discovery to biology. Nat Rev Genet. 2022;23:120–33.
von Mering C, Krause R, Snel B, Cornell M, Oliver SG, Fields S, et al. Comparative assessment of large-scale data sets of protein-protein interactions. Nature 2002;417:399–403.
Koschutzki D, Schreiber F. Centrality analysis methods for biological networks and their application to gene regulatory networks. Gene Regul Syst Bio. 2008;2:193–201.
PubMed PubMed Central Google Scholar
Maleki F, Ovens K, Hogan DJ, Kusalik AJ. Gene set analysis: challenges, opportunities, and future research. Front Genet. 2020;11:654.
Spear BB, Heath-Chiozzi M, Huff J. Clinical application of pharmacogenetics. Trends Mol Med. 2001;7:201–4.
Coleman JJ, Pontefract SK. Adverse drug reactions. Clin Med (Lond). 2016;16:481–5.
Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;21:383–8.
Munoz-Fuentes V, Cacheiro P, Meehan TF, Aguilar-Pimentel JA, Brown SDM, Flenniken AM, et al. The International Mouse Phenotyping Consortium (IMPC): a functional catalogue of the mammalian genome that informs conservation. Conserv Genet. 2018;19:995–1005.
Ndiaye FK, Huyvaert M, Ortalli A, Canouil M, Lecoeur C, Verbanck M, et al. The expression of genes in top obesity-associated loci is enriched in insula and substantia nigra brain regions involved in addiction and reward. Int J Obes (Lond). 2020;44:539–43.
Zeltser LM. Feeding circuit development and early-life influences on future feeding behaviour. Nat Rev Neurosci. 2018;19:302–16.
Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 2012;16:296–309.
Timshel PN, Thompson JJ, Pers TH. Genetic mapping of etiologic brain cell types for obesity. Elife 2020;9:e55851.
O’Brien PD, Hinder LM, Callaghan BC, Feldman EL. Neurological consequences of obesity. Lancet Neurol. 2017;16:465–77.
Niu Y, Chang P, Liu T, Shen X, Zhao H, Zhang M, et al. Obese mice induced by high-fat diet have differential expression of circular RNAs involved in endoplasmic reticulum stress and neuronal synaptic plasticity of hippocampus leading to obesity-associated cognitive impairment. Front Mol Neurosci. 2022;15:1000482.
Seok H, Park HJ, Lee BW, Kim JW, Jung M, Lee SR, et al. Association of annexin A5 polymorphisms with obesity. Biomed Rep. 2013;1:654–8.
Bagchi DP, Nishii A, Li Z, DelProposto JB, Corsa CA, Mori H, et al. Wnt/beta-catenin signaling regulates adipose tissue lipogenesis and adipocyte-specific loss is rigorously defended by neighboring stromal-vascular cells. Mol Metab. 2020;42:101078.
Flanagan SE, Vairo F, Johnson MB, Caswell R, Laver TW, Lango Allen H, et al. A CACNA1D mutation in a patient with persistent hyperinsulinaemic hypoglycaemia, heart defects, and severe hypotonia. Pediatr Diabetes. 2017;18:320–3.
Shen H, Huang X, Zhao Y, Wu D, Xue K, Yao J, et al. The Hippo pathway links adipocyte plasticity to adipose tissue fibrosis. Nat Commun. 2022;13:6030.
Fan F, Wu Y, Hara M, Rizk A, Ji C, Nerad D, et al. Dynamin deficiency causes insulin secretion failure and hyperglycemia. Proc Natl Acad Sci USA 2021;118:e2021764118.
Nishimura Y, Sasagawa S, Ariyoshi M, Ichikawa S, Shimada Y, Kawaguchi K, et al. Systems pharmacology of adiposity reveals inhibition of EP300 as a common therapeutic mechanism of caloric restriction and resveratrol for obesity. Front Pharm. 2015;6:199.
Wu L, Zhao X, Shen Y, Zhang MX, Yan Y, Hou D, et al. Promoter methylation of fas apoptotic inhibitory molecule 2 gene is associated with obesity and dyslipidaemia in Chinese children. Diab Vasc Dis Res. 2015;12:217–20.
Zhu H, Ge K, Lu J, Jia C. Downregulation of GNAI3 Promotes the Pathogenesis of Methionine/Choline-Deficient Diet-Induced Nonalcoholic Fatty Liver Disease. Gut Liver. 2020;14:492–9.
Kochetova OV, Avzaletdinova DS, Korytina GF, Morugova TV, Mustafina OE. The association between eating behavior and polymorphisms in GRIN2B, GRIK3, GRIA1 and GRIN1 genes in people with type 2 diabetes mellitus. Mol Biol Rep. 2020;47:2035–46.
Lappas M. GSK3beta is increased in adipose tissue and skeletal muscle from women with gestational diabetes where it regulates the inflammatory response. PLoS One. 2014;9:e115854.
Zhu H, Guariglia S, Li W, Brancho D, Wang ZV, Scherer PE, et al. Role of extracellular signal-regulated kinase 5 in adipocyte signaling. J Biol Chem. 2014;289:6311–22.
Yang SB, Tien AC, Boddupalli G, Xu AW, Jan YN, Jan LY. Rapamycin ameliorates age-dependent obesity associated with increased mTOR signaling in hypothalamic POMC neurons. Neuron. 2012;75:425–36.
Williams MJ, Eriksson A, Shaik M, Voisin S, Yamskova O, Paulsson J, et al. The obesity-linked gene Nudt3 Drosophila homolog Aps is associated with insulin signaling. Mol Endocrinol. 2015;29:1303–19.
Koch N, Koch D, Krueger S, Troger J, Sabanov V, Ahmed T, et al. Syndapin I Loss-of-Function in Mice Leads to Schizophrenia-Like Symptoms. Cereb Cortex. 2020;30:4306–24.
Huang W, Bansode RR, Bal NC, Mehta M, Mehta KD. Protein kinase Cbeta deficiency attenuates obesity syndrome of ob/ob mice by promoting white adipose tissue remodeling. J Lipid Res. 2012;53:368–78.
Georgescu T, Lyons D, Heisler LK. Role of serotonin in body weight, insulin secretion and glycaemic control. J Neuroendocrinol. 2021;33:e12960.
Tomassoni-Ardori F, Fulgenzi G, Becker J, Barrick C, Palko ME, Kuhn S, et al. Rbfox1 up-regulation impairs BDNF-dependent hippocampal LTP by dysregulating TrkB isoform expression levels. Elife. 2019;8:e49673.
Nordquist N, Gokturk C, Comasco E, Eensoo D, Merenakk L, Veidebaum T, et al. The transcription factor TFAP2B is associated with insulin resistance and adiposity in healthy adolescents. Obes (Silver Spring). 2009;17:1762–7.
Article CAS Google Scholar
Pers TH, Karjalainen JM, Chan Y, Westra HJ, Wood AR, Yang J, et al. Biological interpretation of genome-wide association studies using predicted gene functions. Nat Commun. 2015;6:5890.
Catrysse L, Maes B, Mehrotra P, Martens A, Hoste E, Martens L, et al. A20 deficiency in myeloid cells protects mice from diet-induced obesity and insulin resistance due to increased fatty acid metabolism. Cell Rep. 2021;36:109748.
Guard DB, Swartz TD, Ritter RC, Burns GA, Covasa M. NMDA NR2 receptors participate in CCK-induced reduction of food intake and hindbrain neuronal activation. Brain Res. 2009;1266:37–44.
Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y, et al. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci. 2001;4:507–12.
Wang GJ, Volkow ND, Thanos PK, Fowler JS. Imaging of brain dopamine pathways: implications for understanding obesity. J Addict Med. 2009;3:8–18.
Carlin J, Hill-Smith TE, Lucki I, Reyes TM. Reversal of dopamine system dysfunction in response to high-fat diet. Obes (Silver Spring). 2013;21:2513–21.
Fritz BM, Munoz B, Yin F, Bauchle C, Atwood BK. A High-fat, High-sugar ‘Western’ Diet Alters Dorsal Striatal Glutamate, Opioid, and Dopamine Transmission in Mice. Neuroscience. 2018;372:1–15.
Herman AM, Ortiz-Guzman J, Kochukov M, Herman I, Quast KB, Patel JM, et al. A cholinergic basal forebrain feeding circuit modulates appetite suppression. Nature. 2016;538:253–6.
Martinelli I, Tomassoni D, Roy P, Amenta F, Tayebati SK. Altered Brain Cholinergic and Synaptic Markers in Obese Zucker Rats. Cells 2021;10:2528.
Rasmussen MH. Obesity, growth hormone and weight loss. Mol Cell Endocrinol. 2010;316:147–53.
Huang Z, Wang W, Huang L, Guo L, Chen C. Suppression of Insulin Secretion in the Treatment of Obesity: A Systematic Review and Meta-Analysis. Obes (Silver Spring). 2020;28:2098–106.
Xu W, Wang X, Liu D, Lin X, Wang B, Xi C, et al. Identification and validation of hub genes and potential drugs involved in osteoarthritis through bioinformatics analysis. Front Genet. 2023;14:1117713.
Graham SE, Clarke SL, Wu KH, Kanoni S, Zajac GJM, Ramdas S, et al. The power of genetic diversity in genome-wide association studies of lipids. Nature. 2021;600:675–9.
Villa E, Naves R, Bezares K, Cobena K, Villarroel AC, Guevara C. Pearls & Oy-sters: Managing Cholesterol in a Patient With Statin Intolerance Due to Anti-HMG-CoA Reductase-Associated Myopathy. Neurology. 2022;99:909–13.
Parikh RR, Breve F, Magnusson P, Behzadi P, Pergolizzi J. The Use of Monoclonal Antibody-Based Proprotein Convertase Subtilisin-Kexin Type 9 (PCSK9) Inhibitors in the Treatment of Hypercholesterolemia. Cureus. 2022;14:e25641.
Serralde-Zuniga AE, Gonzalez Garay AG, Rodriguez-Carmona Y, Melendez G. Fluoxetine for adults who are overweight or obese. Cochrane Database Syst Rev. 2019;10:CD011688.
PubMed Google Scholar
McElroy SL, Hudson JI, Malhotra S, Welge JA, Nelson EB, Keck PE Jr. Citalopram in the treatment of binge-eating disorder: a placebo-controlled trial. J Clin Psychiatry. 2003;64:807–13.
McElroy SL, Hudson JI, Capece JA, Beyers K, Fisher AC, Rosenthal NR, et al. Topiramate for the treatment of binge eating disorder associated with obesity: a placebo-controlled study. Biol Psychiatry. 2007;61:1039–48.
Apovian CM, Aronne LJ. Zonisamide for weight reduction in obese adults. JAMA. 2013;310:637–8.
Hui F, Zhang Y, Ren T, Li X, Zhao M, Zhao Q. Role of metformin in overweight and obese people without diabetes: a systematic review and network meta-analysis. Eur J Clin Pharm. 2019;75:437–50.
Luck H, Tsai S, Chung J, Clemente-Casares X, Ghazarian M, Revelo XS, et al. Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab. 2015;21:527–42.
Jia P, Zhao Z. Network assisted analysis to prioritize GWAS results: principles, methods, and perspectives. Hum Genet. 2014;133:125–38.
Wilson JF, Weale ME, Smith AC, Gratrix F, Fletcher B, Thomas MG, et al. Population genetic structure of variable drug response. Nat Genet. 2001;29:265–9.
Fellmann C, Gowen BG, Lin PC, Doudna JA, Corn JE. Cornerstones of CRISPR-Cas in drug discovery and therapy. Nat Rev Drug Discov. 2017;16:89–100.
Download references
Acknowledgements
We gratefully acknowledge the invaluable contributions of members of the National Center for Global Health and Medicine (NCGM), who have provided their expertise, guidance, and support throughout this study.
Open access funding provided by The University of Tokyo.
Author information
Authors and affiliations.
Department of Clinical Genome Informatics, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan
Mia Yang Ang & Norihiro Kato
Department of Gene Diagnostics and Therapeutics, Medical Genomics Center, Research Institute, National Center for Global Health and Medicine, Tokyo, Japan
Mia Yang Ang, Fumihiko Takeuchi & Norihiro Kato
You can also search for this author in PubMed Google Scholar
Contributions
MYA and FT conceived the idea of the study. MYA performed data analysis and interpretation of the results. FT and NK supervised the conduct of this study. All authors reviewed and revised the manuscript draft. All authors approved the final version of the manuscript to be published.
Corresponding author
Correspondence to Mia Yang Ang .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary figure 1, supplementary table 1, supplementary table 2, supplementary table 3, supplementary table 4, supplementary table 5, supplementary table 6, supplementary table 7, supplementary table 8, supplementary table 9, supplementary table 10, supplementary information, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Ang, M.Y., Takeuchi, F. & Kato, N. Deciphering the genetic landscape of obesity: a data-driven approach to identifying plausible causal genes and therapeutic targets. J Hum Genet 68 , 823–833 (2023). https://doi.org/10.1038/s10038-023-01189-3
Download citation
Received : 05 June 2023
Revised : 08 August 2023
Accepted : 15 August 2023
Published : 24 August 2023
Issue Date : December 2023
DOI : https://doi.org/10.1038/s10038-023-01189-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Considerations on efforts needed to improve our understanding of the genetics of obesity.
- Sujoy Ghosh
- Claude Bouchard
International Journal of Obesity (2024)
Weight Loss Programs: Why Do They Fail? A Multidimensional Approach for Obesity Management
- Rabie Khattab
Current Nutrition Reports (2024)
Integrating Genetic Insights, Technological Advancements, Screening, and Personalized Pharmacological Interventions in Childhood Obesity
- Robert Šket
- Barbara Slapnik
- Jernej Kovač
Advances in Therapy (2024)
Relation Between Obesity and Type 2 Diabetes: Evolutionary Insights, Perspectives and Controversies
- Manoj Kumar Gupta
- Gayatri Gouda
- Ramakrishna Vadde
Current Obesity Reports (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Comprehensive Genetic Analysis of Associations between Obesity-Related Parameters and Physical Activity: A Scoping Review
Agata leońska-duniec.
- Author information
- Article notes
- Copyright and License information
Received 2024 Aug 6; Revised 2024 Aug 19; Accepted 2024 Aug 27; Collection date 2024 Sep.
Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( https://creativecommons.org/licenses/by/4.0/ ).
Genetic epidemiological studies have shown that numerous genetic variants cumulatively increase obesity risk. Although genetically predisposed individuals are more prone to developing obesity, it has been shown that physical activity can modify the genetic predisposition to obesity. Therefore, genetic data obtained from earlier studies, including 30 polymorphisms located in 18 genes, were analyzed using novel methods such as the total genetic score and Biofilter 2.4 software to combine genotypic and phenotypic information for nine obesity-related traits measured before and after the realization of the 12-week training program. The results revealed six genes whose genotypes were most important for post-training changes— LEP , LEPR , ADIPOQ , ADRA2A , ADRB3 , and DRD2 . Five noteworthy pairwise interactions, LEP × LEPR , ADRB2 × ADRB3 , ADRA2A × ADRB3 , ADRA2A × ADRB2 , ADRA2A × DRD2 , and three specific interactions demonstrating significant associations with key parameters crucial for health, total cholesterol (TC), high-density lipoprotein (HDL), and fat-free mass (FFM), were also identified. The molecular basis of training adaptation described in this study would have an enormous impact on the individualization of training programs, which, designed according to a given person’s genetic profile, will be effective and safe intervention strategies for preventing obesity and improving health.
Keywords: total genetic score, Biofilter software, obesity, gene–physical-activity interaction
1. Introduction
Obesity is a chronic, multifactorial disease defined as the accumulation of body fat to the extent that it negatively affects health. Obesity has a well-proven genetic basis but requires behavioral, developmental, and/or environmental influences to develop [ 1 , 2 , 3 ]. In recent years, the number of people with overweight and obesity has dramatically increased worldwide, representing a significant public health concern. Given the excess mortality, morbidity, and economic toll, obesity is an illness that warrants increased attention from medical, scientific, and community organizations. Obesity’s status and acceptance as a chronic disease are critical in determining its treatment and the development of comprehensive interventions [ 3 ]. It has been confirmed that systematic physical activity during a diet-induced weight loss program has profound additional metabolic advantages in people with diabetes and obesity [ 4 , 5 ]. However, exercise recommendations do not account for individual genetic variability, increasing risk of these diseases [ 6 ].
Numerous studies have shown that systematic exercise and habitual physical activity have numerous benefits for human health and life expectancy. Physical activity is crucial for reducing the risk of excessive body mass gain, improving the efficiency of fat loss programs, and especially preventing weight regain [ 7 , 8 ]. The physiological and biochemical reactions occurring in the human body after training are well-described. Frequent exercise leads to various metabolic and physical changes, such as alterations in skeletal muscle characteristics, nutrient storage, metabolic enzyme levels, contractile protein quantity, and connective tissue stiffness. The complex process of exercise-induced adaptation is determined by the volume, intensity, and frequency of physical activity [ 9 , 10 ]. In addition, Bouchard (1983) demonstrated significant individual differences in the response to highly standardized and well-controlled exercise programs and that there was a substantial familial aggregation component to the heterogeneity noted above, confirmed by several other authors [ 11 , 12 , 13 ]. However, few studies have focused on the role of specific genes in accounting for the highly prevalent effects of gene-exercise interaction. These authors indicated that training-induced changes in several physical performance and health-related fitness phenotypes may be more effective in individuals with some genotypes than others, confirming that some gene variants influence individual differences in response to regular exercise [ 9 , 11 , 14 ]. However, identifying the genetic markers associated with obesity and explaining the complex mechanisms through which they exert their effects pose challenges. In the future, a better understanding of the molecular basis of training adaptation could significantly affect the customization of training programs to make them more effective and safer. It could also improve recovery, trauma care, medical treatment, diet, or supplementation. The specificity of gene–physical-activity interactions is crucial for sports scientists and offers promising pathways for identifying targets to address obesity. This knowledge holds significant potential for informing both athletic performance and therapeutic interventions [ 9 , 15 ].
There have been several studies over the past 30 years on genetic obesity, which have shown that genetic mutations, polymorphisms, and changes in gene expression all play a role in predisposing individuals to obesity [ 16 ]. To date, more than 600 genes and chromosomal regions have been linked to body mass and composition regulation. The genetic risk of common obesity is associated with the accumulation of various loci , each contributing a small part of the total risk of obesity [ 17 ]. Beyond the large number of possible genetic markers, an additional problem lies in determining their influence on lifestyle-induced changes in obesity-related parameters, separately and together. In earlier studies, the associations of the numerous polymorphisms with training-induced changes in body mass, composition, and biochemical parameters in Caucasian women have been analyzed. However, each of those studies involved a single or a small number of single nucleotide polymorphisms (SNPs), which did not allow comparison of the polymorphisms and led to comprehensive conclusions about their impact on the characteristics and range of the body’s adaptive response to training. Therefore, simultaneously analyzing numerous SNPs is more advantageous than other methods and may offer additional insights for comprehending complex gene–physical-activity interactions. Consequently, in this study, genetic data obtained from earlier studies conducted using 30 polymorphic sites located in 18 genes were used as components of a polygenic profile to find all ‘preferable’ and ‘unpreferable’ genotypes for training-induced body changes, which will provide additional information about people undertaking physical training. In addition, Biofilter software was used to construct a novel picture of the relationships among the genetic architecture and proteins, such as interaction pairs, pathways, and complex phenotypic outcomes, as described in previous biological experiments.
2. Materials and Methods
2.1. overview.
The results obtained from my studies regarding gene–physical-activity interactions were collected, and the functional significance of the genotypes described in the 30 common polymorphic sites connected with obesity risk was determined. The genotypes were assessed regarding their impact on training-induced changes in body mass, body composition, glucose level, and lipid profile in Caucasian females. The functional significance of the individual genotypes was determined based on the various consequences of their presence for achieving the desired health-promoting changes induced by the 12-week training program. Next, the relationships between the genetic variants and metabolic health parameters were studied, providing insights into potential factors influencing individual responses to training interventions.
2.2. Participants
This study is based on the previously published data, which examined training-induced changes in obesity-related parameters in participants. The participants were the same as those in the studies listed in Table 1 in the Section 3 .
The study group consisted of 165–201 healthy females of Caucasian origin (age: 21 ± 1 year). The following inclusion criteria were used: had a low level of physical activity self-reported with the use of the Global Physical Activity Questionnaire; had no metabolic, neuromuscular, or musculoskeletal disorders; were not using supplements or medications; and were nonsmokers. All participants were expected to maintain a balanced diet based on their dietary plan. They took part in the 12-week (36 training units) low- and high-impact aerobics of increasing intensity preceded by a week-long familiarization stage (3 training units). Before the training, participants of the experiment had their maximum heart rate (HRmax) evaluated, on the basis of a continuous graded test on an electronic cycle ergometer (Oxycon Pro, Erich JAEGER GmbH, Hoechberg, Germany). Exercise intensity was designated using HR monitors to control each individual heart rate. The volunteers were instructed to hold an HR or relative value of HRmax within appointed ranges. Each training unit consisted of a warm-up (10 min), aerobic exercise (43 min), and breathing–relaxing exercise with stretching (7 min). The main part was a combination of two styles including high impact (running, jumping, and hopping, with a variety of flight phases) and low impact (movements with at least 1 foot on the floor at all times). The 12-week program was divided as follows:
3 weeks (9 training units), 60 min each, at 50–60% of HRmax, tempo 135–140 BPM;
3 weeks (9 training units), 60 min each, at 60–70% of HRmax, tempo 140–152 BPM;
3 weeks (9 training units), 60 min each, at 65–75% of HRmax, tempo 145–158 BPM;
3 weeks (9 training units), 60 min each, at 65–80% of HRmax, tempo 145–160 BPM.
The training and dietary program was described in detail previously [ 18 , 19 ].
Before and after the completion of the training program, the chosen body mass and composition parameters were assessed via the bioimpedance method using electronic scale Tanita TBF 300 M (Arlington Heights, IL, USA), and biochemical analyses of blood samples were performed [ 20 ]. The following parameters were selected for this study: body mass index (BMI), fat mass (FM, kg), fat-free mass (FFM, kg), total body water (TBW, kg), total cholesterol (TC, mg/dL), triglycerides (TGL, mg/dL), high-density lipoprotein (HDL, mg/dL), low-density lipoprotein (LDL, mg/dL), and blood glucose level (BG, mg/dL).
The experiment was approved by the Ethics Committee of the Regional Medical Chamber in Szczecin (no. 09/KB/IV/2011 and 01/KB/VI/2017). Participants obtained an information sheet about the aim, procedures, benefits, and risks of the experiment, and a written consent form. Pseudonymization was applied as the method of data protection.
2.3. Total Genetic Score
First, the average values of the analyzed variables were compared by calculating their relative change by subtracting the variable’s value after training from the value of the variable before training. Second, the obtained results were used to create a polygenic profile for predicting post-training effects based on genotype data. According to Williams and Folland [ 21 ], each polymorphism site used to calculate the TGS was assigned a score based on the observed genotype (genotype score, GS). Typically, the polymorphisms identified were biallelic, providing 3 possible genotypes assigned GS values of 0, 1, or 2. The ‘optimal’ genotypes associated with beneficial post-training changes in selected variability (meaning that carriers of this genotype showed a relative change in the average values of the analyzed variables closest to the desired post-training effects) were scored 2, ‘intermediate’ genotypes were scored 1, and ‘less optimal’ genotypes (carriers of this genotype showed a change in the mean values of the analyzed variables that were farthest from the desired post-training effects) were scored 0. Due to the low abundance of one of the genotypes, homozygotes of one type were combined with heterozygotes for 6 polymorphisms. These patients only had two possible genotypes: a score of 2 or 0, indicating the least and most ‘optimal’ genotype. A decreasing BMI, FM, TC, TGL, LDL, and BG and increasing FFM, TBW, and LDL were considered favorable changes.
Afterward, the scores of each genotype were summed to generate the total score. The total score was converted to a scale of 0 to 100. The formula for calculating the TGS is as follows:
Greater TGS values indicate a more favorable polygenic profile. Specifically, a TGS of 100 represents a ‘perfect’ profile when the genotype is the best for achieving all investigated post-training effects (all GS values calculated for this genotype are equal to 2), and a TGS of 0 represents the ‘worst’ possible profile in terms of achieving the expected effects (all GS values calculated for this genotype are equal to 0). The words ‘perfect’ and ‘worst’ are, of course, only to be interpreted within the context of this paper. All the other TGS values, ranging between 0 and 100, illustrate the intermediate value of a given genotype in the context of achieving the expected post-training effects.
2.4. Biofilter
Biological information derivation and pairwise interaction modeling Biofilter 2.4 software [ 22 ] was used to derive biological information and construct pairwise interaction models. Initially, a list of SNPs was input into Biofilter software, which subsequently mapped these SNPs to corresponding genes. Next, genes harboring SNPs of interest were interconnected pairwise to explore common sources and groups defined within the Library of Knowledge Integration (LOKI) database. The LOKI database encompasses genes from multiple database repositories [ 22 ]. This integration facilitated the identification of common sources and groups among genes harboring relevant SNPs. Subsequently, the gene models were deconstructed into pairwise combinations of SNPs across genes, specifying the number of LOKI sources and the corresponding groups supporting these models. Each SNP within the interaction pairs was annotated with information from LOKI sources, and common entities were meticulously selected for group characterization.
2.5. Statistical Analysis of SNP Pairs and Interaction Testing
SNP pairs displaying potential interactions supported by biological knowledge were subjected to statistical interaction testing. A mixed-effects model in R ( https://www.R-project.org/ (accessed on 19 January 2024) [ 23 ], specifically the ‘lme4’ package, version 1.1-31) was utilized to assess the impact on all obesity-related parameters. The significance of interactions was assessed through nested models, incorporating models with and without the interaction term and employing a likelihood ratio test. For models demonstrating a statistically significant interaction effect, predictor effect plots were generated using the ‘effects’ (version 4.2-2) library in R.
3.1. Total Genetic Score
The complete list of the 30 SNPs localized in 18 genes associated with obesity-related parameters, the sources of all the information, the relative changes in the mean values of the analyzed variables, the GS for individual parameters, the GS sum, and the TGS values received for each genotype can be viewed in Table 1 . The observed TGS values were in the range of 11–94. The TGS values distinguished five groups of genotypes:
TGS ≥ 80—includes four of the most preferable genotypes for training-induced body changes
TGS of 60–79—includes 21 ‘preferable’ genotypes for training-induced body changes
TGS of 40–59—includes 30 ‘intermediate’ genotypes for training-induced body changes
TGS of 20–39—includes 22 ‘unpreferable’ genotypes for training-induced body changes
TGS < 20—includes five of the most ‘non-preferable’ genotypes for training-induced body changes
The obtained data indicate that the highest TGS values (Group 1) reflecting the most ‘optimal’ genotypes in the context of obtaining all the desired post-training effects were obtained for the following genotypes: ADRA2A rs553668 AA (genotype frequency 3%; 94 TGS), LEPR rs1137101 AA (genotype frequency 29%; 94 TGS), DRD2 rs1076560 AA (genotype frequency 2%; 83 TGS), and LEP rs2167270 GG (genotype frequency 40%; 83 TGS). The lowest TGS values (Group 5) reflecting the ‘worst’ possible profile in terms of achieving the expected effects were obtained for the following genotypes: DRD2 rs1076560 CA (genotype frequency 28%; 11 TGS), ADIPOQ rs266729 GG (genotype frequency 6%; 17 TGS), ADRB3 rs4994 TT (genotype frequency 86%; 17 TGS), ADRA2A rs553668 GG (genotype frequency 66%; 17 TGS), and LEPR rs1137101 AG (genotype frequency 49%; 17 TGS) ( Table 1 ). The other genotypes presented intermediate TGS values.
Polygenic profile of post-training changes in obesity-related parameters according to score.
∆—change in variable (before and after the completion of the training program); SNP—single nucleotide polymorphism; GS—genotype score; TGS—total genotype score; BMI—body mass index; FM—fat mass; FFM—fat-free mass; TBW—total body water; TC—total cholesterol; TGL—triglycerides; LDL—low-density lipoprotein; HDL—high-density lipoprotein.
3.2. Biofilter
Using Biofilter with the LOKI database, five noteworthy pairwise interactions were identified, each revealing potential associations between specific genes and their corresponding SNPs ( Table 2 ).
SNP interactions with scores supported by Biofilter modeling.
The score is a combination of two tallies: the number of original data sources which contained the pair and the number of different groups among those sources. For example, a score of “3–13” indicates that the model appears in thirteen different groups, and those groups originated with three different sources.
Three sources supported all interactions; the groups ranged from 6 to 16. Detailed information about the sources and groups for each interaction SNP pair is shown in Figure 1 A–E. Based on this, comprehensive analyses were conducted to explore the implications of pairwise interactions suggested by Biofilter software, employing mixed-effect models for each obesity-related parameter. The investigation identified three specific interactions demonstrating significant associations with key parameters crucial for metabolic health—TC, HDL, and FFM (see Figure 1 F). Figure 1 F shows predictor effect plots, providing insights into the relationships between changes in predictor variables and corresponding alterations in the predicted response variable. For the TC (the upper panel in Figure 1 F), compound homozygotes with LEP rs2167270 AA and LEPR rs1137101 AA showed a more significant increase in TC than those with other genotypes. For HDL (the middle panel of Figure 1 F), compound genotypes, such as ADRA2A rs553668 AA+AG and ADRB3 rs9449 CC+CT, displayed a more pronounced decrease in HDL cholesterol during intervention than did the other genotypes. For FFM (the bottom panel in Figure 1 F), we explored the interaction between ADRA2A GG and AA+AG based on baseline differences in AA homozygotes in ADRB2 . The interaction of FFM, while detected, appears to be of lesser importance in the context of training response, as it depends on baseline differences in AA homozygotes in ADRB2 between the ADRA2A GG and AA+AG genotypes.
Detailed information about the sources and groups for each interaction SNP pair. ( A ) LEP × LEPR : GO—angiogenesis, positive regulation of protein phosphorylation, protein binding, energy reserve metabolic process, negative regulation of autophagy, sexual reproduction, T cell differentiation, leptin-mediated signaling pathway, regulation of bone remodeling, bone growth, positive regulation of cold-induced thermogenesis; PHARMGKB—Antipsychotics Pathway (Metabolic Side Effects), Pharmacodynamics; REACTOME—R-HSA-2586552; ( B ) ARDB2 × ADRB3 : GO—norepinephrine–epinephrine-mediated vasodilation involved in regulation of systemic arterial blood pressure, desensitization of G-protein-coupled receptor signaling pathway by arrestin, protein binding, plasma membrane, receptor-mediated endocytosis, adenylate-cyclase-modulating G-protein-coupled receptor signaling pathway, activation of adenylate cyclase activity, protein homodimerization activity, receptor complex, positive regulation of MAPK cascade, norepinephrine binding, adenylate-cyclase-activating adrenergic receptor signaling pathway, positive regulation of cold-induced thermogenesis; PFAM—7 transmembrane receptor (rhodopsin family); REACTOME—R-HSA-390696, R-HSA-418555; ( C ) ADRA2A × ADRB3 GO—protein binding, plasma membrane, protein homodimerization activity, receptor complex, positive regulation of MAPK cascade, epinephrine binding, norepinephrine binding, adenylate-cyclase-activating adrenergic receptor signaling pathway; PFAM—7 transmembrane receptor (rhodopsin family); REACTOME—R-HSA-390696; ( D ) ADRA2A × ADRB2 : GO—protein binding, plasma membrane, protein homodimerization activity, receptor complex, positive regulation of MAPK cascade, norepinephrine binding, adrenergic receptor signaling pathway, adenylate-cyclase-activating adrenergic receptor signaling pathway; PFAM—7 transmembrane receptor (rhodopsin family); REACTOME—R-HSA-390696; ( E ) ADRA2A × DRD2 : GO—protein binding, plasma membrane, heterotrimeric G-protein binding, adenylate-cyclase-activating adrenergic receptor signaling pathway; PFAM—7 transmembrane receptor (rhodopsin family); PHARMGKB—Methylphenidate Pathway, Pharmacodynamics; ( F ) predictor effect plots, providing insights into the relationships between changes in predictor variables and corresponding alterations in the predicted response variable; GO (Gene Ontology)—GO is a comprehensive bioinformatics resource that provides structured and standardized terms to describe the functions of genes and proteins in any organism. It categorizes gene functions into three main ontologies: Molecular Function (the molecular activities of gene products), Biological Process (the larger biological goals accomplished by gene products), and Cellular Component (the locations in the cell where gene products are active); PFAM—widely used database that classifies proteins into families based on the presence of specific conserved protein domains or functional units. It provides information about the structure and function of these protein domains; REACTOME—curated and peer-reviewed pathway database that provides insights into biological pathways, reactions, and biomolecule interactions. It covers many biological processes, including signaling pathways, metabolic pathways, and immune system responses.
4. Discussion
According to studies performed in twins, families, and adoptees, the heritability of body mass status ranges from 40% to 50%. However, this value is lower among normal-weight individuals (approximately 30%) and higher in obese people (60–80%) [ 35 ]. Genetic epidemiological studies have shown that numerous genetic loci identified by genome-wide association studies (GWASs) cumulatively increase the risk of obesity [ 17 ] and may influence physical activity and sedentary behavior in daily life [ 36 ]. Although genetically predisposed individuals are more prone to developing obesity, it has been shown that the level of physical activity can modify the genetic predisposition to common obesity. Li et al. [ 37 ] indicated that a physically active lifestyle is associated with a 40% reduction in genetic susceptibility to obesity. The authors emphasized the importance of promoting exercise, particularly in genetically predisposed individuals, as a significant approach to controlling the growing obesity epidemic [ 37 ]. However, we still do not know whether or to what extent habitual physical activity may weaken this genetic susceptibility [ 37 ]. Therefore, my research on gene–physical-activity interactions in obesity for 10 years has resulted in more than 15 published articles [ 15 , 18 , 19 , 20 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 ]. Because the individual studies involved only single or small numbers of polymorphisms, which did not allow for their simultaneous analysis to elucidate the complex associations between the genetic determinants of obesity and physical activity, previously obtained results were used to describe their impact on post-training response comprehensively.
The most important achievement of this work was identifying specific genotypes associated with favorable or undesirable training-induced changes in body mass, body composition, and biochemical parameters (‘preferable’, ‘intermediate’, and ‘unpreferable’, respectively). The data obtained showed that the most significant impact on the effectiveness of a 12-week training program involved genes encoding leptin and a leptin receptor ( LEP and LEPR ), adrenergic receptors ( ADRA2A and ADRB3 ), a dopamine receptor D2 ( DRD2 ), and an adiponectin receptor ( ADIPOQ ). None of the genotypes achieved the TGS value of either 0 or 100, reflecting the ‘worst’ or the ‘perfect’ profile. The best result was 94 TGS for the ADRA2A rs553668 AA and LEPR rs1137101 AA genotypes, which indicated that carriers of these genotypes exhibited a beneficial change in the average values of all the analyzed variables (eight parameters were scored 2, and one was scored 1). In addition, two genotypes, DRD2 rs1076560 AA and LEP rs2167270 GG, had a TGS of 83 and were classified as the most ‘preferable’ genotypes for training-induced body changes. The worst result was 11 TGS for DRD2 rs1076560 CA, and the carriers of this genotype exhibited an unbeneficial change in the mean values of almost all the chosen variables (seven parameters were scored 0, and two were scored 1). The group of individuals with the most ‘unpreferable’ genotypes for training-induced body changes also included genotypes such as ADIPOQ rs266729 GG, ADRB3 rs4994 TT, ADRA2A rs553668 GG, and LEPR rs1137101 AG, for which the TGS value was 17. These results confirm the role of the various genes in the development of obesity described by several authors [ 2 , 13 , 32 ]. However, a polygenic profile aimed at finding all the ‘preferable’ and ‘unpreferable’ genotypes for training-induced body changes was created for the first time. Thus, this study cannot be directly compared to previous studies.
In the 2000s, GWASs allowed the analysis of polymorphic sites in the whole genome to link common and low-frequency genomic variants to phenotypes such as obesity. The first defined obesity susceptibility gene with a more significant influence on body mass to date was the fat mass and obesity-associated ( FTO ) gene. The common FTO polymorphism with a T-to-A change (rs9939609) is strongly associated with an increased risk of obesity development in various populations. Each A allele, a risk allele, is associated with a 1–1.5 kg increase in body weight [ 38 ]. My interventional study confirmed that participants with the AA and AT genotypes had increased BMIs during the entire study period; however, the FTO gene–physical-activity interaction was not demonstrated [ 20 ]. This analysis confirmed that no FTO genotype was associated with better or worse training-induced body changes. Thus, not all obesity-related polymorphisms are crucial for assessing the effectiveness of weight loss programs. Some studies have been unable to demonstrate this interaction [ 39 , 40 ]; however, others have shown that the effect size of FTO variants is up to 80% lower in physically active individuals than in inactive individuals [ 41 , 42 ]. Other SNPs in the LEP , LEPR , ADIPOQ , ADRA2A , and ADRB3 genes, which are described as key for energy intake and fat metabolism [ 2 ], are also important in the body’s adaptive response to training. Adipose tissue plays significant roles in body weight regulation and energy homeostasis, including the production and secretion of numerous cytokines, chemokines, and hormone-like factors known as adipokines [ 43 ]. Leptin and adiponectin are critical in food intake, metabolism, and immunity. Leptin, which acts as an afferent signal in a negative feedback loop by binding to the leptin receptor, plays important roles in regulating body weight by suppressing appetite and stimulating energy expenditure [ 44 , 45 ]. Adiponectin is an essential anti-inflammatory and insulin-sensitizing hormone that promotes lipid oxidation in tissues such as skeletal muscle and liver [ 46 ]. The brain receives signals from adipose tissue, which activates neural circuits controlling energy expenditure and increases sympathetic nerve activity. Adrenergic receptors are part of the sympathetic nervous system and exert their actions by coupling with catecholamines, which are important regulators of lipolysis and energy expenditure [ 47 ]. Considering the multifactorial role of these gene products in regulating energy metabolism, it was expected that they would also contribute to the post-training response, which was confirmed by the analysis. Surprisingly, the genotypes of the rs1076560 polymorphism in the DRD2 gene encoding the dopamine receptor D2 were the genotypes with the highest post-training effect. Although it is associated with essential central nervous system functions, such as cognitive abilities, its impact on exercise-induced changes has rarely been analyzed, making comparison difficult. This analysis highlights that the genes related to the dopaminergic system may play a significant role in the effectiveness of training programs, so continuing research is necessary.
The second part of this analysis, conducted using Biofilter software, confirmed the biological significance of the SNPs. These SNPs are most important, based on the TGS, for the changes in body mass, composition, and biochemical parameters induced by training. Five noteworthy pairwise interactions ( LEP × LEPR , ADRB2 × ADRB3 , ADRA2A × ADRB3 , ADRA2A × ADRB2 , and ADRA2A × DRD2 ) were identified. The core principle guiding the analysis conducted with Biofilter software is that any grouping of genes or proteins, whether a pathway, ontological category, protein family, experimental interaction, or any other classification, implies a potential relationship among the individual elements within that group. When the same two genes repeatedly appear together in various groupings, this signifies a substantial biological relationship. Furthermore, if these genes are present in multiple groups sourced from diverse, independent origins, their probability of being biologically related significantly increases. Biofiltering taps into an extensive repository of such groupings, facilitating the examination of all these associations. The tool discerns pairs of genes or SNPs that co-occur across numerous groupings spanning various original data sources. Consequently, these gene pairs can undergo significance testing within a research dataset, eliminating the need for exhaustive pairwise analyses that would pose computational challenges and the burden of multiple testing. Based on these findings, comprehensive analyses were conducted to explore the implications of pairwise associations suggested by Biofilter software, employing mixed-effect models for each obesity-related parameter. Three specific interactions demonstrating significant associations with key parameters crucial for metabolic health—TC, HDL cholesterol, and FFM—were shown. Carriers of the LEP rs2167270 AA and LEPR rs1137101 AA exhibited greater increases in TC levels during the intervention, suggesting that the average values of these parameters did not change with age. Compound genotypes such as ADRA2A rs553668 AA+AG and ADRB3 rs9449 CC+CT showed a more substantial training-induced decrease in HDL levels and may be classified as unpreferable genotypes. The third interaction, detected for FFM, appears to be of minor importance in training response, as it depends on baseline differences in AA homozygotes in ADRB2 between the ADRA2A GG and AA+AG genotypes. These findings highlight the nuanced relationships between genetic variations and metabolic health parameters, providing insights into potential factors that influence individual responses to training interventions.
GWASs have recognized numerous genetic loci associated with lipid traits. However, these loci explain only 25–30% of the heritability observed at the blood lipid level [ 48 ]. Interactions between genes may explain a part of this missing heritability [ 49 ]. Previously, Holzinger et al. [ 50 ] performed a gene-centric interaction study for four different lipid traits, LDL, HDL, TC, and BG, using a main-effect filter and biofilters. More models passed the selected replication threshold for the main-effect filter analyses. However, for the biofilter analyses, the results were replicated only for the BG trait, with two models passing the significance threshold in a single cohort ( SIK3 rs11216162 × APOA4 rs1263173 and SIK3 rs625145 × APOA4 rs1263173). The authors suggested that biofilter analysis, which creates gene–gene models based on current biological knowledge, allows for more precise interpretations, as the models make biological sense. However, this approach inhibits the discovery of interactions in regions with limited biological knowledge. Using the genetic data from five cohorts of 24,837 individuals, De et al. [ 51 ] combined the quantitative multifactor dimensionality reduction algorithm with two SNP filtering methods to search for interactions between SNPs linked to lipid traits. When SNPs were filtered using Biofilter, two models associated with HDL cholesterol, three associated with LDL cholesterol, one associated with TC, and eight associated with BG were revealed. However, none of these interactions were consistent with the results obtained in this study. These differences may be explained by the fact that previous studies did not address changes in post-training parameters. To the best of my knowledge, this is the first study to analyze the interactions between SNPs in the context of post-training changes in selected parameters, which makes it difficult to compare the results.
The strong point of the study was the comprehensive analysis of numerous genetic data obtained from the experiment consisting of regulation of both food intake and physical activity of a homogeneous Caucasian population, whose body mass and composition, as well as physiological and biochemistry parameters, were analyzed before and after the completion of the 12-week training program. In addition, the use of creative approaches such as the construction of a polygenic profile and Biofilter software has provided novel insights into potential factors influencing individual responses to training interventions. It needs to be highlighted that common obesity is a multifactorial disease, most likely resulting from a complicated interaction of genetic, epigenetic, and environmental components [ 52 , 53 ]. Among the many factors influencing response to exercise training are age, gender, diseases, volume, intensity and frequency of exercise, diet, and many others [ 16 ]. Therefore, a potential limitation of the study is the small size of the participant group, which may not show statistical power sufficient to yield meaningful analysis and interpretation. Another factor that should be considered as a weakness is population-specific characteristics such as one gender, similar age, relatively high physical activity levels as well as a relatively low weight. Previously, studies investigating gene-by-sex interactions for obesity have shown an interplay between sex and obesity-related traits, and specific polymorphisms can be associated with obesity in one sex [ 54 ]. Unfortunately, the experiment only included young Caucasian women, and thus there was no possibility of comparing the results between genders, ethnicities, and age groups. Additionally, Aurich et al. have shown that intervention studies documenting changes in a systemic epigenetic biomarker for obesity susceptibility during weight loss programs make a significant contribution to a better understanding of epigenetic reprogramming in obesity [ 52 ]. Epigenetic modifications include DNA methylation, histone modifications, and non-coding RNAs (microRNAs, miRs) which mediate between environmental and genetic factors. These alterations may be causal for the development of obesity by inducing improper expression or silencing of the obesity-associated genes and regulatory sequences, leading to metabolic balance disorders. Epigenetic changes can also arise as a consequence of obesity and predispose for obesity-associated comorbidities such as cancer [ 52 , 55 , 56 , 57 ]. This study did not examine lifestyle effects on epigenetic remodeling, which is another weak point of the study.
5. Conclusions
In this study, two novel approaches, total genetic score and Biofilter software, were used to combine genotypic and phenotypic information for nine obesity-related traits measured before and after the initiation of a 12-week aerobic training program. The first important finding was the indication of ‘preferable’, ‘intermediate’, and ‘unpreferable’ genotypes for training-induced changes in selected body mass, composition, and biochemical parameters. The genes most important for post-workout changes were LEP , LEPR , ADIPOQ , ADRA2A , ADRB3 , and DRD2 . The second finding involved the identification of five noteworthy pairwise interactions ( LEP × LEPR , ADRB2 × ADRB3 , ADRA2A × ADRB3 , ADRA2A × ADRB2 , and ADRA2A × DRD2 ) and three specific interactions demonstrating significant associations with key parameters crucial for metabolic health—TC, HDL, and FFM. Understanding the genetic architecture and its interactions with lifestyle factors such as physical activity level enables us to clarify individuals’ physical activity criteria. In the future, training programs designed according to a given person’s genetic profile will be effective and safe intervention strategies for preventing obesity and improving health.
Acknowledgments
I would like to extend my sincere thanks to all collaborators involved in the implementation of the grant no. 2012/07/B/NZ7/01155.
Institutional Review Board Statement
The experimental protocols were positively verified by the Ethics Committee of the Regional Medical Chamber in Szczecin (no. 09/KB/IV/2011 and 01/KB/VI/2017), and were conducted according to World Medical Association Declaration of Helsinki and Strengthening the Reporting of Genetic Association studies statement (STREGA).
Informed Consent Statement
Informed consent was obtained from all participants included in the study.
Data Availability Statement
The data presented in this study are available on request from the author. The data are not publicly available due to privacy/ethical restrictions.
Conflicts of Interest
The author declares no conflicts of interest.
Funding Statement
The study was supported by the National Science Centre of Poland (no. 2012/07/B/NZ7/01155). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the paper.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
- 1. Silventoinen K., Jelenkovic A., Sund R., Hur Y.M., Yokoyama Y., Honda C., Hjelmborg J., Möller S., Ooki S., Aaltonen S., et al. Genetic and Environmental Effects on Body Mass Index from Infancy to the Onset of Adulthood: An Individual-Based Pooled Analysis of 45 Twin Cohorts Participating in the COllaborative Project of Development of Anthropometrical Measures in Twins (CODATwins) Study. Am. J. Clin. Nutr. 2016;104:371–379. doi: 10.3945/ajcn.116.130252. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Singh R.K., Kumar P., Mahalingam K. Molecular Genetics of Human Obesity: A Comprehensive Review. Comptes Rendus Biol. 2017;340:87–108. doi: 10.1016/j.crvi.2016.11.007. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Conway B., Rene A. Obesity as a Disease: No Lightweight Matter. Obes. Revs. 2004;5:145–151. doi: 10.1111/j.1467-789X.2004.00144.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Beals J.W., Kayser B.D., Smith G.I., Schweitzer G.G., Kirbach K., Kearney M.L., Yoshino J., Rahman G., Knight R., Patterson B.W., et al. Dietary weight loss-induced improvements in metabolic function are enhanced by exercise in people with obesity and prediabetes. Nat. Metab. 2023;5:1221–1235. doi: 10.1038/s42255-023-00829-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Cai L., Gonzales T., Wheeler E., Kerrison N.D., Day F.R., Langenberg C., Perry J.R.B., Brage S., Wareham N.J. Causal associations between cardiorespiratory fitness and type 2 diabetes. Nat. Commun. 2023;14:3904. doi: 10.1038/s41467-023-38234-w. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Brittain E.L., Han L., Annis J., Master H., Hughes A., Roden D.M., Harris P.A., Ruderfer D.M. Physical Activity and Incident Obesity Across the Spectrum of Genetic Risk for Obesity. JAMA Netw. Open. 2024;7:e243821. doi: 10.1001/jamanetworkopen.2024.3821. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Jakicic J.M. The Role of Physical Activity in Prevention and Treatment of Body Weight Gain in Adults. J. Nutr. 2002;132:3826S–3829S. doi: 10.1093/jn/132.12.3826S. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Chaput J.P., Klingenberg L., Rosenkilde M., Gilbert J.A., Tremblay A., Sjödin A. Physical Activity Plays an Important Role in Body Weight Regulation. J. Obes. 2011;2011:360257. doi: 10.1155/2011/360257. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Coffey V.G., Hawley J.A. The Molecular Bases of Training Adaptation. Sports Med. 2007;37:737–763. doi: 10.2165/00007256-200737090-00001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Hughes D.C., Ellefsen S., Baar K. Adaptations to Endurance and Strength Training. Cold Spring Harb. Perspect. Med. 2018;8:a029769. doi: 10.1101/cshperspect.a029769. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Rankinen T., Bouchard C. Gene-Physical Activity Interactions: Overview of Human Studies. Obesity. 2008;16((Suppl. S3)):S47–S50. doi: 10.1038/oby.2008.516. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Simoneau J.A., Lortie G., Boulay M.R., Marcotte M., Thibault M.C., Bouchard C. Inheritance of Human Skeletal Muscle and Anaerobic Capacity Adaptation to High-Intensity Intermittent Training. Int. J. Sports Med. 1986;7:167–171. doi: 10.1055/s-2008-1025756. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Bouchard C., Rankinen T. Individual Differences in Response to Regular Physical Activity. Med. Sci. Sports Exerc. 2001;33:S446–S451. doi: 10.1097/00005768-200106001-00013. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Ahmad S., Rukh G., Varga T.V., Ali A., Kurbasic A., Shungin D., Ericson U., Koivula R.W., Chu A.Y., Rose L.M., et al. Gene × Physical Activity Interactions in Obesity: Combined Analysis of 111,421 Individuals of European Ancestry. PLoS Genet. 2013;9:e1003607. doi: 10.1371/journal.pgen.1003607. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Leońska-Duniec A., Ahmetov I.I., Zmijewski P. Genetic Variants Influencing Effectiveness of Exercise Training Programmes in Obesity—An Overview of Human Studies. Biol. Sport. 2016;33:207–214. doi: 10.5604/20831862.1201052. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Masood B., Moorthy M. Causes of obesity: A review. Clin. Med. 2023;23:284–291. doi: 10.7861/clinmed.2023-0168. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Goodarzi M.O. Genetics of Obesity: What Genetic Association Studies Have Taught Us about the Biology of Obesity and Its Complications. Lancet Diabetes Endocrinol. 2018;6:223–236. doi: 10.1016/S2213-8587(17)30200-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Leońska-Duniec A., Grzywacz A., Jastrzȩbski Z., Jażdżewska A., Lulińska-Kuklik E., Moska W., Leźnicka K., Ficek K., Rzeszutko A., Dornowski M., et al. ADIPOQ Polymorphisms Are Associated with Changes in Obesity-Related Traits in Response to Aerobic Training Programme in Women. Biol. Sport. 2018;35:165–173. doi: 10.5114/biolsport.2018.72762. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Ficek K., Ciȩszczyk P., Leźnicka K., Kaczmarczyk M., Leońska-Duniec A. Novel Associations between Interleukin-15 Polymorphisms and Post-Training Changes of Body Composition Parameters in Young Nonobese Women. Front. Physiol. 2019;10:876. doi: 10.3389/fphys.2019.00876. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Leońska-Duniec A., Jastrzębski Z., Zarębska A., Maciejewska A., Ficek K., Cięszczyk P. Assessing Effect of Interaction between the FTO A/T Polymorphism (Rs9939609) and Physical Activity on Obesity-Related Traits. J. Sport. Health Sci. 2018;7:459–464. doi: 10.1016/j.jshs.2016.08.013. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Williams A.G., Folland J.P. Similarity of Polygenic Profiles Limits the Potential for Elite Human Physical Performance. J. Physiol. 2008;586:113–121. doi: 10.1113/jphysiol.2007.141887. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. Pendergrass S.A., Frase A., Wallace J., Wolfe D., Katiyar N., Moore C., Ritchie M.D. Genomic Analyses with Biofilter 2.0: Knowledge Driven Filtering, Annotation, and Model Development. BioData Min. 2013;6:25. doi: 10.1186/1756-0381-6-25. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. The R Project for Statistical Computing. [(accessed on 19 January 2024)]. Available online: https://www.r-project.org/
- 24. Cięszczyk P., Jastrzębski Z., Zarębska A., Sawczyn M., Drobnik-Kozakiewicz I., Leońska-Duniec A., Żmijewski P., Murawska-Ciałowicz E., Petr M., Contro V., et al. Association between the ACE I/D Polymorphism and Physical Activity in Polish. TRENDS Sport. Sci. 2016;23:203–210. [ Google Scholar ]
- 25. Leońska-Duniec A., Jastrzębski Z., Jażdżewska A., Moska W., Lulińska-Kuklik E., Sawczuk M., Gubaydullina S.I., Shakirova A.T., Cięszczyk P., Maszczyk A., et al. Individual Responsiveness to Exercise-Induced Fat Loss and Improvement of Metabolic Profile in Young Women Is Associated with Polymorphisms of Adrenergic Receptor Genes. J. Sports Sci. Med. 2018;17:134–144. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Leońska-Duniec A., Maculewicz E., Humińska-Lisowska K., Maciejewska-Skrendo A., Leźnicka K., Cięszczyk P., Sawczuk M., Trybek G., Wilk M., Lepionka W., et al. AMPD1 C34T Polymorphism (RS17602729) Is Not Associated with Post-Exercise Changes of Body Weight, Body Composition, and Biochemical Parameters in Caucasian Females. Genes. 2020;11:558. doi: 10.3390/genes11050558. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Świtała K., Bojarczuk A., Hajto J., Piechota M., Buryta M., Leońska-Duniec A. Impact of the DRD2 Polymorphisms on the Effectiveness of the Training Program. Int. J. Environ. Res. Public Health. 2022;19:4942. doi: 10.3390/ijerph19094942. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Leońska-Duniec A., Świtała K., Ahmetov I.I., Pickering C., Massidda M., Buryta M., Mastalerz A., Maculewicz E. FABP2 Ala54thr Polymorphism and Post-Training Changes of Body Composition and Biochemical Parameters in Caucasian Women. Genes. 2021;12:954. doi: 10.3390/genes12070954. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Leońska-Duniec A., Lepionka W., Brodkiewicz A., Buryta M. Association of the IL1A and IL6 Polymorphisms with Posttraining Changes in Body Mass, Composition, and Biochemical Parameters in Caucasian Women. Biol. Sport. 2024;41:47–56. doi: 10.5114/biolsport.2024.131415. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Leońska-Duniec A., Jastrzębski Z., Jażdżewska A., Ficek K., Cięszczyk P. Leptin and Leptin Receptor Genes Are Associated With Obesity-Related Traits Changes in Response to Aerobic Training Program. J. Strength. Cond. Res. 2018;32:1036–1044. doi: 10.1519/JSC.0000000000002447. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Leońska-Duniec A., Jastrzębski Z., Zarębska A., Smółka W., Cięszczyk P. Impact of the Polymorphism Near MC4R (Rs17782313) on Obesity- and Metabolic-Related Traits in Women Participating in an Aerobic Training Program. J. Hum. Kinet. 2017;58:111–119. doi: 10.1515/hukin-2017-0073. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Maciejewska-Skrendo A., Buryta M., Czarny W., Król P., Spieszny M., Stastny P., Petr M., Safranow K., Sawczuk M. The Polymorphisms of the Peroxisome-Proliferator Activated Receptors’ Alfa Gene Modify the Aerobic Training Induced Changes of Cholesterol and Glucose. J. Clin. Med. 2019;8:1043. doi: 10.3390/jcm8071043. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Leońska-Duniec A., Cieszczyk P., Jastrzębski Z., Jażdżewska A., Lulińska-Kuklik E., Moska W., Ficek K., Niewczas M., Maciejewska-Skrendo A. The Polymorphisms of the PPARD Gene Modify Post-Training Body Mass and Biochemical Parameter Changes in Women. PLoS ONE. 2018;13:e0202557. doi: 10.1371/journal.pone.0202557. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Leońska-Duniec A., Ficek K., Świtała K., Cięszczyk P. Association of the TNF-α -308G/A Polymorphism with Lipid Profile Changes in Response to Aerobic Training Program. Biol. Sport. 2019;36:291–296. doi: 10.5114/biolsport.2019.85456. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Bouchard C. Genetics of Obesity: What We Have Learned Over Decades of Research. Obesity. 2021;29:802–820. doi: 10.1002/oby.23116. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Wang Z., Emmerich A., Pillon N.J., Moore T., Hemerich D., Cornelis M.C., Mazzaferro E., Broos S., Ahluwalia T.S., Bartz T.M., et al. Genome-wide association analyses of physical activity and sedentary behavior provide insights into underlying mechanisms and roles in disease prevention. Nat. Genet. 2022;54:1332–1344. doi: 10.1038/s41588-022-01165-1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 37. Li S., Zhao J.H., Luan J., Ekelund U., Luben R.N., Khaw K.-T., Wareham N.J., Loos R.J.F. Physical Activity Attenuates the Genetic Predisposition to Obesity in 20,000 Men and Women from EPIC-Norfolk Prospective Population Study. PLoS Med. 2010;7:e1000332. doi: 10.1371/journal.pmed.1000332. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Frayling T.M., Timpson N.J., Weedon M.N., Zeggini E., Freathy R.M., Lindgren C.M., Perry J.R.B., Elliott K.S., Lango H., Rayner N.W., et al. A Common Variant in the FTO Gene Is Associated with Body Mass Index and Predisposes to Childhood and Adult Obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Jonsson A., Renström F., Lyssenko V., Brito E.C., Isomaa B., Berglund G., Nilsson P.M., Groop L., Franks P.W. Assessing the Effect of Interaction between an FTO Variant (rs9939609) and Physical Activity on Obesity in 15,925 Swedish and 2,511 Finnish Adults. Diabetologia. 2009;52:1334–1338. doi: 10.1007/s00125-009-1355-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Hakanen M., Raitakari O.T., Lehtimäki T., Peltonen N., Pahkala K., Sillanmaki L., Lagstrom H., Viikari J., Simell O., Ronnemaa T. FTO Genotype Is Associated with Body Mass Index after the Age of Seven Years but Not with Energy Intake or Leisure-Time Physical Activity. J. Clin. Endocrinol. Metab. 2009;94:1281–1287. doi: 10.1210/jc.2008-1199. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Vimaleswaran K.S., Li S., Zhao J.H., Luan J., Bingham S.A., Khaw K.-T., Ekelund U., Wareham N.J., Loos R.J. Physical Activity Attenuates the Body Mass Index-Increasing Influence of Genetic Variation in the FTO Gene. Am. J. Clin. Nutr. 2009;90:425–428. doi: 10.3945/ajcn.2009.27652. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Rampersaud E., Mitchell B.D., Pollin T.I., Fu M., Shen H., O’connell J.R., Ducharme J.L., Hines S., Sack P., Naglieri R., et al. Physical Activity and the Association of Common FTO Gene Variants With Body Mass Index and Obesity. Arch. Intern. Med. 2008;168:1791–1797. doi: 10.1001/archinte.168.16.1791. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Trayhurn P., Wood I.S. Adipokines: Inflammation and the Pleiotropic Role of White Adipose Tissue. Br. J. Nutr. 2004;92:347–355. doi: 10.1079/BJN20041213. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Lenard N.R., Berthoud H.R. Central and Peripheral Regulation of Food Intake and Physical Activity: Pathways and Genes. Obesity. 2008;16((Suppl. S3)):S11–S22. doi: 10.1038/oby.2008.511. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Sahu A. Minireview: A Hypothalamic Role in Energy Balance with Special Emphasis on Leptin. Endocrinology. 2004;145:2613–2620. doi: 10.1210/en.2004-0032. [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Yamauchi T., Kamon J., Minokoshi Y., Ito Y., Waki H., Uchida S., Yamashita S., Noda M., Kita S., Ueki K., et al. Adiponectin Stimulates Glucose Utilization and Fatty-Acid Oxidation by Activating AMP-Activated Protein Kinase. Nat. Med. 2002;8:1288–1295. doi: 10.1038/nm788. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Lowell B.B., Bachman E.S. β-Adrenergic Receptors, Diet-Induced Thermogenesis, and Obesity. J. Biol. Chem. 2003;278:29385–29388. doi: 10.1074/jbc.R300011200. [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Kathiresan S., Willer C.J., Peloso G.M., Demissie S., Musunuru K., Schadt E.E., Kaplan L., Bennett D., Li Y., Tanaka T., et al. Common Variants at 30 Loci Contribute to Polygenic Dyslipidemia. Nat. Genet. 2009;41:56–65. doi: 10.1038/ng.291. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Manolio T.A., Collins F.S., Cox N.J., Goldstein D.B., Hindorff L.A., Hunter D.J., McCarthy M.I., Ramos E.M., Cardon L.R., Chakravarti A., et al. Finding the Missing Heritability of Complex Diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Holzinger E.R., Verma S.S., Moore C.B., Hall M., De R., Gilbert-Diamond D., Lanktree M.B., Pankratz N., Amuzu A., Burt A., et al. Discovery and Replication of SNP-SNP Interactions for Quantitative Lipid Traits in over 60,000 Individuals. BioData Min. 2017;10:25. doi: 10.1186/s13040-017-0145-5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. De R., Verma S.S., Holzinger E., Hall M., Burt A., Carrell D.S., Crosslin D.R., Jarvik G.P., Kuivaniemi H., Kullo I.J., et al. Identifying Gene–Gene Interactions That Are Highly Associated with Four Quantitative Lipid Traits across Multiple Cohorts. Hum. Genet. 2017;136:165–178. doi: 10.1007/s00439-016-1738-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Aurich S., Müller L., Kovacs P., Keller M. Implication of DNA methylation during lifestyle mediated weight loss. Front. Endocrinol. 2023;14:1181002. doi: 10.3389/fendo.2023.1181002. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 53. Silveira A., Gomes J., Roque F., Fernandes T., de Oliveira E.M. MicroRNAs in Obesity-Associated Disorders: The Role of Exercise Training. Obes. Facts. 2022;15:105–117. doi: 10.1159/000517849. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. Kyrgiafini A.M., Sarafidou T., Giannoulis T., Chatziparasidou A., Christoforidis N., Mamuris Z. Gene-by-Sex Interactions: Genome-Wide Association Study Reveals Five SNPs Associated with Obesity and Overweight in a Male Population. Genes. 2023;14:799. doi: 10.3390/genes14040799. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. Barrero M.J., Cejas P., Long H.W., Ramirez de Molina A. Nutritional epigenetics in cancer. Adv. Nutr. 2022;13:1748–1761. doi: 10.1093/advances/nmac039. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 56. Duncan G.E., Avery A., Maamar M.B., Nilsson E.E., Beck D., Skinner M.K. Epigenome-wide association study of systemic effects of obesity susceptibility in human twins. Epigenetics. 2023;18:2268834. doi: 10.1080/15592294.2023.2268834. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 57. Cannataro R., Perri M., Gallelli L., Caroleo M.C., De Sarro G., Cione E. Ketogenic Diet Acts on Body Remodeling and MicroRNAs Expression Profile. Microrna. 2019;8:116–126. doi: 10.2174/2211536608666181126093903. [ DOI ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
- View on publisher site
- PDF (917.3 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES
VIDEO
COMMENTS
The candidate gene approach was first applied in the mid-1990s and aimed to validate genes identified through human and animal models of extreme obesity for a role in common obesity (Fig. 3). Common variants in such candidate genes were tested for association with obesity risk, BMI or other body composition traits.
In the context of the complex etiology of human obesity, epigenetic mechanisms based on, e.g. DNA methylation or histone modifications and gene-environment interactions are important to be considered in order to better understand the role of genetics in the development of this multifactorial disease.
The candidate gene approach was first applied in the mid-1990s and aimed to validate genes identified through human and animal models of extreme obesity for a role in common obesity (Fig. 3 ...
NGS is now in use and emerging as a useful tool to search for candidate genes for obesity in clinical settings. Keywords: obesity, genetics, monogenic, polygenic, Prader-Willi, syndrome. 1. Introduction. Obesity is a major health problem worldwide. It is more common in established countries but is on the increase in developing countries.
Researchers were able to locate a specific gene variant correlated with some people's weight gain, a new study showed. Obesity isn’t just a matter of food and exercise — it may be in your ...
Obesity is closely linked to genetics and environmental factors. The newest studies in the field of epigenetics further our understanding of the effect of the environment on genetics. This article describes the genetic causes of obesity, including syndromic, monogenic, and polygenic causes, and cites specific examples of epigenetic ...
Abstract. The prevalence of obesity has tripled over the past four decades, imposing an enormous burden on people's health. Polygenic (or common) obesity and rare, severe, early-onset monogenic obesity are often polarized as distinct diseases. However, gene discovery studies for both forms of obesity show that they have shared genetic and ...
Over the past twenty years a growing number of genes have been described in which loss of function mutations are consistently associated with the development of severe obesity beginning in early childhood. Whilst individually these disorders are rare, cumulatively at least 10% of children with severe obesity have rare chromosomal abnormalities, nonsense mutations, or missense mutations that ...
Previous research has investigated the genetic regulation of blood pressure regulatory genes using post-GWAS data , but similar investigations for obesity remain limited.
Therefore, my research on gene–physical-activity interactions in obesity for 10 years has resulted in more than 15 published articles [15,18,19,20,24,25,26,27,28,29,30,31,32,33,34]. Because the individual studies involved only single or small numbers of polymorphisms, which did not allow for their simultaneous analysis to elucidate the ...