
- Book Solutions
- State Boards

Case Study Questions Class 9 Science Matter in our Surroundings
Case study questions class 9 science chapter 1 matter in our surroundings.
CBSE Class 9 Case Study Questions Science Matter in our Surroundings. Important Case Study Questions for Class 9 Exam. Here we have arranged some Important Case Base Questions for students who are searching for Paragraph Based Questions Matter in our Surroundings.
At Case Study Questions there will given a Paragraph. In where some Questions will made on that respective Case Based Study. There will various types of marks will given 1 marks, 2 marks, 3 marks or 4 marks.
CBSE Case Study Questions Class 9 Science – Matter in our Surroundings
Case study 1:.
1.) A matter is anything that has mass and occupies space. Pen, paper, clips, sand, air, ice, etc. are different forms of matter. Every matter is made up of small particles. These particles are so tiny that they can’t be seen with naked eyes. Let’s see about the different characteristics of particles of matter.
- All matter is made up of very small particles.
- .Particles of matter has spaces between them.
- Particles of matter are continuously moving.
- Particles of matter attract each other.
Answer the following questions by referring above paragraph.
i.) Which of following is not matter?
c.) smell of perfume
d.) None of these
ii.) Thoughts coming in our mind are example of matter. True or false
c.) None of these
iii.) Which of the following is true about particles of matter?
a.) Particles of matter has spaces between them
b.) Particles of matter are continuously moving
c.) Particles of matter attract each other
d.) All of these
iv.) Give 5 examples of matter in our surroundings
v.) Enlist all properties of particles of matter
Answer key-1
iv.) pen, pencil, notebook, ice and water
v.) Different characteristics of particles of matter are
Case Study 2:
2.) There are three states of matter – solid, liquid and gas.
Solids have a definite shape, distinct boundaries and fixed volumes, that is, have negligible compressibility. Solids have a tendency to maintain their shape when subjected to outside force. Solids may break under force but it is difficult to change their shape, so they are rigid.
Liquids have no fixed shape but have a fixed volume. They take up the shape of the container in which they are kept. Liquids flow and change shape, so they are not rigid but can be called fluid.
Gas as has indefinite shape, no fixed volume. Gas gets the shape and volume of container.
Gas has very low density hence are light. Gas can flow easily and hence are called fluid.
i.) Which of the following state of matter takes shape of container in which it is filled?
d.) Both b and c
ii.) Distance between particles of matter least in
iii.) Compressibility is least in case of
iv.) Give properties of solids.
v.) Give properties of Gases.
Answer key-2
iv.) properties of solid are given below
- Solid has fixed volume.
- Solid has fixed shape.
- Solid has high density.
- Solids are heavy.
- Solid does not flow.
v.) Properties of gases are
- Gas has indefinite shape
- Gas has no fixed volume.
- Gas gets the shape and volume of container.
- Gas fills the container completely.
- Gas has very low density.
- Because of low density gas are light.
- Gas can flow easily and hence are called fluid.
Case Study 3:
3.) What happens inside the matter during change of state? On increasing the temperature of solids, the kinetic energy of the particles increases. Due to the increase in kinetic energy, the
Particles start vibrating with greater speed. The energy supplied by heat overcomes the forces of attraction between the particles. The particles leave their fixed positions and start moving more freely. A stage is reached when the solid melts and is converted to a liquid. The minimum temperature at which a solid melts to become a liquid at the atmospheric pressure is called its melting point.
The temperature of the system does not change after the melting point is reached, till all the ice melts. This happens even though we continue to heat the beaker, that is, we continue to supply heat. This heat gets used up in changing the state by overcoming the forces of attraction between the particles. The amount of heat energy that is required to change 1 kg of a solid into liquid at atmospheric pressure at its melting point is known as the latent heat of fusion. So, particles in water at 0 0 C (273 K) have more energy as compared to particles in ice at the same temperature.
The temperature at which a liquid starts boiling at the atmospheric pressure is known as its boiling point. Boiling is a bulk phenomenon. Particles from the bulk of the liquid gain enough energy to change into the vapour state. A change of state directly from solid to gas without changing into liquid state is called sublimation and the direct change of gas to solid without changing into liquid is called deposition.
i.) A change of state directly from solid to gas without changing into liquid state is called
a.) Sublimation
b.) Deposition
c.) Boiling point
ii.) The direct change of gas to solid without changing into liquid is called
iii.) The energy supplied by heat to solid is used to overcome the forces of attraction between the particles. True or false
iv.) Define melting point and boiling point
v.) Define latent heat of fusion
Answer key-3
iv.) The minimum temperature at which a solid melts to become a liquid at the atmospheric pressure is called its melting point.
The temperature at which a liquid starts boiling at the atmospheric pressure is known as its boiling point.
v.) The amount of heat energy that is required to change 1 kg of a solid into liquid at atmospheric pressure at its melting point is known as the latent heat of fusion.
Case Study 4:
4 .) Do we always need to heat or change pressure for changing the state of matter? Can you quote some examples from everyday life where change of state from liquid to vapour takes place without the liquid reaching the boiling point? In the case of liquids, a small fraction of particles at the surface, having higher kinetic energy, is able to break away from the forces of attraction of other particles and gets converted into vapour. This phenomenon of change of a liquid into vapors at any temperature below its boiling point is called evaporation.
i.) Evaporation of liquid takes place at
a.) Boiling point
b.) Above boiling point
c.) Below boiling point
ii.) Evaporation takes place at surface of liquid because
a.) They are heavy as compare to other particles
b.) They have sufficient kinetic energy to break the force
c.) They are light weight as compare to other particles
iii.) During evaporation particles of liquid change into vapour
a.) From the surface
b.) From the bottom
c.) From all over the liquid
iv.) Define evaporation.
v.) Explain process of evaporation
Answer key-4
iv.) The phenomenon of change of a liquid into vapors at any temperature below its boiling point is called evaporation.
v.) In the case of liquids, a small fraction of particles at the surface, having higher kinetic energy, is able to break away from the forces of attraction of other particles and gets converted into vapour. This phenomenon of change of a liquid into vapors at any temperature below its boiling point is called evaporation.
Case Study 5:
5.) You must have observed that the rate of evaporation increases with–
- an increase of surface area:
- We know that evaporation is a surface phenomenon. If the surface area is increased, the rate of evaporation increases. For example, while putting clothes for drying up we spread them out.
- an increase of temperature:
With the increase of temperature, more number of particles get enough kinetic energy to go into the vapour state.
In an open vessel, the liquid keeps on evaporating. The particles of liquid absorb energy from the surrounding to regain the energy lost during evaporation. This absorption of energy from the surroundings makes the surroundings cold. What happens when you pour some acetone (nail polish remover) on your palm? The particles gain energy from your palm or surroundings and evaporate causing the palm to feel cool. After a hot sunny day, people sprinkle water on the roof or open ground because the large latent heat of vaporization of water helps to cool the hot surface.
i.) Evaporation is surface phenomenon. True or false
ii.) As temperature increases the rate of evaporation is
a.) increases
b.) decreases
c.) remains constant
iii.) The rate of evaporation increases with
a.) Increase in wind speed
b.) Decrease in wind speed
c.) Does not have any effect from wind speed
iv.) What happens when you pour some acetone (nail polish remover) on your palm?
v.) We are able to sip hot tea from saucer than from cup. Why?
Answer key-5
iv.) The particles gain energy from your palm or surroundings and evaporate causing the palm to feel cool.
v.) We are able to sip hot tea from saucer than from cup. This is because saucer has large surface area, due to large surface area as compare to cut area tea evaporates at faster rate.
Thank you It helped me a lot
Why smell of Perfume is not a matter?
Because there is no particle
Because their are perfume particles suspended in air
These all case study questions are really helpful . Thanks
This is my first I was so nervous but these questions help me alot thank you
Smell of perfume is a matter because it have gas particles means perfume particles
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
We have a strong team of experienced Teachers who are here to solve all your exam preparation doubts
New trends in history and civics class 6 solutions chapter 5 the mauryan empire, of studies 2 marks questions answers, chhattisgarh state class 9 math chapter 13 solution, ncert joyful mathematics class 1 worksheet 2 – what is long, what is round.
Sign in to your account
Username or Email Address
Remember Me
Class 9 Science Chapter 1 Case Based Questions - Matter in Our Surroundings
(I) Read the given passage and answer the questions that follows based on the passage and related studied concepts. Matter is anything that occupies space and has mass. Matter is classified into solid, liquid and gas. In solid state particles are closely packed and have very strong force of attraction, particles can only vibrate and rotate around fixed positions. In liquid state, particles are less closely packed and have strong force of attraction but less than solids, particles can move throughout the liquid. In Gaseous state, particles are far apart with weak force of attraction and are in state of constant random motion. Gases can be easily compressed where as solids and liquids are incompressible. Q1: An inflated balloon is placed in refrigerator, what will happen? (a) Balloon will shrink and particles will move faster and become closer. (b) Balloon will expand and particles will move faster and become far apart. (c) Balloon will shrink, particles will move slower and become close together. (d) Balloon will expand, particles will move slower and come closer therefore, volume of balloon will decrease. Ans: (c) Balloon will shrink, particles will move slower and become close together.
When an inflated balloon is placed in the refrigerator, the temperature decreases. This causes the gas particles inside the balloon to move slower and come closer together, resulting in the balloon shrinking.
Q2: When solid changes into vapours, the process is called. (a) Evaporation (b) Boiling (c) Sublimation (d) Vapourisation Ans: (c)
Sublimation is the process by which a solid changes directly into a gas without passing through the liquid state.
Q3: A substance melts at 5°C and boils at 150°C. What will be its physical state at room temperature? Ans: Room temperature (around 25°C) is between the melting point (5°C) and the boiling point (150°C) of the substance. Therefore, the substance will be in the liquid state at room temperature.
Q4: Why do we feel more cold after taking bath with hot water? Ans: We feel more cold after taking a bath with hot water because when we step out of the hot water, the water on our skin starts to evaporate. The evaporation process requires heat, which is taken from our body, causing a cooling effect and making us feel colder.
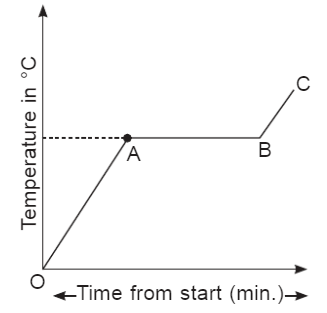
Q2: What does AB represent? Ans: It represents a mixture of liquid and vapours at the boiling point of the liquid.
Q3: Why does temperature remain constant at boiling point? Ans: The temperature remains constant at the boiling point because the heat of vaporization is used to overcome the force of attraction between liquid particles.
Q4: What does BC represent? Ans: It represents a vapour state, and particles absorb heat and become more energetic. The temperature of the gas will rise.
(III) Read the following information and answer the questions based on information and related studied concepts Substance – 1. is brittle. Substance – 2. melts at 5°C and boils at 150°C. Substance – 3. has high melting point of 800°C. Substance – 4. has melting point –169°C and boiling point –104°C.
Q1: What is physical state of substance – 4 at –150°C and –100°C? Ans:
- At –150°C: Substance – 4 will be in the solid state.
- At –100°C: Substance – 4 will be in the gaseous state.
The melting point of Substance – 4 is –169°C, and its boiling point is –104°C. Therefore, at –150°C (which is above its melting point but below its boiling point), Substance – 4 will be a solid. At –100°C (which is above its boiling point), it will be a gas.
Q2: What is physical state of substance – 1 and 3 at room temperature? Ans:
- Substance – 1: The physical state cannot be determined solely based on the information that it is brittle. However, substances that are brittle are often solids at room temperature.
- Substance – 3: Solid.
Substance – 1: Brittle substances are typically solid (e.g., certain metals, minerals, and salts). Substance – 3: With a high melting point of 800°C, it will be in the solid state at room temperature (~25°C).
Q3: What is physical state of substance – 2 at 100°? Ans: It will be in a liquid state.
The melting point of Substance – 2 is 5°C, and its boiling point is 150°C. Therefore, at 100°C, Substance – 2 will be in the liquid state.
Q4: Out of substances – 1, 2, 3, 4 which one has strongest force of attraction? Ans: Substance 3 has strongest forces of attraction.
The strength of the force of attraction between particles can be inferred from the melting point. Substance – 3 has the highest melting point (800°C), indicating that it has the strongest force of attraction between its particles.
Top Courses for Class 9
Faqs on class 9 science chapter 1 case based questions - matter in our surroundings, previous year questions with solutions, objective type questions, important questions, viva questions, extra questions, study material, video lectures, mock tests for examination, shortcuts and tricks, practice quizzes, past year papers, sample paper, semester notes.

Case Based Question Answer: Matter in Our Surroundings Free PDF Download
Importance of case based question answer: matter in our surroundings, case based question answer: matter in our surroundings notes, case based question answer: matter in our surroundings class 9 questions, study case based question answer: matter in our surroundings on the app, welcome back, create your account for free.

Forgot Password
Change country.
myCBSEguide
- Class 9 Science Case...

Class 9 Science Case Study Questions
Table of Contents
myCBSEguide App
Download the app to get CBSE Sample Papers 2023-24, NCERT Solutions (Revised), Most Important Questions, Previous Year Question Bank, Mock Tests, and Detailed Notes.
If you are wondering how to solve class 9 science case study questions, then myCBSEguide is the best platform to choose. With the help of our well-trained and experienced faculty, we provide solved examples and detailed explanations for the recently added Class 9 Science case study questions.
You can find a wide range of solved case studies on myCBSEguide, covering various topics and concepts. Class 9 Science case studies are designed to help you understand the application of various concepts in real-life situations.
The rationale behind Science
Science is crucial for Class 9 students’ cognitive, emotional, and psychomotor development. It encourages curiosity, inventiveness, objectivity, and aesthetic sense.
In the upper primary stage, students should be given a variety of opportunities to engage with scientific processes such as observing, recording observations, drawing, tabulating, plotting graphs, and so on, whereas in the secondary stage, abstraction and quantitative reasoning should take a more prominent role in science teaching and learning. As a result, the concept of atoms and molecules as matter’s building units, as well as Newton’s law of gravitation, emerges.
Science is important because it allows Class 9 Science students to understand the world around us. It helps to find out how things work and to find solutions to problems at the Class 9 Science level. Science is also a source of enjoyment for many people. It can be a hobby, a career, or a source of intellectual stimulation.
Case study questions in Class 9 Science
The inclusion of case study questions in Class 9 science CBSE is a great way to engage students in critical thinking and problem-solving. By working through real-world scenarios, Class 9 Science students will be better prepared to tackle challenges they may face in their future studies and careers. Class 9 Science Case study questions also promote higher-order thinking skills, such as analysis and synthesis. In addition, case study questions can help to foster creativity and innovation in students. As per the recent pattern of the Class 9 Science examination, a few questions based on case studies/passages will be included in the CBSE Class 9 Science Paper. There will be a paragraph presented, followed by questions based on it.
Examples of Class 9 science class case study questions
Class 9 science case study questions have been prepared by myCBSEguide’s qualified teachers. Class 9 case study questions are meant to evaluate students’ knowledge and comprehension of the material. They are not intended to be difficult, but they will require you to think critically about the material. We hope you find Class 9 science case study questions beneficial and that they assist you in your exam preparation.
The following are a few examples of Class 9 science case study questions.
Class 9 science case study question 1
- due to its high compressibility
- large volumes of a gas can be compressed into a small cylinder
- transported easily
- all of these
- shape, volume
- volume, shape
- shape, size
- size, shape
- the presence of dissolved carbon dioxide in water
- the presence of dissolved oxygen in the water
- the presence of dissolved Nitrogen in the water
- liquid particles move freely
- liquid have greater space between each other
- both (a) and (b)
- none of these
- Only gases behave like fluids
- Gases and solids behave like fluids
- Gases and liquids behave like fluids
- Only liquids are fluids
Answer Key:
- (d) all of these
- (a) shape, volume
- (b) the presence of dissolved oxygen in the water
- (c) both (a) and (b)
- (c) Gases and liquids behave like fluids
Class 9 science case study question 2
- 12/32 times
- 18 g of O 2
- 18 g of CO 2
- 18 g of CH 4
- 1 g of CO 2
- 1 g of CH 4 CH 4
- 2 moles of H2O
- 20 moles of water
- 6.022 × 1023 molecules of water
- 1.2044 × 1025 molecules of water
- (I) and (IV)
- (II) and (III)
- (II) and (IV)
- Sulphate molecule
- Ozone molecule
- Phosphorus molecule
- Methane molecule
- (c) 8/3 times
- (d) 18g of CH 4
- (c) 1g of H 2
- (d) (II) and (IV)
- (c) phosphorus molecule
Class 9 science case study question 3
- collenchyma
- chlorenchyma
- It performs photosynthesis
- It helps the aquatic plant to float
- It provides mechanical support
- Sclerenchyma
- Collenchyma
- Epithelial tissue
- Parenchyma tissues have intercellular spaces.
- Collenchymatous tissues are irregularly thickened at corners.
- Apical and intercalary meristems are permanent tissues.
- Meristematic tissues, in its early stage, lack vacuoles, muscles
- (I) and (II)
- (III) and (I)
- Transpiration
- Provides mechanical support
- Provides strength to the plant parts
- None of these
- (a) Collenchyma
- (b) help aquatic plant to float
- (b) Sclerenchyma
- (d) Only (III)
- (c) provide strength to plant parts
Cracking Class 9 Science Case Study Questions
There is no one definitive answer to Class 9 Science case study questions. Every case study is unique and will necessitate a unique strategy. There are, nevertheless, certain general guidelines to follow while answering case study questions.
- To begin, double-check that you understand the Class 9 science case study questions. Make sure you understand what is being asked by reading it carefully. If you’re unclear, seek clarification from your teacher or tutor.
- It’s critical to read the Class 9 Science case study material thoroughly once you’ve grasped the question. This will provide you with a thorough understanding of the problem as well as the various potential solutions.
- Brainstorming potential solutions with classmates or other students might also be beneficial. This might provide you with multiple viewpoints on the situation and assist you in determining the best solution.
- Finally, make sure your answer is presented simply and concisely. Make sure you clarify your rationale and back up your claim with evidence.
A look at the Class 9 Science Syllabus
The CBSE class 9 science syllabus provides a strong foundation for students who want to pursue a career in science. The topics are chosen in such a way that they build on the concepts learned in the previous classes and provide a strong foundation for further studies in science. The table below lists the topics covered in the Class 9 Science syllabus of the Central Board of Secondary Education (CBSE). As can be seen, the Class 9 science syllabus is divided into three sections: Physics, Chemistry and Biology. Each section contains a number of topics that Class 9 science students must study during the course.
CBSE Class 9 Science (Code No. 086)
Theme: Materials Unit I: Matter-Nature and Behaviour Definition of matter; solid, liquid and gas; characteristics – shape, volume, density; change of state-melting (absorption of heat), freezing, evaporation (cooling by evaporation), condensation, sublimation. Nature of matter: Elements, compounds and mixtures. Heterogeneous and homogenous mixtures, colloids and suspensions. Particle nature and their basic units: Atoms and molecules, Law of constant proportions, Atomic and molecular masses. Mole concept: Relationship of mole to mass of the particles and numbers. Structure of atoms: Electrons, protons and neutrons, valency, the chemical formula of common compounds. Isotopes and Isobars.
Theme: The World of the Living Unit II: Organization in the Living World Cell – Basic Unit of life: Cell as a basic unit of life; prokaryotic and eukaryotic cells, multicellular organisms; cell membrane and cell wall, cell organelles and cell inclusions; chloroplast, mitochondria, vacuoles, endoplasmic reticulum, Golgi apparatus; nucleus, chromosomes – basic structure, number. Tissues, Organs, Organ System, Organism: Structure and functions of animal and plant tissues (only four types of tissues in animals; Meristematic and Permanent tissues in plants).
Theme: Moving Things, People and Ideas Unit III: Motion, Force and Work Motion: Distance and displacement, velocity; uniform and non-uniform motion along a straight line; acceleration, distance-time and velocity-time graphs for uniform motion and uniformly accelerated motion, derivation of equations of motion by graphical method; elementary idea of uniform circular motion. Force and Newton’s laws: Force and Motion, Newton’s Laws of Motion, Action and Reaction forces, Inertia of a body, Inertia and mass, Momentum, Force and Acceleration. Elementary idea of conservation of Momentum. Gravitation: Gravitation; Universal Law of Gravitation, Force of Gravitation of the earth (gravity), Acceleration due to Gravity; Mass and Weight; Free fall. Floatation: Thrust and Pressure. Archimedes’ Principle; Buoyancy. Work, energy and power: Work done by a Force, Energy, power; Kinetic and Potential energy; Law of conservation of energy. Sound: Nature of sound and its propagation in various media, speed of sound, range of hearing in humans; ultrasound; reflection of sound; echo.
Theme: Food Unit IV: Food Production Plant and animal breeding and selection for quality improvement and management; Use of fertilizers and manures; Protection from pests and diseases; Organic farming.
PRESCRIBED BOOKS:
- Science-Textbook for class IX-NCERT Publication
- Assessment of Practical Skills in Science-Class IX – CBSE Publication
- Laboratory Manual-Science-Class IX, NCERT Publication
- Exemplar Problems Class IX – NCERT Publication
myCBSEguide: A true helper
There are numerous advantages to using myCBSEguide to achieve the highest results in Class 9 Science.
- myCBSEguide offers high-quality study materials that cover all of the topics in the Class 9 Science curriculum.
- myCBSEguide provides practice questions and mock examinations to assist students in the best possible preparation for their exams.
- On our myCBSEguide app, you’ll find a variety of solved Class 9 Science case study questions covering a variety of topics and concepts. These case studies are intended to help you understand how certain principles are applied in real-world settings
- myCBSEguide is that the study material and practice problems are developed by a team of specialists who are always accessible to assist students with any questions they may have. As a result, students may be confident that they will receive the finest possible assistance and support when studying for their exams.
So, if you’re seeking the most effective strategy to study for your Class 9 Science examinations, myCBSEguide is the place to go!
Test Generator
Create question paper PDF and online tests with your own name & logo in minutes.
Question Bank, Mock Tests, Exam Papers, NCERT Solutions, Sample Papers, Notes
Related Posts
- Competency Based Learning in CBSE Schools
- Class 11 Physical Education Case Study Questions
- Class 11 Sociology Case Study Questions
- Class 12 Applied Mathematics Case Study Questions
- Class 11 Applied Mathematics Case Study Questions
- Class 11 Mathematics Case Study Questions
- Class 11 Biology Case Study Questions
- Class 12 Physical Education Case Study Questions
Leave a Comment
Save my name, email, and website in this browser for the next time I comment.

IMAGES
VIDEO