- Research article
- Open access
- Published: 03 February 2021

Balloon occluded TACE (B-TACE) vs DEM-TACE for HCC: a single center retrospective case control study
- Pierleone Lucatelli ORCID: orcid.org/0000-0002-7448-1404 1 ,
- Gianluca De Rubeis 1 ,
- Bianca Rocco 1 ,
- Fabrizio Basilico 1 ,
- Alessandro Cannavale 1 ,
- Aurelio Abbatecola 2 ,
- Pier Giorgio Nardis 1 ,
- Mario Corona 1 ,
- Stefania Brozzetti 3 ,
- Carlo Catalano 1 &
- Mario Bezzi 1
BMC Gastroenterology volume 21 , Article number: 51 ( 2021 ) Cite this article
3543 Accesses
14 Citations
Metrics details
A Correction to this article was published on 09 July 2021
This article has been updated
To compare oncological results and safety profile of balloon micro-catheter trans-arterial chemoembolization (b-TACE) and drug-eluting-microsphere (DEM-TACE) in patients with hepatocellular-carcinoma (HCC).
This is a case–control, retrospective, single-center study. Between January-2015/March-2019, 149 patients (131 males [87.9%]) with 226 HCC were treated, 22 patients (35 HCC; 19 [86.4%] males) with b-TACE and 127 with DEM-TACE (191 HCC, 112 [88.2%] males). Embolization protocol was standardized (sequential 100 ± 25 and 200 ± 25 μm microspheres). Results were evaluated by modified-response-evaluation-criteria-in-solid-tumor [mRECIST] at 1, 3–6 and 9–12 months and time to recurrence after complete response [TTR] at 1 years. Cox’s regression weighted with tumor dimensions was performed. Adverse events (AEs) were recorded.
mRECIST oncological response at all time points (1, 3–6 and 9–12 months) for both treatments were similar, with the exception of Objective response rate at 9-12 months. Objective response at 1 and 3–6 months between b-TACE vs DEM-TACE [23/35 (65.7%) vs 119/191 (62.3%), 21/29 (72.4%) vs 78/136 (57.4%) ( p > 0.05), respectively]. On the contrary, at 9–12 months, it was significantly higher in b-TACE subgroup than DEM-TACE (15/19 [78.9%] vs 48/89 [53.9%], p = 0.05). TTR for complete response at 1 year had a better trend for b-TACE vs DEM-TACE (278.0 days [196.0–342.0] vs 219.0 days [161.0–238.0], OR 0.68 [0.4–1.0], p = 0.10). The use of balloon micro-catheter reduced the relative risk of the event of recurrence by 0.63 [CI95% 0.38–1.04]; p = 0.07). No significant differences were found in AEs rate.
b-TACE showed a trend of better oncological response over DEM-TACE with and longer TTR with a similar adverse events rate, in patients presenting with larger tumors.
Peer Review reports
Hepatocellular carcinoma (HCC), with definitive diagnosis (LI-RADS-5) according to Liver Imaging Reporting and Data System (LI-RADS)[ 1 , 2 ] are staged according to Barcelona Clinic Liver Cancer (BCLC) staging system [ 3 ]. BCLC algorithm treatment of choice of Intermediate stage (B stage) HCC is trans-arterial chemoembolization (TACE).
Recently, the use of a balloon micro-catheter for temporary arterial occlusion has been proposed for TACE (named b-TACE procedure) [ 4 ]. The temporary arterial occlusion may enhance treatment success, due to its ability to redistribute flow towards lower resistance vascular territories (i.e. hyper-vascular HCC), thus allowing a pressure-gradient driven embolization [ 4 ] The increased accumulation of embolic particles within the tumor may lead to increased necrosis and increased rates of complete tumor response.
To date there are no randomized controlled trials comparing TACE to b-TACE in terms of oncological response; moreover, some retrospective studies reported conflicting results. [ 5 ] Ogawa et a l[ 6 ] and Irie et al. [ 7 ] showed a better tumor response for b-TACE performed with Lipiodol in comparison to Lipiodol TACE performed with a standard catheter. Maruyama et al. [ 8 ], on the other hand, failed to demonstrate a difference in tumor control between the two techniques.
The literature regarding the use of this balloon micro-catheter in combination with Drug Eluting microsphere (DEM-TACE) is scarce. To our knowledge, only two studies reported the use of DEM-TACE with a balloon micro-catheter [ 9 , 10 ] with an objective response of 90% and 100%, respectively. There is currently no evidence on which patients should be offered b-TACE, particularly when the procedure is performed with DEM. This is of particular relevance because the patients included in the BCLC B stage may have a broad spectrum of disease presentations, which may result in lower effectiveness of catheter-based treatments; for example, large tumors (> 50 mm), multiple tumors (> 3) and elevated baseline α-fetoprotein level are all associated with failure to achieve a complete response [ 11 , 12 , 13 ]. This is extremely important to understand, since HCC patients with initial complete response after TACE have the longest overall survival, in comparison to other mRECIST response categories [ 11 ].
The purpose of our work was to retrospectively analyse in a case–control, retrospective, single center study the results obtained in two groups of HCC patients who underwent catheter based treatment with drug eluted microsphere with a standard micro-catheter and with the use of a balloon micro-catheter (DEM-TACE versus b-TACE).
The primary outcome was to compare results in patients treated with b-TACE and DEM-TACE, in terms of oncological response, and time to recurrence (TTR) after complete response. The secondary outcome was to compare differences in terms of safety profile between the two techniques including post-procedural changes of liver function tests, post-embolic syndrome (PES) and incidence of adverse events.
This study was approved by the ethical review board of our Institution. Informed consent for the procedure and for anonymized publication of non-sensitive data was obtained from all individual patients.
This is a case–control, retrospective, single center study.The data of 159 consecutive patients with 248 LI-RADS-5 HCC tumors managed in our tertiary center for liver cancer treatment between January 2015 and March-2019 were reviewed. All TACE indications were discussed at the multidisciplinary tumor board comprising a transplant surgeon, an interventional radiologist, body radiologist and a hepatologist, according to the Quality Improvement Guidelines for Hepatic Transarterial Chemoembolization of the CIRSE [ 12 ].
Inclusion criteria were: Child–Pugh score up to B8, Barcelona Clinic Liver Cancer (BCLC) stage up to B,not eligible for curative treatments (surgical resection or percutaneous ablative treatments). Patients presenting with Child–Pugh > B8, BCLC stage C, portal vein thrombosis (defined as the complete or partial obstruction of blood flow in the portal vein, due to the presence of a chronic, acute or neoplastic thrombus), extrahepatic secondary lesions, and high-flow arterioportal or arteriovenous shunts, previous systemic treatments, platelet count < 50,000, and bilirubin level > 3 mg/dL, were not considered suitable for the procedure.
Ten patients who underwent TACE with degradable starch microsphere were excluded. The final study population included 149 patients with 226 HCC. Twenty-two patients (35 HCC tumors; median of 1.6 tumor/patient) were treated with b-TACE (DEM TACE with balloon occlusion) while 127 patients with 191 HCC tumors (median of 1.5 tumors per patient) received standard catheter DEM-TACE without balloon occlusion. Patients’ demographic and clinical characteristics are reported in Table 1 .
All DEM TACE procedures from January 2015 to April 2018 were performed without the use of a balloon micro-catheter for temporary arterial occlusion. The balloon micro-catheter was available at our Institution from April 2018. Considering that there are no recommendations or guidelines for using a balloon micro-catheter for temporary arterial occlusion during DEM-TACE, the decision to use it was left to the Interventional Radiologist preference at the time of the procedure. The embolization protocol at our institution (see following paragraph) was standardized since January 2015.
DEM-TACE and B-TACE technique
All procedures were performed via femoral access by two experienced Interventional Radiologist (experience > 10 years). After positioning a 4F angiographic catheter in the common/proper hepatic artery, a detailed tumor’s feeder map was performed by digitally subtraction angiography and dual-phase cone beam CT.
After careful identification of the tumor feeders, super-selective catheterization was performed with a 2.7 F micro-catheter (Progreat; Terumo Europe NV, Leuven, Belgium) for DEB-TACE and with a 2.8 F balloon micro-catheter (Occlusafe, Terumo Europe NV, Leuven, Belgium) for B-TACE [ 10 ].
The embolization protocol used, for both B-TACE and DEM-TACE, was highly standardized since January-2015. The protocol consisted, as previously reported[ 14 ], in a sequential embolization, starting with 100 ± 25 μm PEG microspheres, immediately followed by a second embolization with 200 ± 50 μm, PEG microspheres when needed.
The technical embolization endpoint differs in the two procedures: for DEB-TACE was flow stasis considered as stasis for 10 heartbeats. If stasis was achieved with the injection of 100 μm ± 25 particles, the adjunctive injection of 200 μm ± 50 microspheres was not performed. For b-TACE, the endpoint was different, due to the presence of the inflated balloon micro-catheter that impaired the assessment of flow stasis. Therefore, for this procedure, we used a composite endpoint: upstream reflux of microspheres despite balloon inflation, visualization of vascular anastomosis that could determine potential non-target embolization and manual perception of resistance to the injection of the microspheres [ 10 ].
Follow-up imaging
Imaging follow-up was performed using either contrast enhanced multi-detector computed tomography (MDCT) or contrast enhanced magnetic resonance imaging (CE-MRI) with the use of hepatobiliary contrast agents, according to our institutional protocol (follow-up at 1 month, 3 months and after that every 3–6 months). The response was evaluated according to mRECIST criteria by a radiologists with > 20 years’ experience in CT/MR body imaging as follow: Complete Response (CR) was considered as disappearance of any intra-tumoral arterial enhancement in all target lesions; Partial Response (PR) as a decrease > 30% in the sum of diameters of viable target lesions (taking as reference the baseline sum of the diameters of target lesions); Stable disease (SD) as any cases that do not qualify for either PR or progressive disease (PD), and PD as an increase of at least 20% in the sum of the diameters of viable target lesions (taking as reference the smallest sum of the diameters of viable target lesions recorded since treatment started). Objective response is defined as CR + PR rate; disease control (DC) is defined as CR + PR + SD rate [ 15 , 16 ].
Study outcomes and potential confounders
The primary outcome was to compare the oncological results according to mRECIST criteria for patients treated with b-TACE vs DEB-TACE, in terms of oncological response and TTR after complete response. The TTR was calculated at the 1-year follow-up check-point.
Hepatic function of the patients and radiological tumors’ characteristics were potential confounders. Therefore, differences in hepatic function (summarized in Table 2 ) and radiological tumors’ characteristics (summarized in Table 3 ) between the two cohorts were considered as co-variants in the statistical analysis only if statistically different; in particular tumor size, which is considered the most important predictive factor for TACE outcome [ 12 ].
The secondary outcome was to compare differences in terms of safety profile between the two techniques including modifications of post-procedural liver function test, occurrence of post-embolic syndrome (PES) and adverse event. PES was defined as fever and/or nausea and/or pain presenting up to 48 h after the procedures[ 10 ]. Adverse events (AEs) were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAEv5) [ 17 ].
Statistical analysis
The Kolmogorov–Smirnov Z test was used to assess normality distribution for all variables tested. Continuous normal variables were expressed as mean ± standard deviation. Continuous non-normal variables were expressed as median and confidential interval (CI) 95%. Oncological response was compared using chi-square test at three time points (1 months, 3–6 months, and 9–12 months) on nodule-based analysis (Bonferroni’s correction for post-hoc analysis). For matching pre and post laboratory analysis, the Student T test and the Wilcoxon rank-sum test were used as appropriate according to distribution. A logistic regression was performed for analyzing the impact of hepatic status (MELDNa), gender, age, biochemical tumor spread (AFP), radiological tumor impact (DM max) and presence of micro-balloon catheter on objective response a 9–12 months. For comparing laboratory analysis (in fold modification) and oncological response, between DEM-TACE and B-TACE, Student T test and a Mann–Whitney test were used as appropriate. Chi-square test was used for likening adverse events between the two groups. The PFS was evaluated with Kaplan–Meier curve and Cox’s regression using as tumor dimensions as covariate. Statistical analysis was performed, and the graph was plotted using MedCalc 18.2.1 (MedCalc Software bvba, Ostend, Belgium). P values < 0.05 were considered statistically significant, and all P values were calculated using a two-tailed significance level.
The study cohort was composed of 149 patients with 226 HCC tumors (B-TACE vs DEM-TACE, 22 vs 127 patients, 35 vs 191 HCC tumors, respectively).
The only statistical difference variable between b-TACE and DEM-TACE cohorts was the median maximum diameter of HCC tumors treated in the B-TACE group compared to DEM-TACE arm (27.0 mm [CI 95% 21.6–32.4] vs 19.0 mm [CI 95% 17.0–20.0]; p < 0.0001; median difference: 8.0 mm [CI95% 4.0–12.0]). All the other tumor and clinical characteristics where similar in both groups (see Tables 1 and 3 for details).
Oncological results
Per-nodule analysis demonstrated no significant differences in the oncological response at all time points (1, 3–6 and 9–12 months) for both treatments, with the exception of Objective response rate at 9-12 months. In particular: Complete response was [b-TACE vs DEM-TACE] 14/35 (40.0%) vs 81/191 (42.4%) at 1 month, 13/29 (44.8%) vs 62/136 (45.6%) at 3–6 months and 13/19 (68.4%) vs 45/89 (50.6%) at 9–12 months ( p > 0.05). Objective response was similar at 1 and 3–6 months between b-TACE vs DEM-TACE [23/35 (65.7%) vs 119/191 (62.3%), 21/29 (72.4%) vs 78/136 (57.4%) ( p > 0.05), respectively]. On the contrary, at 9–12 months, it was significantly higher in b-TACE subgroup than DEM-TACE (15/19 [78.9%] vs 48/89 [53.9%], p = 0.05) (see Table 4 for detailed data) (Fig. 1 .). Stable disease was significantly higher for DEM-TACE vs b-TACE at 9–12 months (30.3% vs 0%, p = 0.0006), however disease control remained not statistically significant due to compensation of Complete and Objective Response in b-TACE group. No statistical significancy were found regarding the presence of the balloon micro-catheter in the logistic regression for objective response at 9–12 months (OR 1.70 [CI95% 0.32–8.96], p = 0.53) and for the remaining parameters (MELDNa, gender, AFP, age and max diameter; OR 0.82 [CI95% 0.66–1.03]; 4.27 [CI95% 0.78–23.4]; 1.00 [CI95% 0.99–1.00]; 1.01 [CI95% 0.95–1.06]; 1.01 [CI95% 0.97–1.06]).
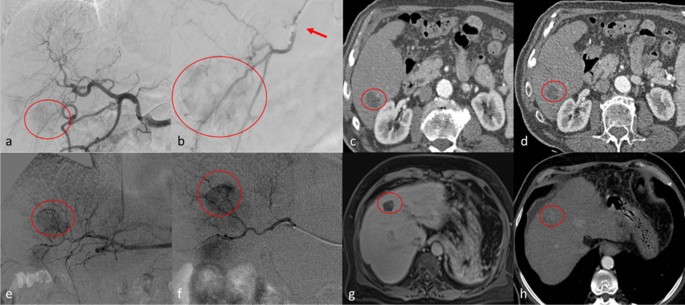
Top row. Clinical case of a 54 years old male with hepatocellular carcinoma [HCC] (diameters: 25 × 23 mm) in segment 6. a digital subtraction angiography (DSA) obtained with injection from common hepatic artery demonstrates a hyper vascular tumor (circle); b selective DSA with inflated balloon micro-catheter (arrow) confirms the HCC (circle). c , d show the arterial phase of contrast enhanced computed tomography which demonstrate complete response at 1 month ( c ) and persisting complete response at 6 months ( d ). Bottom row. Clinical case of a 61 years old female with HCC (diameters: 22 × 21 mm) in the segment 4. e , f DSA from common hepatic artery and super-selective DSA with micro-catheter, respectively, demonstrate the HCC (circle). g MR imaging follow-up in hepatobiliary phase shows complete response at 1 month (circle); h at 6 months follow-up, computed tomography in arterial phase shows only a partial response
The median follow-up time was 143 days (CI95% 132.0–154.0), which is higher in b-TACE comparing with DEM-TACE (162.5 days [CI95% 134.2–227.9] vs 132.0 days [98.6–154.0], p = 0.03). Only 108/226 (47.8%) reached the timeframe of 9–12 months follow-up. There was a trend for better median TTR for b-TACE vs DEM-TACE for complete response at 9–12 months (278.0 days [196.0–342.0] vs 219.0 days [161.0–238.0], odd ratio [OR] 0.68 [0.4–1.0], p = 0.10). (Fig. 2 .). This higher trend of TTR was confirmed by Cox-regression with a relative risk of event of 0.63 (CI95% 0.38–1.04, p = 0.07) for the presence of the micro-balloon catheter and of 1.0 (CI95% 0.99–1.02, p = 0.46) for tumors’ dimension (Fig. 2 .).

Kaplan Meier analysis of time to recurrence for complete response at 9–12 months follow-up ( a ). b Showed the Cox-regression analysis weighted for the presence/absence of micro-balloon catheter and tumor dimension confirming the better trend of time to recurrence for b-TACE comparing to DEM-TACE
- Safety profile
Adverse events were observed without significant difference between B-TACE and DEM-TACE (grade 3: 1/22 [4.5%] vs 3/127 [2.4%] and grade 2: 4/22 [18.1%] vs 20/127 [15.7%], p > 0.05, respectively). In particular, a pseudo-aneurysm was recorded for a B-TACE procedure, and an intra-hepatic artery dissection and acute renal insufficiency requiring dialysis were observed in two DEM-TACE procedures. PES was experienced by 8/22 (36.4%) of B-TACE patients and 32/127 (25.2%) of DEM-TACE ( p = 0.28).
Regarding laboratory values, no statistically significant differences were found between the two interventions for all parameters considered at post-procedural evaluation (see Table 2 . For details).
The analysis of our data shows that b-TACE has a trend of better oncological response over DEM-TACE. This was supported by an improvement in long term oncological response (Objective response 78,9% [b-TACE] vs 58,9% [DEM-TACE] at 9–12 months, p = 0.05) and a longer Time to Progression (TTR) after Complete Response over standard non-occluded DEM-TACE. This is of particular relevance, considering that b-TACE cohort included larger size tumors (mean diameter: 27 mm [b-TACE] vs 19 mm [DEM-TACE]), and that the adverse events rate were comparable between two techniques.
Transarterial chemoembolization (TACE) represent the standard of care for intermediate HCC. Its aim is to locally deliver to the target lesion the maximum amount of drugs and non-re-absorbable microspheres, thus permitting local tumor control. Recently b-TACE, thanks to its ability to redistribute flow towards lower resistance vascular territories and allowing a pressure-gradient driven embolization, has demonstrated to be capable to improve drug delivery to target lesion[ 4 ]. This technical benefit should theoretically enhance the ability to locally control tumor growth. Despite this, literature evidence on oncological response of b-TACE over standard non occluded TACE is controversial[ 5 , 7 , 8 , 18 ]
In order to evaluate the adjunctive value of b-TACE we retrospectively evaluated the results of patients treated in our institution with b-TACE and compared them with an historical cohort treated with non-occluded DEM-TACE. b-TACE and DEM-TACE were performed by the same team under dual phase CBCT guidance i.e..: better tumor/feeders visualization) [ 19 ], with rigorous standardization of the embolization procedure (sequential embolization with 100 and 200 microns particles[ 14 ]), being the only technical variable the balloon micro-catheter employment. Moreover by comparing our study to the one reported by Irie et al.[ 7 ], the only that compared superselective b-TACE to superselective TACE (both performed with Lipiodol emulsion), emerges several differences. First, the embolic agent is different; second we enrolled a larger control population; third, mean diameter of the treated nodule are different, in particular: in our study treated nodule are smaller in both group (b-TACE 27 mm; TACE 19 mm) if compared to the Irie’s one (b-TACE 39 mm; TACE 40 mm); finally nodule treated with TACE in the Irie’s series were not naïve. All these variables render direct comparison of the study results limited.
With regards to the clinical response, b-TACE demonstrated an improvement in oncological response at 9–12 months (Objective response 78.9% [b-TACE] vs 58,9% [DEM-TACE], p = 0.05), whereas at other time points (1, 3–6 months) we didn’t observed statistically different response rates. Moreover, B-TACE cohort’s tumor had a larger median diameter compared to DEM-TACE (8.0 mm [CI95% 4.0–12.0]). This is particularly relevant considering that tumors’ size is one the major factors influencing oncological response after TACE (odds ratio per centimeters [OR] 2.85, p = 0.002) [ 20 ] and overall response (OR) is strongly correlated with positive clinical outcomes (recurrence rate: 35.8% [non-responder and tumors > 3 cm] vs 11.9% [responder and tumors > 3 cm]) [ 21 ]. Although, the logistic regression using objective response at 9–12 months as outcome showed no significancy for the presence of the balloon micro-catheter.
B-TACE had a trend for higher TTR after an initial complete response vs DEM-TACE at 1-year, confirmed also by the Cox-regression analyses weighted for the presence of micro-balloon catheter and tumors’ diameter. This should be explained by several reasons: i) B-TACE procedures were performed by positioning the device proximal to all tumor’s feeders, thus less selective than DEM-TACE procedures, therefore allowing for better pharmacological coverage of the area immediately surrounding the HCC tumors; ii) complete response tumors received a more targeted dose of drug and particles due to pressure gradient driven embolization that improves distribution to the tumoral vasculature [ 5 ]. This result is of particular importance considering that a complete response after first chemoembolization is still the most robust predictor for long-term favorable outcome (Overall Survival) in hepatocellular carcinoma according to Kim et al.[ 11 ]. In addition, it could play a role in maintaining patients in active transplantation list for longer time.
No differences were observed between B-TACE and DEM-TACE in terms of AEs. Of note, the grade 3 AEs (pseudo-aneurysm) observed in the B-TACE subgroup occurred during the learning curve of balloon micro-catheter usage (within the first five cases) [ 10 , 22 ]. It is to be noted that also during DEM-TACE procedures grade 3 hepatic artery injury (defined as occlusions) occurred [8/205 (3.9%) after 2 sessions of TACE] as reported by Suh et al.[ 23 ]. Regarding PES, both groups had a similar percentage of incidence (36.4% and 25.2%), and this is in accordance with the existing literature regarding DEM-TACE (range 24.7%-75%)[ 24 ]. Both sub-groups of this study experienced a transient rise of AST, ALT, and neutrophils, and no single parameter increased more than 1.5 fold (CTCAEv5 grade 1). This findings are comparable with published literature[ 24 ]. Moreover B-TACE patients experienced a slight increase of bilirubin and direct bilirubin (fold: 1.2 (0.7–1.3) and 1.2 (0.8–1.4), respectively), reflecting a possible major impact of the embolization performed with the micro-balloon on the biliary tree. In fact, the peri-biliary plexus is one of the intrahepatic collateral pathways that open after balloon inflation[ 5 ]. For this reasons several authors [ 8 ] [ 25 ], advised extra caution when using a balloon micro-catheter to perform-TACE in patients with bile duct dilatation[ 5 ].
This study presents some limitations. First, the nature of the study is retrospective and observational without randomization. Second, groups were not homogenous, though this limitation was overcome by weighting differences as co-variate in statistical analysis.
Conclusions
B-TACE had a better objective response at 9–12 months and higher TTR after CR at 1-year in comparison to DEM-TACE, with a similar AEs rate, in patients presenting with larger tumors. These findings suggest a potential advantage of B-TACE for patients with larger tumors. If these results will be confirmed in on-going large-scale studies, B-TACE may be offered as a safe and effective alternative to current standard catheter TACE in selected patients.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Change history
09 july 2021.
A Correction to this paper has been published: https://doi.org/10.1186/s12876-021-01861-y
Abbreviations
trans-catheter-arterial chemoembolization
balloon-occluded trans-catheter arterial chemoembolization
drug eluting microsphere trans arterial chemoembolization
hepatocellular-carcinoma
modified-response-evaluation-criteria-in-solid-tumor
time to recurrence
barcelona clinic liver cancer
post-embolic syndrome
multi detector computed tomography
contrast enhanced magnetic resonance imaging
complete response
partial response
stable disease
progressive disease
adverse events
common terminology criteria for adverse events
Elsayes KM, Kielar AZ, Elmohr MM, Chernyak V, Masch WR, Furlan A, Marks RM, Cruite I, Fowler KJ, Tang A, et al. White paper of the Society of Abdominal Radiology hepatocellular carcinoma diagnosis disease-focused panel on LI-RADS v2018 for CT and MRI. Abdom Radiol (New York). 2018;43(10):2625–42.
Article Google Scholar
Abdel Razek AAK, El-Serougy LG, Saleh GA, Shabana W, Abd El-Wahab R. Liver imaging reporting and data system version 2018: what radiologists need to know. J Comput Assist Tomogr. 2020;44(2):168–77.
Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30(1):61–74.
Article CAS Google Scholar
Irie T, Kuramochi M, Takahashi N. Dense accumulation of lipiodol emulsion in hepatocellular carcinoma nodule during selective balloon-occluded transarterial chemoembolization: measurement of balloon-occluded arterial stump pressure. Cardiovasc Intervent Radiol. 2013;36(3):706–13.
Hatanaka T, Arai H, Kakizaki S. Balloon-occluded transcatheter arterial chemoembolization for hepatocellular carcinoma. World J Hepatol. 2018;10(7):485–95.
Ogawa M, Takayasu K, Hirayama M, Miura T, Shiozawa K, Abe M, Matsumoto N, Nakagawara H, Ohshiro S, Yamamoto T, et al. Efficacy of a microballoon catheter in transarterial chemoembolization of hepatocellular carcinoma using miriplatin, a lipophilic anticancer drug: Short-term results. Hepatol Res. 2016a;46(3):E60-69.
Irie T, Kuramochi M, Kamoshida T, Takahashi N. Selective balloon-occluded transarterial chemoembolization for patients with one or two hepatocellular carcinoma nodules: retrospective comparison with conventional super-selective TACE. Hepatol Res. 2016;46(2):209–14.
Maruyama M, Yoshizako T, Nakamura T, Nakamura M, Yoshida R, Kitagaki H. Initial experience with balloon-occluded trans-catheter arterial chemoembolization (B-TACE) for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2016;39(3):359–66.
Goldman DT, Singh M, Patel RS, Nowakowski FS, Bishay V, Ranade M, Lookstein RA, Fischman AM. Balloon-occluded transarterial chemoembolization for the treatment of hepatocellular carcinoma: a single-center US preliminary experience. J Vasc Interv Radiol. 2019;30(3):342–6.
Lucatelli P, Ginnani Corradini L, De Rubeis G, Rocco B, Basilico F, Cannavale A, Nardis PG, Corona M, Saba L, Catalano C, et al. Balloon-occluded transcatheter arterial chemoembolization (b-TACE) for hepatocellular carcinoma performed with polyethylene-glycol epirubicin-loaded drug-eluting embolics: safety and preliminary results. Cardiovasc Intervent Radiol. 2019;42(6):853–62.
Kim BK, Kim SU, Kim KA, Chung YE, Kim MJ, Park MS, Park JY, Kim DY, Ahn SH, Kim MD, et al. Complete response at first chemoembolization is still the most robust predictor for favorable outcome in hepatocellular carcinoma. J Hepatol. 2015;62(6):1304–10.
Golfieri R, Renzulli M, Mosconi C, Forlani L, Giampalma E, Piscaglia F, Trevisani F, Bolondi L. Hepatocellular carcinoma responding to superselective transarterial chemoembolization: an issue of nodule dimension? J Vasc Intervent Radiol. 2013;24(4):509–17.
Golfieri R, Cappelli A, Cucchetti A, Piscaglia F, Carpenzano M, Peri E, Ravaioli M, D’Errico-Grigioni A, Pinna AD, Bolondi L. Efficacy of selective transarterial chemoembolization in inducing tumor necrosis in small (<5 cm) hepatocellular carcinomas. Hepatology. 2011;53(5):1580–9.
Lucatelli P, Argirò R, De Rubeis G, Rocco B, Corradini SG, Corona M, Nardis PG, Saba L, Mennini G, Fiorentino F. Polyethylene glycol epirubicin-loaded transcatheter arterial chemoembolization procedures utilizing a combined approach with 100 and 200 μm microspheres: a promising alternative to current standards. J Vasc Interv Radiol. 2019;30(3):305–13.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60.
Abdel Razek AAK, El-Serougy LG, Saleh GA, Abd El-Wahab R, Shabana W. Interobserver agreement of magnetic resonance imaging of liver imaging reporting and data system version 2018. J Comput Assist Tomogr. 2020;44(1):118–23.
Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
Ogawa M, Takayasu K, Hirayama M, Miura T, Shiozawa K, Abe M, Matsumoto N, Nakagawara H, Ohshiro S, Yamamoto T. Efficacy of a microballoon catheter in transarterial chemoembolization of hepatocellular carcinoma using miriplatin, a lipophilic anticancer drug: Short-term results. Hepatol Res. 2016b;46(3):E60–9.
Lucatelli P, Argiro R, Bascetta S, Saba L, Catalano C, Bezzi M, Levi Sandri GB. Single injection dual phase CBCT technique ameliorates results of trans-arterial chemoembolization for hepatocellular cancer. Transl Gastroenterol Hepatol. 2017;2:83.
Hu HT, Kim JH, Lee LS, Kim KA, Ko GY, Yoon HK, Sung KB, Gwon DI, Shin JH, Song HY. Chemoembolization for hepatocellular carcinoma: multivariate analysis of predicting factors for tumor response and survival in a 362-patient cohort. J Vasc Intervent Radiol JVIR. 2011;22(7):917–23.
Kim DJ, Clark PJ, Heimbach J, Rosen C, Sanchez W, Watt K, Charlton MR. Recurrence of hepatocellular carcinoma: importance of mRECIST response to chemoembolization and tumor size. Am J Transpl. 2014;14(6):1383–90.
Matsumoto T, Endo J, Hashida K, Ichikawa H, Kojima S, Takashimizu S, Watanabe N, Yamagami T, Hasebe T. Balloon-occluded transarterial chemoembolization using a 1.8-French tip coaxial microballoon catheter for hepatocellular carcinoma: technical and safety considerations. Min Invas Therapy Allied Technol. 2015;24(2):94–100.
Suh CH, Shin JH, Yoon HM, Yoon HK, Ko GY, Gwon DI, Kim JH, Sung KB. Angiographic evaluation of hepatic arterial injury after cisplatin and Gelfoam-based transcatheter arterial chemoembolization for hepatocellular carcinoma in a 205 patient cohort during a 6-year follow-up. Br J Radiol. 2014;87(1041):20140054–20140054.
Xie Z-B, Wang X-B, Peng Y-C, Zhu S-L, Ma L, Xiang B-D, Gong W-F, Chen J, You X-M, Jiang J-H, et al. Systematic review comparing the safety and efficacy of conventional and drug-eluting bead transarterial chemoembolization for inoperable hepatocellular carcinoma. Hepatol Res. 2015;45(2):190–200.
Hatanaka T, Arai H, Shibasaki M, Tojima H, Takizawa D, Toyoda M, Takayama H, Abe T, Sato K, Kakizaki S. Factors predicting overall response and overall survival in hepatocellular carcinoma patients undergoing balloon-occluded transcatheter arterial chemoembolization: a retrospective cohort study. Hepatol Res. 2018;48(2):165–75.
Download references
Acknowledgements
Author information, authors and affiliations.
Vascular and Interventional Radiology Unit, Department of Diagnostic Service, Sapienza University of Rome, Viale Regina Elena 324, 00161, Rome, Italy
Pierleone Lucatelli, Gianluca De Rubeis, Bianca Rocco, Fabrizio Basilico, Alessandro Cannavale, Pier Giorgio Nardis, Mario Corona, Carlo Catalano & Mario Bezzi
Gastroenterology Division, Department of Clinical Medicine, Sapienza University of Rome, Rome, Italy
Aurelio Abbatecola
Pietro Valdoni Surgery Department, Sapienza” University of Rome, Rome, Italy
Stefania Brozzetti
You can also search for this author in PubMed Google Scholar
Contributions
Conception of the study (PL, GDR, BR, FB, AC, AA, PGN, MC, SB, CC, MB); design of the work (PL, GDR, BR, FB, AC, AA, PGN, MC, SB, CC, MB); acquisition, analysis (PL, GDR, BR, FB, AA, PGN), interpretation of data (PL, GDR, BR, FB, AC, AA, PGN, MC, SB, CC, MB); drafted the work (PL, GDR, BR, FB, AC, AA, PGN, MC, SB, CC, MB); substantively revised it (PL, GDR, BR, FB, AC, AA, PGN, MC, SB, CC, MB); approved the submitted version (and any substantially modified version that involves the author's contribution to the study) (PL, GDR, BR, FB, AC, AA, PGN, MC, SB, CC, MB); have agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature (PL, GDR, BR, FB, AC, AA, PGN, MC, SB, CC, MB). All authors read and approved the final manuscript.
Corresponding author
Correspondence to Pierleone Lucatelli .
Ethics declarations
Ethics approval.
The Ethical Committee of the “Azienda Ospedaliera Universitaria Policlinico Umberto I of Rome” approved the study with reference code: Ref Ce 5291. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Written informed consent for the procedure and to participate in this study was obtained from all individual participants included in the study.
Consent to publish
Written consent for the publication of identifying images or other personal or clinical details of participants that compromise anonymity, was waived.
Competing interests
All authors have no conflict of interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been updated to correct the name of Stefania Brozzetti
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Lucatelli, P., De Rubeis, G., Rocco, B. et al. Balloon occluded TACE (B-TACE) vs DEM-TACE for HCC: a single center retrospective case control study. BMC Gastroenterol 21 , 51 (2021). https://doi.org/10.1186/s12876-021-01631-w
Download citation
Received : 27 September 2020
Accepted : 27 January 2021
Published : 03 February 2021
DOI : https://doi.org/10.1186/s12876-021-01631-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Trans-catheter-arterial chemoembolization (TACE)
- Balloon-occluded trans-catheter arterial chemoembolization (b-TACE)
- Drug eluting microsphere trans arterial chemoembolization (DEM-TACE)
- Balloon micro-catheter
- Hepatocellular-carcinoma (HCC)
- Oncological comparison
BMC Gastroenterology
ISSN: 1471-230X
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Help & FAQ
High Risk Features Contributing to 30-Day Readmission After Acute Ischemic Stroke: A Single Center Retrospective Case-Control Study
- Icahn School of Medicine at Mount Sinai
- Medical Education
- Friedman Brain Institute
- Institute for Medical Education
Research output : Contribution to journal › Article › peer-review
Background and Purpose: Risk of 30-day stroke readmission has been attributed to medical comorbidities, stroke severity, and hospitalization metrics. The leading etiologies appear to vary across institutions and remain a moving target. We hypothesized that patients with increased medical complexity have higher odds of 30-day readmission and the immediate time after discharge may be most vulnerable. We aimed to characterize patients with 30-day readmission after acute ischemic stroke (IS) and identify predictors of post-IS readmission. Methods: We performed a retrospective case-control study analyzing post-IS 30-day readmission between January 2016-December 2019 using data from Mount Sinai Hospital’s Get With The Guidelines database. We performed chi square analyses and multivariate adjusted logistic regression model including age, sex, coronary artery disease (CAD), renal insufficiency (RI), history of prior stroke or TIA, length of stay (LOS) > 7, and NIHSS ≥ 5. Results: 6.7% (n = 115) of 1,706 IS encounters had 30-day readmission. The 115 cases were compared to 1,591 controls without 30-day readmission. In our adjusted model, CAD (OR = 1.7, p = 0.01), history of prior stroke or TIA (OR = 1.6, p = 0.01), LOS >7 (OR = 1.7, p = 0.02), and NIHSS ≥ 5 (OR = 4.5, p < 0.001) predicted 30-day readmission. 65% (n = 75) of readmitted patients had readmission within 14 days post-discharge. Conclusions: Patients with post-IS 30-day readmission were more likely to have complex medical comorbidities and history of stroke or TIA compared to controls. Patients with more severe stroke and longer LOS may benefit from individualized transition of care plans and closer follow up during the vulnerable 30-day post-stroke period.
- ischemic stroke
- quality improvement
- stroke readmissions
Access to Document
- 10.1177/19418744211027746
Fingerprint
- Patient Readmission Medicine & Life Sciences 100%
- Case-Control Studies Medicine & Life Sciences 69%
- Stroke Medicine & Life Sciences 59%
- Length of Stay Medicine & Life Sciences 16%
- Coronary Artery Disease Medicine & Life Sciences 11%
- Comorbidity Medicine & Life Sciences 11%
- Logistic Models Medicine & Life Sciences 9%
- Patient Transfer Medicine & Life Sciences 8%
T1 - High Risk Features Contributing to 30-Day Readmission After Acute Ischemic Stroke
T2 - A Single Center Retrospective Case-Control Study
AU - Loebel, Emma M.
AU - Rojas, Mary
AU - Wheelwright, Danielle
AU - Mensching, Connor
AU - Stein, Laura K.
N1 - Publisher Copyright: © The Author(s) 2021.
PY - 2022/1
Y1 - 2022/1
N2 - Background and Purpose: Risk of 30-day stroke readmission has been attributed to medical comorbidities, stroke severity, and hospitalization metrics. The leading etiologies appear to vary across institutions and remain a moving target. We hypothesized that patients with increased medical complexity have higher odds of 30-day readmission and the immediate time after discharge may be most vulnerable. We aimed to characterize patients with 30-day readmission after acute ischemic stroke (IS) and identify predictors of post-IS readmission. Methods: We performed a retrospective case-control study analyzing post-IS 30-day readmission between January 2016-December 2019 using data from Mount Sinai Hospital’s Get With The Guidelines database. We performed chi square analyses and multivariate adjusted logistic regression model including age, sex, coronary artery disease (CAD), renal insufficiency (RI), history of prior stroke or TIA, length of stay (LOS) > 7, and NIHSS ≥ 5. Results: 6.7% (n = 115) of 1,706 IS encounters had 30-day readmission. The 115 cases were compared to 1,591 controls without 30-day readmission. In our adjusted model, CAD (OR = 1.7, p = 0.01), history of prior stroke or TIA (OR = 1.6, p = 0.01), LOS >7 (OR = 1.7, p = 0.02), and NIHSS ≥ 5 (OR = 4.5, p < 0.001) predicted 30-day readmission. 65% (n = 75) of readmitted patients had readmission within 14 days post-discharge. Conclusions: Patients with post-IS 30-day readmission were more likely to have complex medical comorbidities and history of stroke or TIA compared to controls. Patients with more severe stroke and longer LOS may benefit from individualized transition of care plans and closer follow up during the vulnerable 30-day post-stroke period.
AB - Background and Purpose: Risk of 30-day stroke readmission has been attributed to medical comorbidities, stroke severity, and hospitalization metrics. The leading etiologies appear to vary across institutions and remain a moving target. We hypothesized that patients with increased medical complexity have higher odds of 30-day readmission and the immediate time after discharge may be most vulnerable. We aimed to characterize patients with 30-day readmission after acute ischemic stroke (IS) and identify predictors of post-IS readmission. Methods: We performed a retrospective case-control study analyzing post-IS 30-day readmission between January 2016-December 2019 using data from Mount Sinai Hospital’s Get With The Guidelines database. We performed chi square analyses and multivariate adjusted logistic regression model including age, sex, coronary artery disease (CAD), renal insufficiency (RI), history of prior stroke or TIA, length of stay (LOS) > 7, and NIHSS ≥ 5. Results: 6.7% (n = 115) of 1,706 IS encounters had 30-day readmission. The 115 cases were compared to 1,591 controls without 30-day readmission. In our adjusted model, CAD (OR = 1.7, p = 0.01), history of prior stroke or TIA (OR = 1.6, p = 0.01), LOS >7 (OR = 1.7, p = 0.02), and NIHSS ≥ 5 (OR = 4.5, p < 0.001) predicted 30-day readmission. 65% (n = 75) of readmitted patients had readmission within 14 days post-discharge. Conclusions: Patients with post-IS 30-day readmission were more likely to have complex medical comorbidities and history of stroke or TIA compared to controls. Patients with more severe stroke and longer LOS may benefit from individualized transition of care plans and closer follow up during the vulnerable 30-day post-stroke period.
KW - ischemic stroke
KW - quality improvement
KW - stroke
KW - stroke readmissions
UR - http://www.scopus.com/inward/record.url?scp=85110104987&partnerID=8YFLogxK
U2 - 10.1177/19418744211027746
DO - 10.1177/19418744211027746
M3 - Article
AN - SCOPUS:85110104987
SN - 1941-8744
JO - The Neurohospitalist
JF - The Neurohospitalist

- Vol 10, No 1 (January 31, 2021) /
The management of elderly patients with lung cancer: a single center retrospective study
Ping Wang 1# , Chunyan Li 2# , Yang An 1# , Xiaoqian Wang 2 , Zhixin Liang 1 , Liang’an Chen 1
1 Department of Respiratory and Critical Care Medicine, the Eighth Medical Center of PLA General Hospital , Beijing , China ; 2 Department of Respiratory and Critical Care Medicine, the First Medical Center of PLA General Hospital , Beijing , China
Contributions: (I) Conception and design: L Chen; (II) Administrative support: Z Liang; (III) Provision of study materials or patients: P Wang, Y An; (IV) Collection and assembly of data: C Li, X Wang; (V) Data analysis and interpretation: P Wang; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
# These authors contributed equally to this work.
Background: The incidence of lung cancer in patients aged over 80 years accounts for 30% of the entire lung cancer population. However, the emphasis on the treatment and prognosis of this subpopulation remains poorly investigated. This study evaluated outcomes associated with treatment strategies for these patients.
Methods: A retrospective analysis was performed on the overall survival and treatment of deceased patients over 80 years of age, diagnosed with lung cancer in our hospital. Treatment and overall survival were evaluated using logistic regression, the Kaplan-Meier method, and multivariable Cox proportional hazard models.
Results: A total of 56 patients were included in this study, with 30 (53.6%) patients diagnosed with stage IV at the time of detection. One-third of the patients refused any form of treatment. The majority (n=27, 48.2%) of the included patients with stage I–IV lung cancer received chemotherapy or tyrosine kinase inhibitors (TKIs). The median overall survival was determined to be 9.067±1.2477 months, with the median survival time of small cell lung cancer (SCLC) patients calculated as 7.167±3.797 months for the entire cohort. The majority of patients exhibited lesions in the left upper lung and displayed the longest overall survival. For the over 80 yrs with lunch cancer patients, that who chose not to receive any treatment exhibited a shorter overall survival than those who received treatment.
Conclusions: Most patients in this study presented with advanced disease. Treatment-naïve patients exhibited a poorer prognosis compared to their counterparts who received treatment, highlighting the need for this subpopulation to access further treatment.
Keywords: Lung cancer; elder; patient; therapy
Submitted Sep 24, 2020. Accepted for publication Dec 23, 2020.
doi: 10.21037/apm-20-2125
Introduction
Lung cancer is ranked as the leading cause of cancer mortality among malignant tumors ( 1 ). In China, lung cancer was the third leading cause of years of life lost in 2017, exceeding chronic obstructive pulmonary disease, another common respiratory disease ( 2 ). However, the focus on the treatment and prognosis of lung cancer patients in people over 80 years of age is still poorly investigated, resulting in limited treatment options for this subpopulation. Our study collected the clinical data of lung cancer patients who expired in our hospital from 1998 to 2020, to evaluate the therapeutic significance and influencing factors of these patients. The study was intended to provide recommendations for clinicians regarding the treatment options available for lung cancer patients aged 80 years and older.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-2125 ).
The study was approved by the PLA General Hospital Medical Ethics Committee (S2020-447-01). All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived. Demographic and treatment information from 1998 to 2020 of deceased patients aged 80 years and older diagnosed with lung cancer were retrospectively collected in our hospital. Pathology was confirmed by histopathology and cytopathology. Patients without pathological confirmation were excluded. All patients were re-staged according to the International Association for the Study of Lung Cancer’s (IASLC) TNM classification of lung cancer (8th edition) ( 3 ).
We first evaluated the overall cohort of patients aged 80 years and older with clinical stages I to IV lung cancer [non-small cell lung cancer (NSCLC) + small cell lung cancer (SCLC)], including staging and histology. Predictors of survival were determined using a multivariable logistic regression model. The variables in this adjusted model were age, sex, smoking, comorbidity condition, and history of previous malignancy.
Overall survival of the different subgroups (stages I–IV) stratified by treatment types (i.e., chemotherapy, or tyrosine kinase inhibitor (TKI), radiation, chemoradiation, surgery, and no treatment) was assessed using the Kaplan-Meier analysis and multivariable Cox models adjusting for clinical stage, tumor location, and histology.
Statistical analysis
For all comparisons, a P value =0.05 was used to define statistical significance. Statistical analysis was performed using SPSS software, version 25.0 (IBM).
Patient cohort
From 1998 to 2020, 56 patients with lung cancer were enrolled into our study. These patients had died past 80 years of age, and had complete, clinically relevant data. Lung cancer was confirmed by pathology (histopathology and cytopathology), 7 patients exhibited SCLC, and 49 had NSCLC. Adenocarcinoma was the most common pathological type. Table 1 provides additional patient characteristics. In our patient cohort, most patients displayed concurrent diseases of other systems. The most common comorbidities were hypertension and coronary heart disease. Seven patients were identified to concurrently exhibit tumors in other systems. Approximately 40% of patients had an Eastern Cooperative Oncology Group (ECOG) score of 2 points and above at the time of diagnosis.
Clinical characteristics and survival
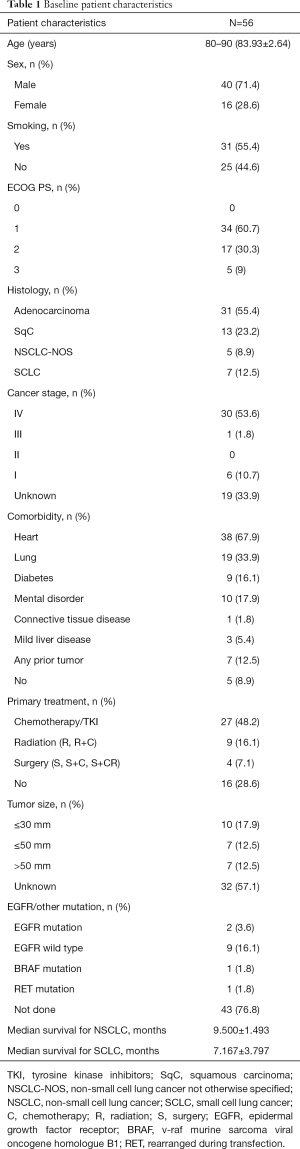
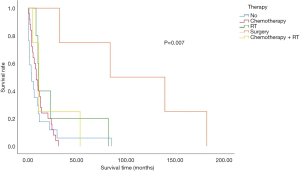
The overall median survival time in the subject population was 9.067±1.2477 months. The median survival time of SCLC patients was 7.167±3.797 months, whereas NSCLC cancer patients displayed an overall survival of 9.500±1.493 months. The intergroup differences were not found to be significant ( Figure 1 and Table 2 ). The effects of age and gender on the overall survival of patients were also not significant. However, smoking was shown to be a risk factor affecting the overall survival of patients (P<0.05) ( Table 3 ). The effect of complications on patient survival was not significant.
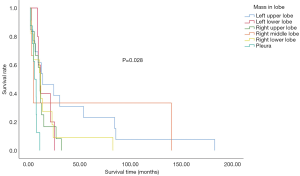
Primary tumor characteristics and survival
In the study population, the left upper lung was the most affected site of lung cancer. Furthermore, the primary site of lung cancer significantly affected overall survival. Patients with the primary site at the left upper lung displayed the longest median survival time ( Figure 2 ). Within our cohort, 76.8% of patients were not tested for driver gene mutations, only 2 patients exhibited epidermal growth factor receptor (EGFR) mutations, 1 displayed the v-raf murine sarcoma viral oncogene homologue B1 (BRAF) mutations, while another had receptor tyrosine kinase rearranged during transfection (RET) mutations. Furthermore, 9 had no mutations detected ( Table 1 ).
Treatment and survival
In the overall study population, 16 patients did not receive any treatment after diagnosis confirmation. The most common treatment was chemotherapy. Four patients underwent surgical treatment, while 9 received radiation and chemoradiation. Meanwhile, 16 of the 56 patients aged 80 years or older with stage I–IV NSCLC did not receive any therapy. Survival analysis revealed a significantly worse survival rate for patients who did not receive any treatment, compared to their treated counterparts. The patients who underwent surgery displayed the longest survival duration ( Figure 3 ).
Staging and survival
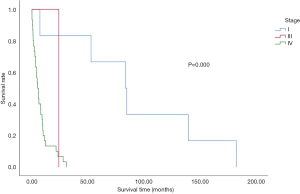
The effects of staging on patient survival are illustrated in Figure 4 . In the overall population, 53.6% of patients were at stage IV at the time of diagnosis. Additionally, 33.9% of the total analysed population refused any examination to assess their condition ( Table 1 and Figure 4 ). There was a significant correlation between progression-free survival (PFS) and overall survival. Both PFS1 obtained after the first treatment and PFS2 obtained after progression were significantly associated with survival time. However, no correlation was noted between the diagnosis-and-treatment interval and overall survival ( Figure 5 ).
Conclusions
In the present study, the clinical data, treatment and survival time of 56 deceased patients with lung cancer diagnosed at our center were analyzed. Approximately one-third of patients refused tumor assessment and one-third refused treatment. An increased ECOG score, increased TNM stage, and concern for treatment side effects were the main factors for their refusal for treatment and examination. However, our study still included more treated patients compared to other investigations ( 4 ). After rigorous review, we concluded that this difference may be attributed to the development of targeted drugs with lower toxicity, which was more easily accepted by vulnerable, elderly patients. EGFR gene mutations were detected in only 2 patients, and were treated with EGFR-TKI therapy. Another 15 patients received the same treatment without gaining clear benefit. One of the limitations of the present is the small sample size, which affected the conclusion.
Our findings indicate that overall survival of treated patients exceeded that of their treatment-naïve counterparts. However, patients who received surgical removal at stage I predominantly benefited most, which was consistent with other studies ( 5 , 6 ). Since economic evaluation was not performed, benefit from treatment in patients with high ECOG scores and advanced TNM stages remains to be determined for future investigations.
The gradual increase in the average age of lung cancer patients, along with the growing proportion of people aged over 80 years in this population highlights the need for further evaluations into the treatment options of this group. At present, an increasing number of studies have analyzed the therapeutic effect of these patients; however, the majority of these studies were retrospective ( 7 - 9 ). Reports of prospective clinical drug trials, solely targeting this population are still limited ( 10 ). Furthermore, the number of clinical studies that included this population is low ( 11 , 12 ). Further studies are expected to evaluate the treatment and prognosis of octogenarians.
The present study retrospectively evaluated the current status of treatment in lung cancer patients, aged 80 years and older. To minimize bias, we selected data from deceased patients. Due to the retrospective nature of the study, the absence of some indicators might have affected the results. In the present study, the left upper lung was the most common site of primary lesions in these patients, with surgery observed as the best treatment for stage I patients. However, over half of the patients were at stage IV, and chemotherapy can benefit such patients. Our findings indicate that the treatment of lung cancer in patients aged 80 years and above requires re-evaluation, with multiple factors, in addition to age, needing to be considered for the most effective treatment.
Acknowledgments
Funding: This work was supported by the Tanslational Medicine Programme of Chinese PLA General Hospital (2017TM-011).
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-2125
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-2125
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-2125 ). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the PLA General Hospital Medical Ethics Committee (S2020-447-01). All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/ .
- Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Abate D, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2019;5:1749-68. [ Crossref ] [ PubMed ]
- Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019;394:1145-58. [ Crossref ] [ PubMed ]
- Rami-Porta R, Bolejack V, Giroux DJ, et al. The IASLC lung cancer staging project: the new database to inform the eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2014;9:1618-1624.
- Yang CJ, Brown AB, Deng JZ, et al. The Oldest Old: A National Analysis of Outcomes for Patients 90 Years or Older With Lung Cancer. Ann Thorac Surg 2020;109:350-7. [ Crossref ] [ PubMed ]
- Nanda RH, Liu Y, Gillespie TW, et al. Stereotactic body radiation therapy versus no treatment for early stage non-small cell lung cancer in medically inoperable elderly patients: A National Cancer Data Base analysis. Cancer 2015;121:4222-30. [ Crossref ] [ PubMed ]
- Arnold BN, Thomas DC, Rosen JE, et al. Effectiveness of local therapy for stage I non-small-cell lung cancer in nonagenarians. Surgery 2017;162:640-51. [ Crossref ] [ PubMed ]
- Battisti NML, Sehovic M, Extermann M. Assessment of the External Validity of the National Comprehensive Cancer Network and European Society for Medical Oncology Guidelines for Non-Small-Cell Lung Cancer in a Population of Patients Aged 80 Years and Older. Clin Lung Cancer 2017;18:460-71. [ Crossref ] [ PubMed ]
- Hino H, Karasaki T, Yoshida Y, et al. Risk factors for postoperative complications and long-term survival in lung cancer patients older than 80 years. Eur J Cardiothorac Surg 2018;53:980-6. [ Crossref ] [ PubMed ]
- Muchnik E, Loh KP, Strawderman M, et al. Immune Checkpoint Inhibitors in Real-World Treatment of Older Adults with Non-Small Cell Lung Cancer. J Am Geriatr Soc 2019;67:905-12. [ Crossref ] [ PubMed ]
- clinicaltrials.gov [Internet]. Maryland: U. S. National Library of Mdedicine; c2020 [cited 2020 Nov 9]. Available online: https://clinicaltrials.gov/
- Ball D, Mai GT, Vinod S, et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol 2019;20:494-503. [ Crossref ] [ PubMed ]
- West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20:924-37. [ Crossref ] [ PubMed ]
(English Language Editors: E. Tan and J. Gray)
Article Options
- PDF 798 views
- Full Text 1467 views
- Reporting Checklist 163 views
- Data Sharing Statement 176 views
- COI Form 184 views

Download Citation
- Share on Facebook
- Share on Twitter
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Brief Communication
- Open access
- Published: 28 March 2022
Case series of outcomes in advanced cancer patients with single pathway alterations receiving N-of-One therapies
- Diviya Gupta 1 na1 ,
- Razelle Kurzrock ORCID: orcid.org/0000-0003-4110-1214 1 , 2 , 3 na1 ,
- Suzanna Lee 2 ,
- Ryosuke Okamura ORCID: orcid.org/0000-0001-7352-8621 2 , 3 ,
- Hyo Jeong Lim 4 ,
- Ki Hwan Kim 5 ,
- Jason K. Sicklick ORCID: orcid.org/0000-0003-4403-0271 1 , 2 , 6 &
- Shumei Kato 1 , 2 , 3
npj Precision Oncology volume 6 , Article number: 18 ( 2022 ) Cite this article
1539 Accesses
1 Citations
3 Altmetric
Metrics details
- Cancer genomics
Though advanced cancers generally display complex molecular portfolios, there is a subset of patients whose malignancies possess only one genomic alteration or alterations in one oncogenic pathway. We assess how N-of-One therapeutic strategies impact outcomes in these patients. From 12/2012 to 9/2018, 429 therapy-evaluable patients with diverse treatment-refractory cancers were presented at Molecular Tumor Boards at Moores Cancer Center at UC San Diego. The clinical benefit rate, defined by RECIST1.1, was assessed for patients with solid tumors who underwent next-generation sequencing (NGS) profiling revealing one genomic or pathway alteration, subsequently managed with N-of-One therapies. Nine of 429 patients (2.1%) met evaluation criteria. Using matched therapy indicated by NGS, the clinical benefit rate (stable disease ≥ 6 months/partial/complete response) was 66.7%. Median progression-free survival was 11.3 months (95% CI: 3.4–not evaluable). Thus, a small subset of diverse cancers has single pathway alterations on NGS testing. These patients may benefit from customized therapeutic matching.
Similar content being viewed by others

Evolutionary trajectories of small cell lung cancer under therapy
Julie George, Lukas Maas, … Roman K. Thomas

PRMT5 is an actionable therapeutic target in CDK4/6 inhibitor-resistant ER+/RB-deficient breast cancer
Chang-Ching Lin, Tsung-Cheng Chang, … Carlos L. Arteaga
The evolution of metastatic upper tract urothelial carcinoma through genomic-transcriptomic and single-cell protein markers analysis
Kentaro Ohara, André Figueiredo Rendeiro, … Juan Miguel Mosquera
Recent advances in precision medicine have quickly transformed treatment strategies for patients with advanced cancers. Currently, genomic testing allows physicians to identify mutated genes and tailor treatment to precisely target the alterations in their malignancies. This approach has been therapeutically beneficial for several cancer types with particular genomic alterations, including BCR-ABL kinase inhibition for chronic myeloid leukemia and KIT inhibition for gastrointestinal stromal tumor (GIST), as well as BRAF and Her2 inhibition for multiple tumor types 1 , 2 . Furthermore, immunotherapies, such as anti-PD-1/PD-L1 blockades, have shown durable responses among patients with high tumor mutational burden (TMB), microsatellite instability-high (MSI-high), and PD-L1 overexpression/amplification 3 , 4 , 5 .
Despite the identification of numerous biomarkers for targeted therapy, oncology medications have significantly higher drug development attrition rates than medications for non-oncology indications 6 . This is partly because drugs that fail to elicit a durable response in a significant subgroup of patients are frequently abandoned, even if the drug exhibits significant activity in a small proportion of people 7 . Two of the major obstacles to treatment are intra-tumor heterogeneity, in which multiple genomic clonal populations exist within a neoplasm, and inter-tumor heterogeneity, in which multiple tumors in the same patient possess different co-occurring genomic alterations. In fact, patients with advanced cancer harbor a median of five unique oncogenic alterations, suggesting that therapeutics should be individualized and, if indicated, utilize a combination approach 8 . Still, when multiple co-driver alterations exist, it seems likely that patients will exhibit primary or secondary resistance to targeted therapeutic strategies.
Accordingly, we hypothesized that patients whose advanced cancers harbored a single alteration or alterations in a single genomic pathway on interrogation with next-generation sequencing (NGS) would respond especially well to cognate targeted therapy. Herein, we show that such individuals, while uncommon, can often achieve objective and durable responses when administered agents that are well matched to their molecular alteration(s) across a spectrum of cancer types and genomic abnormalities.
Overall, 715 distinct patients with advanced cancer were discussed at face-to-face Molecular Tumor Board (MTB) meetings. Among 429 patients who were subsequently treated and evaluable for outcome analysis, nine patients had a single genomic alteration or alterations in one molecular pathway that were treated with matched targeted therapy (Fig. 1 ). All nine patients had NGS performed on tissue by Foundation Medicine (FoundationOne™, Cambridge, Massachusetts, http://www.foundationmedicine.com ) (Clinical Laboratory Improvement Amendments (CLIA)-certified). The FoundationOne™ tissue assay utilized during the study period interrogated between 182 and 324 cancer-related genes. Median patient age was 41 years (range, 14–72 years). They received a median of two lines of therapy, including matched therapy indicated by NGS results (range, 1–5).
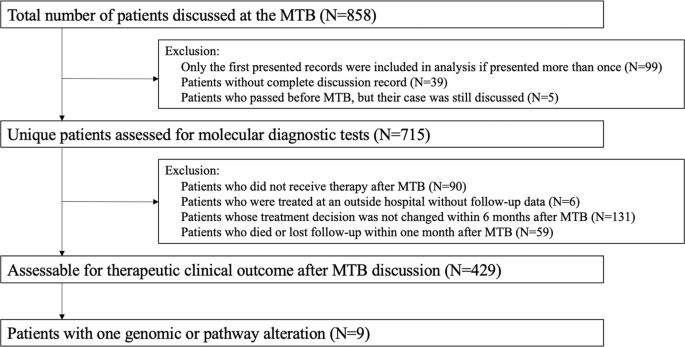
Only solid tumors were included. Patients who had only one alteration on an initial profiling test but subsequently had more genomic profiling after MTB presentation and demonstrated additional alterations were excluded. All included patients had NGS profiling. Patients were treated within six months of MTB. Patients who received immunotherapy based on MSI-high or TMB-high were not included in the current analysis 9 .
Eight patients received small molecule targeted agent(s), while one patient with PDL1 amplification received immune checkpoint blockade. Among the nine patients, three patients achieved partial response (PR) and an additional three patients achieved stable disease (SD) ≥ 6 months. The remaining three patients had progressive disease (PD). Altogether, the clinical benefit rate was 66.7% (i.e., 6/9 patients) (Table 1 ). Median progression-free survival (PFS) was 11.3 months (95% CI: 3.4–not evaluable), while median overall survival (OS) was not reached (95% confidence interval (CI): 8.4–not evaluable) (Fig. 2 ). Only Patient #5467 (Table 1 ), who received immunotherapy, experienced a serious adverse event (SAE), namely Grade 3 pancreatitis. No other Grade 3–4 adverse events were observed, according to Common Terminology Criteria for Adverse Events.
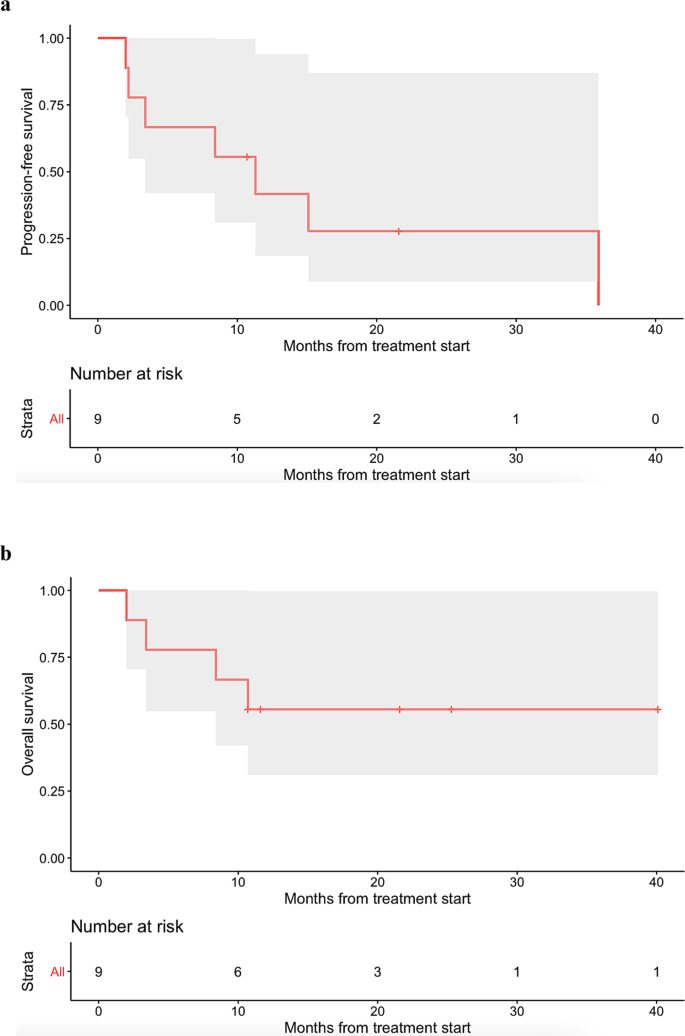
The gray shade areas represent the 95% confidence intervals, and bars denote censored observations. Median PFS was 11.3 months (95% CI: 3.4–not evaluable) ( a ), while median OS was not reached (95% CI: 8.4–not evaluable) ( b ).
Recent literature on molecular profiling technologies has revealed that advanced cancer patients harbor a median of five molecular alterations 8 . Although rare, some patients may harbor only one molecular alteration after interrogation of several hundred oncogenic markers with NGS 9 . In this study, the administration of N-of-One treatments was retrospectively reviewed to assess how this approach impacted clinical benefit rates (i.e., PR + SD ≥ 6 months), PFS, and OS in patients harboring one alteration or alterations in one oncogenic pathway.
Overall, 2.1% (9/429) of evaluable patients had one gene/pathway alteration targeted with molecularly matched agents. Six of these nine patients achieved clinical benefit. The pathways successfully targeted included BRAF/MEK, CDK4/6, FGFR, KIT, and RET pathways, as well as PD-L1 amplification-associated immune suppression. Notably, the patient with a BRAF V600E mutant ovarian serous carcinoma tumor (i.e., not melanoma) was successfully treated with BRAF inhibitor dabrafenib and MEK inhibitor trametinib (PR ongoing at 10.7+ months). Furthermore, one of two patients with alterations expected to activate CDK4/6 was successfully treated with a CDK4/6 inhibitor as monotherapy (ovarian undifferentiated neuroendocrine cancer, PR lasting 8.4 months); this is in contrast to observations suggesting that matched CDK4/6 inhibitor monotherapy, such as palbociclib, is ineffective 10 , possibly for patients with multiple co-occurring mutations, unlike the patient discussed above. On the other hand, three of the nine patients did poorly. It is plausible that, despite a single pathway alteration on genomics, other important driver pathways were altered at the transcript or protein level in these patients. As such, in addition to tissue genomic profiling, more comprehensive analysis that includes cell-free DNA, transcriptomics, epigenetics, and immune profiling may be considered in the care of future patients.
Limitations of this paper include the retrospective nature of analysis, small sample size, and lack of controls. Despite these limitations, this study provides a window into the opportunity to leverage NGS to prescribe personalized matched therapies for patients with incurable malignancies whose cancers have not yet shown complicated genomic evolution. Trials such as NCI-MATCH 11 and MSK-IMPACT 12 have demonstrated the viability of deploying NGS to triage patients to targeted therapeutics, while I-PREDICT 8 and WINTHER 13 have evaluated the outcomes of patients receiving therapies that prioritize combination therapy matched to complex genomic and/or transcriptomic profiles. The current study suggests that there is a small subset of patients (~2%) with advanced metastatic disease whose tumors still demonstrate only single pathway alterations on NGS, and that such cancers remain amenable to focused pathway targeting.
Patient selection
We investigated the molecular profiling status (performed by CLIA-certified laboratories) and clinical outcomes of patients with advanced cancer presented at MTB meetings from December 2012 to September 2018, following guidelines of the institutional review board-approved Profile Related Evidence Determining Individualized Cancer Therapy (PREDICT) study (NCT02478931; ClinicalTrials.gov; Posted June 23, 2015) and any investigational therapies for which patients gave consent. Weekly in-person MTBs were held at the Moores Cancer Center at UC San Diego (UCSD) Health and followed protocols as previously described 14 . We studied patients with solid tumors harboring one genomic or pathway alteration managed with matched targeted therapy 8 , 15 . We excluded patients who received immunotherapy based on MSI-high or TMB-high. However, patients treated with checkpoint blockade were included if the agent targeted discrete alterations such as PD-L1 amplification 4 . Patients who had only one alteration on an initial profiling test but subsequently received additional NGS profiling that revealed further mutations after MTB discussions were excluded from this analysis.
Endpoints and statistics
In accordance with RECIST 1.1 criteria, all patients were assessed with the outcome endpoints of clinical benefit rate [i.e., stable disease (SD) ≥ 6 months, partial response (PR), or complete response (CR)] as determined by the treating physician. Median PFS and median OS were also evaluated by the Kaplan–Meier method. PFS was defined as the time from the start of therapy to disease progression or last follow-up date if progression-free (the latter being censored). OS was defined as the time from the start of therapy to death or last follow-up if alive (the latter being censored).
Declaration of ethical approval
This retrospective case series involves patients enrolled in the UCSD Study of Profile Related Evidence Determining Individualized Cancer Therapy (PREDICT). This study was performed in accordance with UCSD IRB guidelines, and for any investigational treatments for which patients gave consent. All patients underwent informed consent and signed consented forms in their native languages via licensed medical interpreters.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
De-identified data will be made available on reasonable request. Should qualified researchers contact the corresponding author for the de-identified dataset, they will not need to obtain ethical approval or sign a data usage agreement as patient confidentiality will be maintained.
Padma, V. V. An overview of targeted cancer therapy. BioMedicine 5 , 19 (2015).
Kato, S., Subbiah, V. & Kurzrock, R. Counterpoint: successes in the pursuit of precision medicine: biomarkers take credit. J. Natl Compr. Cancer Netw. 15 , 863–866 (2017).
Article Google Scholar
Goodman, A. M. et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol. Cancer Therapeutics 16 , 2598–2608 (2017).
Article CAS Google Scholar
Goodman, A. M. et al. Prevalence of PDL1 amplification and preliminary response to immune checkpoint blockade in solid tumors. JAMA Oncol. 4 , 1237 (2018).
Marcus, L., Lemery, S. J., Keegan, P. & Pazdur, R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin. Cancer Res. 25 , 3753–3758 (2019).
Hutchinson, L. & Kirk, R. High drug attrition rates—where are we going wrong? Nat. Rev. Clin. Oncol. 8 , 189–190 (2011).
Iyer, G. et al. Genome sequencing identifies a basis for everolimus sensitivity. Science 338 , 221–221 (2012).
Sicklick, J. K. et al. Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Nat. Med. 25 , 744–750 (2019).
Kato, S. et al. Prognostic implications of RAS alterations in diverse malignancies and impact of targeted therapies. Int. J. Cancer 146 , 3450–3460 (2020).
Edelman, M. J. et al. SWOG S1400C (NCT02154490)—a phase II study of palbociclib for previously treated cell cycle gene alteration–positive patients with stage IV squamous cell lung cancer (Lung-MAP Substudy). J. Thorac. Oncol. 14 , 1853–1859 (2019).
Flaherty, K. T. et al. Molecular landscape and actionable alterations in a genomically guided cancer clinical trial: national cancer institute molecular analysis for therapy choice (NCI-MATCH). J. Clin. Oncol. 38 , 3883–3894 (2020).
Cheng, D. T. et al. Memorial sloan kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT). J. Mol. Diagnostics 17 , 251–264 (2015).
Rodon, J. et al. Genomic and transcriptomic profiling expands precision cancer medicine: the WINTHER trial. Nat. Med. 25 , 751–758 (2019).
Kato, S. et al. Real-world data from a molecular tumor board demonstrates improved outcomes with a precision N-of-One strategy. Nat. Commun. 11 , 4965 (2020).
Wheler, J. J. et al. Cancer therapy directed by comprehensive genomic profiling: a single center study. Cancer Res. 76 , 3690–3701 (2016).
Download references
Acknowledgements
Funded in part by the Joan and Irwin Jacobs Fund and by National Cancer Institute grants P30 CA023100 (RK, JKS). The authors also acknowledge the support of NIH K08CA168999 and R21CA192072, as well as Pedal the Cause, David Foundation, and Kristen Ann Carr Fund (JKS).
Author information
These authors contributed equally: Diviya Gupta, Razelle Kurzrock.
Authors and Affiliations
School of Medicine, University of California San Diego, La Jolla, CA, USA
Diviya Gupta, Razelle Kurzrock, Jason K. Sicklick & Shumei Kato
Center for Personalized Cancer Therapy, UC San Diego Moores Cancer Center, La Jolla, CA, USA
Razelle Kurzrock, Suzanna Lee, Ryosuke Okamura, Jason K. Sicklick & Shumei Kato
Division of Hematology/Oncology, UC San Diego, San Diego, CA, USA
Razelle Kurzrock, Ryosuke Okamura & Shumei Kato
Department of Internal Medicine, Veterans Health Service Medical Center, Seoul, Republic of Korea
Hyo Jeong Lim
Division of Hematology and Medical Oncology, Seoul National University Boramae Medical Center, Seoul, Republic of Korea
Ki Hwan Kim
Department of Surgery, Division of Surgical Oncology, UC San Diego, San Diego, CA, USA
Jason K. Sicklick
You can also search for this author in PubMed Google Scholar
Contributions
Study conception and design: D.G., R.K., S.K. Acquisition of the data: S.L., R.O., H.J.L., K.H.K. Data analysis and interpretation: D.G., R.K., J.K.S., S.K. Writing—original draft preparation: D.G., R.K., S.K. Writing—review and edits: S.L., R.O., H.J.L., K.H.K., J.K.S. Created visualizations: D.G. Project administration: R.K., S.K. All authors approve this submitted version, agree to be accountable for their contributions, and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. D.G. and R.K. are listed as co-first authors. The method used in assigning the authorship order among co-first authors D.G. and R.K. was based on the individual who contributed most to writing the manuscript.
Corresponding authors
Correspondence to Razelle Kurzrock or Shumei Kato .
Ethics declarations
Competing interests.
The authors declare no competing non-financial interests but the following competing financial interests: D.G., S.L., R.O., H.J.L., and K.H.K. have nothing to disclose. R.K. has received research funding from Incyte, Genentech, Merck Serono, Pfizer, Sequenom, Foundation Medicine, Guardant Health, and Konica Minolta, as well as consultant fees from LOXO, X-Biotech, Actuate Therapeutics, Roche, and NeoMed. She receives speaker fees from Roche, owns stock in IDbyDNA, and has an ownership interest in CureMatch, Inc. J.K.S. receives research funding from Novartis Pharmaceuticals, Amgen Pharmaceuticals, and Foundation Medicine; consultant fees from Grand Rounds, Loxo, and Deciphera; and speaker’s fees from Roche and Deciphera. He also owns stocks in Personalis. S.K. serves as a consultant for Foundation Medicine and receives speaker’s fees from Roche.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Gupta, D., Kurzrock, R., Lee, S. et al. Case series of outcomes in advanced cancer patients with single pathway alterations receiving N-of-One therapies. npj Precis. Onc. 6 , 18 (2022). https://doi.org/10.1038/s41698-022-00259-7
Download citation
Received : 07 September 2021
Accepted : 11 February 2022
Published : 28 March 2022
DOI : https://doi.org/10.1038/s41698-022-00259-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Nonsense-mediated rna decay: an emerging modulator of malignancy.
- Dwayne G. Stupack
- Miles F. Wilkinson
Nature Reviews Cancer (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
- Research article
- Open access
- Published: 06 May 2020
A single-center, retrospective study of COVID-19 features in children: a descriptive investigation
- Huijing Ma 1 na1 ,
- Jiani Hu 2 na1 ,
- Jie Tian 3 na1 ,
- Xi Zhou 4 ,
- Maxwell Thomas Laws 2 ,
- Luke David Wesemann 2 ,
- Baiqi Zhu 1 ,
- Wei Chen 6 , 7 ,
- Rafael Ramos 2 ,
- Jun Xia 4 &
- Jianbo Shao 1
BMC Medicine volume 18 , Article number: 123 ( 2020 ) Cite this article
18k Accesses
84 Citations
104 Altmetric
Metrics details
A Commentary to this article was published on 05 June 2020
A Commentary to this article was published on 28 May 2020
Compared to adults, there are relatively few studies on COVID-19 infection in children, and even less focusing on the unique features of COVID-19 in children in terms of laboratory findings, locations of computerized tomography (CT) lesions, and the role of CT in evaluating clinical recovery. The objective of this study is to report the results from patients at Wuhan Children’s Hospital, located within the initial center of the outbreak.
Clinical, imaging, and laboratory data of 76 children were collected retrospectively and analyzed with the Fisher exact test and Cox regression statistical methods.
Among 50 children with a positive COVID-19 real-time reverse-transcriptase polymerase chain reaction (PCR), five had negative PCR results initially but showed positive results in subsequent tests. Eight (16%) patients had lymphopenia, seven (14%) with thrombocytopenia, four (8%) with lymphocytosis, two (4%) with thrombocytosis, ten (20%) with elevated C-reactive protein, four (8%) with hemoglobin above, and six (12%) with below standard reference values. Seven (14%) of the 50 had no radiologic evidence of disease on chest CT. For the 43 patients who had abnormal CT findings, in addition to previously reported patterns of ground-glass opacity (67%), local patchy shadowing (37%), local bilateral patchy shadowing (21%), and lesion location of lower lobes (65%), other CT features include that an overwhelming number of pediatric patients had lesions in the subpleural area (95%) and 22 of the 28 lower lobe lesions were in the posterior segment (78%). Lesions in most of the 15 patients (67%) who received chest CT at discharge were not completely absorbed, and 26% of these pediatric patients had CT lesions that were either unchanged or worse.
Conclusions
There were a few differences between COVID-19 children and COVID-19 adults in terms of laboratory findings and CT characteristics. CT is a powerful tool to detect and characterize COVID-19 pneumonia but has little utility in evaluating clinical recovery for children. These results oppose current COVID-19 hospital discharge criteria in China, as one requirement is that pulmonary imaging must show significant lesion absorption prior to discharge. These differences between pediatric and adult cases of COVID-19 may necessitate pediatric-specific discharge criteria.
Peer Review reports
Since initially identified in Wuhan city of China’s Hubei province in December 2019, the coronavirus disease 2019 (COVID-19) has resulted in 466,836 confirmed cases and 21,152 deaths as of March 25, 2020. Two months prior, on January 23, 2020, there were only 581 reported cases. COVID-19 can rapidly spread from human-to-human and is more contagious than other notable members of the coronavirus family, such as severe acute respiratory syndrome (SARS) and Middle Eastern respiratory syndrome (MERS) [ 1 , 2 ]. The World Health Organization recently declared COVID-19 a global pandemic, and the USA has declared a national emergency. Even though the incidence of COVID-19 infection in children is less than it is in adults, the total number of pediatric cases is expected to increase rapidly in the coming weeks.
Compared to adults [ 3 , 4 , 5 , 6 , 7 ], there are a few studies on the COVID-19 in children. Although mortality in children has been reported [ 8 ], studies have demonstrated that COVID-19 is generally less severe compared to adults in terms of both symptoms and computerized tomography (CT) manifestations [ 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 ]. The common chest CT patterns are ground-glass opacities (GGO) followed by local bilateral shadowing (LPS), in contrast to a large percentage of bilateral patchy shadowing (BPS) pattern in adults [ 19 , 20 ]. However, there are no studies that quantitatively examine the location of lung lesions in COVID-19-positive pediatric patients [ 21 ]. Most of the pediatric patients are at the early stages of the disease when admitted to hospitals. Thus, a detailed localization study is meaningful both clinically and scientifically, as it could help pinpoint lung regions that are particularly susceptible to COVID-19 infection.
Several studies have reported on the laboratory findings of children infected with COVID-19. However, the interpretations of these results vary substantially [ 15 , 22 , 23 , 24 ]. The discrepancy in laboratory interpretations could be attributed to the studies each referring to a different set of reference values. Of note, the range of normal lab values changes depending upon the age of the child, i.e., a 1-year-old has a different set of reference values than a 9-year-old. Confounding these results is the fact that the reference values used among the studies lack consistency and appear to be hospital-self-defined values [ 15 , 22 , 23 , 24 ]. This inconsistency of reference values makes any systemic review of the published data less meaningful [ 23 ].
There is also no research on the role of CT in monitoring clinical recovery in children. CT has been widely used in the clinical management of adult patients due to its ability to reveal detailed features of pneumonia [ 25 , 26 , 27 , 28 ]. Because of how many unknowns there were about the disease, particularly at the beginning of the COVID-19 outbreak, CT was frequently used in the clinical management and diagnosis of children in China. Notably, repeated use of CT can be harmful, particularly for children [ 29 , 30 ].
The objective of this study is to report relevant findings from the COVID-19-positive patients treated at Wuhan Children’s Hospital. Specifically, we attempt to answer three questions based on the patient’s clinical, laboratory, diagnostic, and treatment outcome data. The questions are, in hospitalized COVID-19 children, (i) what are the typical laboratory findings, (ii) is there any unique CT feature, and (iii) is CT necessary for evaluating clinical recovery?
Study design and patient selection
For this retrospective, single-center study, patients were recruited from January 21 to February 14, 2020, at Wuhan Children’s Hospital in Wuhan, China. Real-time reverse-transcriptase polymerase chain reaction (PCR) was performed on children 16 years of age and under who had a family or social history of COVID-19 exposure. Subsequently, these patients received a chest CT examination to evaluate lung pathology. Based on the PCR and CT results, these patients were stratified into groups A–C (Fig. 1 ). This study was approved by the Ethics Committee of Wuhan Children’s Hospital (Wuhan Maternal and Child Health Care Hospital # WHCH 2020005). Written informed parental/guardian consent and child assent (where appropriate) were obtained prior to enrollment in the study.
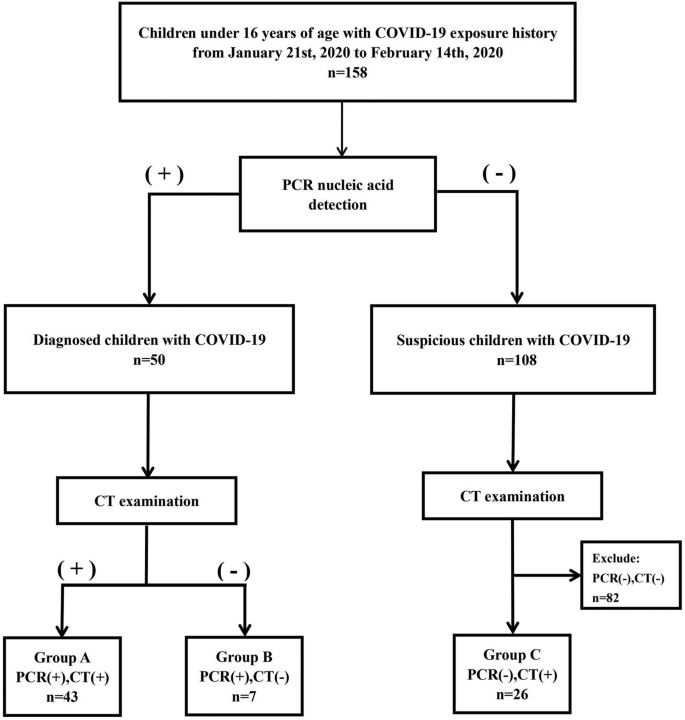
Flow chart for patient selection. Group A: 43 children with COVID-19 exposure history, positive CT, and positive PCR. Group B: seven children with COVID-19 exposure history, negative CT, and positive PCR. Group C: 26 children with COVID-19 exposure history, positive CT, and persistently negative PCR results
We obtained demographic information, clinical symptoms, laboratory results, management, and outcome data from each patient’s electronic medical records. Clinical outcomes were followed up to February 17, 2020.
Chest CT without intravenous contrast was performed on all patients using a Siemens SOMATOM Definition AS128 or GE Optima CT 660 with a 1-mm or 0.625-mm slice thickness, respectively. Children under 5 years old, as well as uncooperative children, received oral chloral hydrate sedation (0.5 ml/kg) prior to CT. Cooperative children above 5 years old were trained with breathing exercises prior to CT.
All CT images were reviewed by at least two radiologists with more than 10 years of experience. Imaging was reviewed independently. When the opinions on the CT features were inconsistent, the two radiologists discussed and decided together. Only final decisions reached by consensus are reported. No negative control cases were examined.
PCR confirmation of COVID-19 was performed at two different institutions: Hubei Center for Disease Control and Prevention and Wuhan Children’s Hospital.
Patient discharge
Criteria for discharging pediatric patients in this hospital were normal body temperature for 3 days, two negative PCR results at 24-h intervals, and resolution of all clinical symptoms.
Statistical analysis
The Fisher exact test method was used to determine whether there is a significant difference in CT image characteristics and lesion locations between group A and group C. The Cox regression analysis was used to determine whether changes in CT images during treatment were associated with clinical outcomes for children with COVID-19 infection. All analyses were performed using EmpowerStats ( http://www.empowerstats.com ) and the statistical package R (version 3.2.3). p value of less than 0.05 was considered to indicate a statistically significant difference.
From January 21 to February 14, 2020, 158 children at Wuhan Children’s Hospital were radiologically examined with chest CT, and respiratory secretions were obtained and subsequently tested for COVID-19 with PCR. A CT scan was considered positive when at least one lesion was identified. Among them, 43 had a positive CT and positive PCR (group A), 7 had a negative CT and positive PCR (group B), and 26 had a positive CT and at least two negative consecutive PCR results (group C, Fig. 1 ).
PCR-positive groups A and B ( n = 50) were chosen to interpret clinical and chest CT features because group C patients were not deemed COVID-19 positive by PCR. Over half of the patients were males (56%, Table 1 ). The most common symptoms at the onset of illness (Table 1 ) were fever (64%) and cough (44%); less common symptoms were rhinorrhea (16%), abdominal pain (4%), diarrhea (6%), fatigue (4%), and pharyngalgia (2%). Six children (12%) were asymptomatic. After treatment, 38 (76%) children were discharged.
Laboratory reference normal ranges were age- and gender-adjusted according to values in Reference Range Values for Pediatric Care 2nd edition , pages 92–98 [ 31 ]. On laboratory assessment, eight (16%) and seven (14%) patients had lymphopenia and thrombocytopenia, respectively. In contrast, four (8%) were noted to have lymphocytosis, and two (4%) had thrombocytosis. Overall, leukopenia was observed in 19 (38%) patients and elevated C-reactive protein in ten (20%) patients. A small set of patients had hemoglobin abnormalities, four (8%) with elevated hemoglobin, and six (12%) with anemia (Table 2 ).
Of the 26 patients in group C, all had more than two negative consecutive PCR results. However, they all had a history of exposure to COVID-19 infection (or strongly suspected infection), and their chest CT had similar patterns to confirmed patients in group A (Table 2 ). Fisher exact test results indicated that there was no significant difference in CT characteristics (ground-glass opacity [ p > 0.05], local patchy shadowing [ p > 0.05], bilateral patchy shadowing [ p > 0.05], interstitial abnormalities [ p > 0.05]) and lesion location (parallel pleura [ p > 0.05], visible vascular thickening [ p > 0.05], subpleural [ p > 0.05], lower lobe of the lung [ p > 0.05], middle lobe of the lung [ p > 0.05], upper lobe of the lung [ p > 0.05]) between groups A and C (Table 3 ).
Among the 50 children with positive PCR results, five of them (10%) had negative initial PCR results but showed positive results in subsequent tests. Two of the 50 (4%) had no clinical symptoms and no radiologic findings. Seven of the 50 (14%) were negative for any abnormal CT findings. The spectrum of COVID-19 severity was two (4%) had no symptoms or radiologic signs, five (10%) very mild, 41 (82%) mild, and two (4%) critically ill with one having multiple organ dysfunction syndrome (MODS) and another with renal failure (Table 1 ). There were no patient mortalities in this study. The critically ill patient with renal failure has since fully recovered.
Among the 43 children with positive PCR results and abnormal CT findings, 41 patients had lesions present in the subpleural area (95%) and lower lung lobes (65%), especially in the posterior segment of the lower lung lobes (22 [78%] of 28). Ground-glass opacities (GGO) were the most common radiologic lesion identified on chest CT (67%). Local patchy shadowing (37%) was the second most common radiologic lesion, followed by local bilateral patchy shadowing (21%, Table 2 ). Interstitial lesions were rare (7%). Pleural fluid was observed in one case, and no lymphadenopathy was noted (Table 2 ). Appearances of lesions were irregular shaped, flaky, wedge-shaped, or strip-shaped. The long axis of some lesions (49%) was parallel to the pleura. However, lesions did not follow the segment of the lung lobe, single or multiple, and diffuse consolidation was rare. Bilateral lesions can be seen radiating around the bronchial blood vessels or showing large areas of consolidation, which can be traced by the lung segment into the bronchial tube.
Among the 50 confirmed children (groups A and B), 29 patients (including 23 discharged children) had more than one chest CT. Nineteen of the 29 patients (65%) had improved CT presentations after treatment, and lesions in two of the 19 patients completely disappeared. Two of the 29 patients (7%) showed no change in CT lesions, and 8 of the 29 patients (28%) had more CT lesions after treatment.
Figure 2 illustrates typical radiographic features of COVID-19 pneumonia in children. Figure 3 shows chest CT before and after treatment from three COVID-19 children. Cox regression results (Table 4 ) indicated that there is no association between changes in CT lesions (completely absorbed [ p > 0.05], partially absorbed [ p > 0.05], worse [ p > 0.05]). Table 5 lists changes in CT lesions during treatment. Table 6 lists the normal ranges for children of different ages based on Reference Range Values for Pediatric Care 2nd edition pages 92–98 [ 31 ].
Chest CT images depicting typical radiographic findings of COVID-19 pneumonia in children. 2A A unilateral chest CT from a 14-year-old boy with a cough. Ground-glass opacities under and parallel to the pleura (thick green arrow) in the inferior lobes of the left lungs. Ground-glass opacities distributed along the bronchovascular bundle (thin green arrow). 2B Bilateral ground-glass opacities with vascular thickening (arrowheads) in the subpleural area from a 13-year-old boy with a fever and a cough. 2C Local patchy shadowing (green arrow) image from a 6 month-old girl with a fever and a cough. 2D Lesions in the lower lobe of both lungs (green arrows) on chest CT obtained from a 15-year-old boy with a fever and a cough
Chest CT findings at initial presentation and at discharge. 3A , 3B Chest CT scans obtained from a 1-year-old boy, presenting with fever and diarrhea, at arrival ( 3A ) and after ( 3B ) treatment. The first CT scan shows a large, patchy shadow in the left inferior lobe (green arrow). The second CT scan shows no lesions. The patient was hospitalized for 17 days prior to discharge. 3C , 3D Chest CT scans from a 4-month-old girl, who presented with a fever and a cough at arrival. The first CT scan reveals multiple ground-glass opacities under the pleura in the left superior lobe (green arrows). The second CT scan reveals that the range of original lesions was enlarged and extended to the center. The girl was hospitalized for 13 days and subsequently discharged. 3E , 3F Chest CT scans from a 14-year-old boy, presenting with rhinorrhea and a cough, at arrival and discharge. The first CT scan reveals a patchy shadow in the left middle lobe (arrowhead). There were no obvious changes in the areas of pulmonary consolidation on the second CT scan. The boy was hospitalized for 11 days and then discharged
The symptoms in children with COVID-19 infection have been well described in the literature [ 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 ]. Our results are consistent with these previous reports. For example, the clinical symptoms from our study versus the recent study with the most pediatric patients are similar [ 10 ]: fever, 64% versus 41.5%; cough, 44% versus 48.5%; diarrhea, 6% versus 8.8%; and fatigue, 4% versus 7.6%. Results including ours indicate that COVID-19 symptoms in children follow a similar pattern in adults, albeit much less severe.
Our results of abnormal laboratory findings for children infected with COVID-19 contrast with recently published ones [ 15 , 22 , 23 , 24 ]. For example, our results for lymphopenia compared to Zheng et al. are 16% versus 40% [ 22 ]. Their normal reference values for lymphocytes were (2.1–5.7) × 10 9 /L (< 3 years), (1.4–4.2) × 10 9 /L (4–6 years), and (1.1–3.2) × 10 9 /L (≥ 6 years). To date, this is the only paper that has explicitly listed the normal ranges for children of different age ranges [ 22 ]. Thus, the differences between ours and those in the literature are most likely due to different normal ranges used for children of different ages or the small number of children who participated in their studies.
Like clinical symptoms, the laboratory findings in COVID-19-positive pediatric patients can vary from adult patients. Guan et al. [ 25 ] noted that 731 (82%) of 890 adult patients had lymphopenia, whereas only eight (16%) children had lymphopenia in this study. Similarly, 481 (61%) of 793 adult patients were found to have an elevated C-reactive protein. In contrast, only ten (20%) children in this study had elevated C-reactive protein. Some laboratory findings were consistent between children and adult groups: leukopenia 38% versus 36% and thrombocytopenia 14% versus 18%. The mechanism behind the observations is unknown and might provide an explanation for the differences between pediatric and adult patients.
The most common pattern of chest CT is ground-glass opacities, followed by local patchy shadowing and then local bilateral patchy shadowing, which is consistent with published data [ 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 ]. Our study indicates that chest CT manifested with a predominance of lesions in the subpleural area (41 [95%] of 43) and in lower lung lobes (28 [65%] of 43), especially in the posterior segment (22 [78%] of 28), an area with a relatively dense amount of bronchioles, blood vessels, and alveoli. To the best of our knowledge, these are the first quantitative results on the locations of chest CT lesions for COVID-19 children [ 21 ]. COVID-19 is less severe in children than in adults, and the children infected with COVID-19 were at the early stages of the disease when admitted to the hospital. The fact that an overwhelming percentage of pediatric patients had lesions in the subpleural area suggests this site is the first target for the COVID-19 virus.
The current gold standard for the diagnosis of COVID-19 is PCR. However, it has been documented that patients with a negative PCR result cannot be definitively ruled out for COVID-19 infection [ 11 , 26 ]. Our results are consistent with the literature. Among the 50 hospitalized children with positive PCR results, five of them (10%) had negative initial PCR results but showed positive results in subsequent tests. Moreover, 26 patients in group C never had a positive PCR result but had histories of contact with COVID-19 patients. Most of them exhibited clinical symptoms such as fever (81%) and cough (73%). Although they received a negative PCR result at least twice, all 26 patients had similar CT patterns to the PCR-positive COVID-19 patients in group A. Twenty-one (81%) had ground-glass opacities (GGO). Seven (27%) had local patchy shadowing. Five (19%) had bilateral patchy shadowing. Furthermore, our Fisher exact analysis indicated that there was no significant difference in CT image characteristics and lesion location between groups A and C. Although a positive CT alone cannot rule out the possibility of other causes of virus-induced pneumonia [ 11 , 26 ], all 26 children were hospitalized and given immediate antiviral and supportive therapy. Whether or not a child presents with pneumonia is one of the key considerations for clinical management, and it is crucial to start treatment as early as possible, considering that many deaths in the adult population are due to complications resulting from severe pneumonia [ 3 , 4 , 5 , 6 , 7 ].
It has been well documented that chest CT is a powerful tool to identify and characterize pneumonia for COVID-19 adult patients [ 25 , 26 , 27 , 28 ]. However, there is no publication to study its usefulness in evaluating clinical recovery for children with COVID-19 infection. To determine whether CT is necessary, we investigated the data of 23 patients who had been discharged after effective treatment and had at least two CT scans. All patients had normal body temperatures for more than 3 days at the time of discharge, clinical symptoms disappeared, and PCR tests all returned negative twice at 24-h intervals. Of the 23 children, eight patients did not receive CT scans within the 2 days before their discharge. However, in their most recent CT scan performed in the hospital, most children either still had lesions (50%), or more developed lesions since the previous scan (37%). The remaining 15 discharged children had a CT obtained within 2 days of discharge. Again, ten patients had lesions that were not completely absorbed (67%), two were the same (13%), and lesions in another two became worse (13%). These results indicate that CT may not be better than symptoms in evaluating recovery. Our Cox regression analysis further showed that there was no association between changes in CT lesions and clinical outcomes. The results are consistent with the knowledge that clinical improvement predates radiographic improvement by weeks for children with community-acquired pneumonia.
When deciding whether to use CT on children, the harmful effects that radiation may have on a growing body must be considered. Hong et al., in a study of 12,068,821 children aged 0 to 19 years, found a statistically significant increase in cancer in children exposed at least once to diagnostic low-dose ionizing radiation after adjusting for age and sex [ 29 , 30 ]. Based on our data, we do not recommend using CT for determining clinical recovery unless it is necessary to evaluate the status of pneumonia. For comparison, the current criteria for discharging adult patients infected with COVID-19 in China are (1) normal body temperature for 3 days, (2) two negative PCR tests at 24-h intervals, (3) resolution of clinical symptoms (these three are the current criteria for discharging pediatric patients in this hospital), plus (4) a chest imaging requirement: pulmonary imaging must show significant absorption of lesions. To date, there are no child-specific discharge criteria for COVID-19 in China.
Our study had a few limitations. First, this study has a small sample size and was conducted at a single-center in Wuhan, China, located at the center of the outbreak. The clinical severity of pediatric patients outside Wuhan may be less severe. Indeed, it is reported that there is a lower death rate of adult patients outside Wuhan areas. Second, long-term follow-up was not done because of the short time for data collection.
The severity of COVID-19 infection in children is less than it is in adults in terms of symptoms, lung consolidation as visualized by CT, and laboratory abnormalities. COVID-19 has a preference for subpleural areas of the lung in pediatric patients. Chest CT is an excellent tool to detect and characterize COVID-19 pneumonia but not to evaluate the resolution of illness for children.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Bilateral patchy shadowing
Coronavirus disease 2019
- Computerized tomography
Ground-glass opacities
Local bilateral shadowing
Middle Eastern respiratory syndrome
Multiple organ dysfunction syndrome
Polymerase chain reaction
Severe acute respiratory syndrome
Cao Q, Chen YC, Chen CL, Chiu CH. SARS-CoV-2 infection in children: transmission dynamics and clinical characteristics. J Formos Med Assoc. 2020;119:670–3.
Article CAS Google Scholar
Lee PI, Hu YL, Chen PY, Huang YC, Hsueh PR. Are children less susceptible to COVID-19? J Microbiol Immunol. 2020. https://doi.org/10.1016/j.jmii.2020.02.011 .
Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J Med Virol. 2020. https://doi.org/10.1002/jmv.25678 .
Paules CI, Marston HD, Fauci AS. Coronavirus infections—more than just the common cold. JAMA. 2020. https://doi.org/10.1001/jama.2020.0757 .
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020. https://doi.org/10.1056/NEJMoa2001017 .
Chen N, Zhou M, Dong X, Qu J, Han Y, Qiu Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020. https://doi.org/10.1016/S0140-6736(20)30211-7 .
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan. JAMA. 2020;323(11):1061–9.
WHO-China Joint Mission. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). Geneva 2020. https://www.who.int/docs/default-source/coronaviruse/who-china-jointmission-on-covid-19-final-report.pdf . Accessed 1 Mar 2020.
Cai J, Xu J, Lin D, Yang Z, Xu L, Qu Z, et al. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciaa198 .
Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. SARS-CoV-2 infection in children. N Engl J Med. 2020. https://doi.org/10.1056/NEJMc2005073 .
Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol. 2020. https://doi.org/10.1002/ppul.24718 .
Ji LN, Chao S, Wang YJ, Li XJ, Mu XD, Lin MG, et al. Clinical features of pediatric patients with COVID-19: a report of two-family cluster cases. World J Pediatr. 2020. https://doi.org/10.1007/s12519-020-00356-2 .
Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, Tong S. Epidemiology of COVID-19 among children in China. Pediatrics. 2020. https://doi.org/10.1542/peds.2020-0702 .
Li W, Cui H, Li K, Fang Y, Li S. Chest computed tomography in children with COVID-19 respiratory infection. Pediatr Radiol. 2020. https://doi.org/10.1007/s00247-020-04656-7 .
Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020. https://doi.org/10.1016/S1473-3099(20)30198-5 .
Kelvin AA, Halperin S. COVID-19 in children: the link in the transmission chain. Lancet Infect Dis. 2020. https://doi.org/10.1016/S1473-3099(20)30236-X .
Liu H, Liu F, Li J, Zhang T, Wang D, Lan W. Clinical and CT imaging features of the COVID-19 pneumonia: focus on pregnant women and children. J Inf Secur. 2020. https://doi.org/10.1016/j.jinf.2020.03.007 .
Shen Q, Guo W, Guo T, Li J, He W, Ni S, Ouyang X, Liu J, Xie Y, Tan X, et al. Novel coronavirus infection in children outside of Wuhan, China. Pediatr Pulmonol. 2020. https://doi.org/10.1002/ppul.24762 .
Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology. 2020;2020. https://doi.org/10.1148/radiol.2020200230 .
Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;2020. https://doi.org/10.1148/radiol.2020200463 .
Pan Y, Guan H, Zhou S, Wang Y, Li Q, Zhu T, et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020. https://doi.org/10.1007/s00330-020-06731-x .
Zheng F, Liao C, Fan QH, Chen HB, Zhao XG, Xie ZG, Li XL, Chen CX, Lu XX, Liu ZS, et al. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr Med Sci. 2020. https://doi.org/10.1007/s11596-020-2172-6 .
Henry BM, Lippi G, Plebani M. Laboratory abnormalities in children with novel coronavirus disease 2019. Clin Chem Lab Med. 2020. https://doi.org/10.1515/cclm-2020-0272 .
Li Y, Guo F, Cao Y, Li L, Guo Y. Insight into COVID-2019 for pediatricians. Pediatr Pulmonol. 2020. https://doi.org/10.1002/ppul.24734 .
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of 2019 novel coronavirus infection in China. NEJM. 2020. https://doi.org/10.1056/NEJMoa2002032 .
Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020. https://doi.org/10.1148/radiol.2020200343 .
Fang Y, Zhang H, Xu Y, et al. CT manifestations of two cases of 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;2020. https://doi.org/10.1148/radiol.2020200343 .
Pan F, Ye T, Sun P, Gui S, Liang B, Li L, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020. https://doi.org/10.1148/radiol.2020200370 .
Hong JY, Han K, Jung JH, Kim JS. Association of exposure to diagnostic low-dose ionizing radiation with risk of cancer among youths in South Korea. JAMA Netw Open. 2019. https://doi.org/10.1001/jamanetworkopen.2019.10584 .
Kalra MK, Maher MM, Rizzo S, Kanarek D, Shephard JO. Radiation exposure from chest CT: issues and strategies. J Korean Med Sci. 2004;19(2):159–66.
Article Google Scholar
Soghier LM, Fratantoni K, Reyes C. Reference range values for pediatric care. 2nd ed. Itasca: American Academy of Pediatrics; 2019. p. 92–8.
Google Scholar
Download references
Acknowledgements
We would like to thank Pin He for her help in image processing.
The authors received no specific funding for this work.
Author information
Huijing Ma, Jiani Hu, and Jie Tian are joint first authors.
Authors and Affiliations
Imaging Center, Wuhan Children’s Hospital (Wuhan Maternal and Child Healthcare Hospital), Tongji Medical College, Huazhong University of Science & Technology, No.100 Hongkong Road, Wuhan, 430016, China
Huijing Ma, Baiqi Zhu & Jianbo Shao
Department of Radiology, School of Medicine, Wayne State University, Detroit, MI, 48201, USA
Jiani Hu, Maxwell Thomas Laws, Luke David Wesemann & Rafael Ramos
Beijing Advanced Innovation Center for Big Data-Based Precision Medicine, School of Medicine and Engineering, Beihang University, Beijing, 100191, China
Department of Radiology, Shenzhen Second People’s Hospital, The First Affiliated Hospital of Shenzhen University Health Science Center, 3002 SunGang Xi Road West, Shenzhen, 518035, China
Xi Zhou & Jun Xia
Medical department, Wuhan Children’s Hospital (Wuhan Maternal and Child Healthcare Hospital), Tongji Medical College, Huazhong University of Science & Technology, No.100 Hongkong Road, Wuhan, 430016, China
Department of Radiology, Tongji Hospital, Tongji University School of Medicine, Shanghai, 200065, China
Pingshan District People’s Hospital, Pingshan General Hospital of Southern Medical University, Shenzhen, 518118, Guangdong, China
You can also search for this author in PubMed Google Scholar
Contributions
HM, JH, JT, JX, and JS had roles in the study design, data analysis, data interpretation, literature search, and writing of the manuscript. XZ, HL, MTL, LDW, BZ, WC, and RR had roles in the data analysis, data interpretation, literature search, and writing of the manuscript. JS, HM, HL, and BZ had roles in clinical management, patient recruitment, and data collection and had full access to all of the data in the study and take responsibility for the integrity of the data. HM, JH, and JT contributed equally. The authors read and approved the final manuscript.
Corresponding authors
Correspondence to Jun Xia or Jianbo Shao .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the Ethics Committee of Wuhan Children’s Hospital (Wuhan Maternal and Child Health Care Hospital # WHCH 2020005). Written informed parental/guardian consent and child assent (where appropriate) were obtained prior to enrollment in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Ma, H., Hu, J., Tian, J. et al. A single-center, retrospective study of COVID-19 features in children: a descriptive investigation. BMC Med 18 , 123 (2020). https://doi.org/10.1186/s12916-020-01596-9
Download citation
Received : 26 March 2020
Accepted : 16 April 2020
Published : 06 May 2020
DOI : https://doi.org/10.1186/s12916-020-01596-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Coronavirus
- Epidemiology
- Clinical features
BMC Medicine
ISSN: 1741-7015
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Research article
- Open access
- Published: 25 January 2018
Secondary pulmonary alveolar proteinosis: a single-center retrospective study (a case series and literature review)
- Dongmei Zhang 1 , 5 ,
- Xinlun Tian ORCID: orcid.org/0000-0003-4307-6665 1 ,
- Ruie Feng 2 ,
- Xiaobei Guo 1 ,
- Peng Wang 3 ,
- Yusen Situ 4 ,
- Yi Xiao 1 &
- Kai-Feng Xu 1
BMC Pulmonary Medicine volume 18 , Article number: 15 ( 2018 ) Cite this article
3612 Accesses
15 Citations
2 Altmetric
Metrics details
Secondary pulmonary alveolar proteinosis (sPAP) is an extremely rare disease. The clinical features of sPAP patients remain to be summarizeds.
Patients pathologically diagnosed with PAP and with negative results for anti-granulocyte macrophage colony stimulating factor (GM-CSF) autoantibodies from Peking Union Medical College Hospital between January 2000 and July 2016 were retrospectively studied. The PubMed database was also searched for literature to collect published cases.
In our center, nine patients were diagnosed as sPAP with a median age of 37 years. Hematological disorders, including myelodysplastic syndrome (MDS), chronic myelogenous leukemia (CML), and pulmonary tuberculosis (TB) infection were the underlying diseases. Cases secondary to MDS had very poor prognosis as all of them survived less than 2 years after their diagnosis, while those secondary to TB had favorable prognosis. Only 33.3% of cases showed interlobular septal thickening in our sPAP group. Through literature review, 164 sPAP cases were collected. The age at diagnosis was 45.0 ± 14.8 years old and the gender radio was 1.20:1 (M:F). 61.9% of cases were diagnosed by bronchoscopy. MDS and CML were common underlying diseases in 34.1% and 15.2% of patients, respectively. Patients with sPAP secondary to hematological diseases had a short survival time and half of them died within 14.95 months after diagnosis.
Conclusions
MDS and TB infection were the most frequent underlying causes of sPAP in this single-center research in China, with cases secondary to MDS having a poor survival rate. sPAP was more likely to be secondary to hematological disorders, especially MDS and CML and had a fairly poor prognosis in published cases. sPAP should be suspected in PAP patients whose CT scan presents only ground-glass opacities without interlobular septal thickening.
Peer Review reports
Pulmonary alveolar proteinosis (PAP), a rare disease with an estimated prevalence of 3.7 to 6.2 cases per million persons [ 1 , 2 ], is characterized by the accumulation of lipoprotein material in alveoli and terminal respiratory airways [ 3 ]. Current knowledge about PAP is based on case studies and individual case reports, and as such, the prevalence of PAP may vary due to differences in medical systems and facilities in different countries. According to the pathogenesis, PAP can be classified in three ways: congenital PAP, secondary PAP (sPAP) and autoimmune PAP (or acquired PAP, or idiopathic PAP) [ 4 ]. sPAP mainly develops as a result of hematological diseases, the use of immunosuppression drugs, dust inhalation and certain chronic infections with impaired alveolar macrophage function. Autoimmune PAP, which accounts for over 90% of PAP cases, is an autoimmune disease with autoantibodies targeting granulocyte macrophage colony stimulating factor (GM-CSF) [ 5 ].
According to previous reports, sPAP is different from autoimmune PAP in aspects besides the pathogenesis. For example, sPAP has different features on high-resolution computed tomography (HRCT) compared to autoimmune PAP [ 6 ] and sPAP has a much poorer prognosis compared with autoimmune PAP [ 7 ]. Knowledge of the clinical characteristics of sPAP are still limited as the majority of current literature is in the form of case reports, the largest series being a report by Ishii H which included 40 Japanese patients [ 7 ]. As we do not know whether the prevalence of sPAP in China has any differences compared to other countries, we retrospectively collected sPAP patients hospitalized from 2000 to 2016 in our hospital, listed their clinical features and symptoms, and we also reviewed all published cases in literature to learn more about the characteristics of sPAP around the world.
Study design and participants
This is a case study of patients who were diagnosed with PAP in Peking Union Medical College Hospital (PUMCH) between Jan. 1st, 2000 and July 1st, 2016. Medical files were retroactively collected on Feb. 2017. The study was approved by the Ethical Committee of PUMCH (S-K-215), and universal informed consent forms were signed when each subject was admitted to our hospital. The patients or their kin have given written informed consent to publish these case details. Patients had a clinical diagnosis of PAP, which was further confirmed pathologically by testing for amorphous periodic Acid-Schiff (PAS)-positive granules, found either in milky broncho-alveolar lavage fluid (BALF) or in alveolar structures of lung biopsy tissues. Patients under 18 years old were excluded. Serum GM-CSF autoantibodies were evaluated using an enzyme-linked immunosorbent assay, as previously described [ 8 , 9 , 10 ]. Patients with positive serum GM-CSF autoantibodies (> 5 μg/mL) were also excluded.

Methods of data analysis
The patients’ quantitative characteristics such as age at diagnosis were summarized by median and range. The qualitative characteristics such as general appearance, imaging features and other organ complications were presented as a frequency distribution or percentage. Authors had no access to information that could identify individual participants during or after data collection.
Demographic characteristics
In total, among 157 hospitalized PAP cases, 9 patients were diagnosed with sPAP. Their clinical characteristics are summarized in Table 1 . In these 9 patients, 3 were female and 6 were male, and they ranged in age from 20 to 56 years (median age 37 years). At the time of administration, 5 (5/9) of the cases complained of fever and cough, followed by weakness (4/9), weight loss (3/9), and dyspnea or exertional dyspnea (3/9). Diagnostic procedures included BALF (3/9), CT guided biopsy (2/9), open lung biopsy (2/9), transbronchial lung biopsy (TBLB) plus BALF (1/9), and video-assisted thoracic surgery (VATS) (1/9).
Primary diseases
The primary diseases of these 9 sPAP cases were variable, as 5 cases arose from hematologic disorders (4 myelodysplastic syndrome (MDS) and 1 chronic myelogenous leukemia (CML)) and 4 cases from tuberculosis (TB). Mycobacterium chelonei infection was detected in one patient with underlying MDS using a subcutaneous nodule biopsy, and another patient was affected by hospital-acquired pneumonia with obscure pathogens, and neither of their sputum nor BALF cultures tested positive.
CT features
As shown in Table 1 , all of our cases presented bilateral ground-glass opacities (GGO) on their CT scans. Among them, GGO with a diffuse pattern was the most frequent pattern, presenting in 5 (5/9) patients, while a patchy geographic pattern and crazy paving pattern each presented in 2 (2/9) patients respectively. Small scattered nodules were found in both TB (2/4) and MDS patients (2/4). The patient infected with M. chelonei showed enlarged mediastinal lymph nodes. Interlobular septal thickening, which was thought to be a typical feature of PAP, could only be recognized in three (3/9) of our sPAP patients.
Pulmonary function tests
At the time of diagnosis, 8 patients underwent arterial blood gas tests and the mean arterial partial oxygen pressure (PaO 2 ) was 62.2 mmHg (34.1, 97.4 mmHg) in room air. In 7 patients who performed pulmonary function tests during administration, 6 had normal forced vital capacity (FVC) and forced expiratory volume in the first 1 s (FEV 1 ). All 5 cases evaluated for diffusing function showed a remarkable reduction in diffusing capacity for carbon monoxide (DL CO ) with a mean of 43.1% (22.1%, 58.9%) of the normal predicted value.
Treatment and prognosis
The median follow-up duration was 4.7 years in these patients. All 4 patients with underlying MDS died within 2 years after diagnosis, and whole lung lavage (WLL) was performed in 2 of them, but symptoms only improved temporarily. Both the symptoms and the CT scans improved in 3 of the 4 patients with TB after anti-TB therapy, and the remaining patient with TB remains stable throughout 8 years of follow-up. Unfortunately, the patient with CML could not be followed-up on. The patient with M.chelonei complications (patient 5) had his condition worsen rapidly even with antibiotic administration based on drug sensitivity tests and died 3 months after diagnosis.
Literature reviews
Texts were searched for using the terms “pulmonary alveolar lipoproteinosis [Title/abstract]” or “pulmonary alveolar proteinosis [Title/abstract]” in the PubMed database. Cases published only in abstract without full-text were excluded. For cases with a history of dust inhalation, only those that tested negative for GM-CSF autoantibodies were included as dust exposure was not a specified factor of sPAP [ 11 ].
Using the above search strategy, 1291 articles on PAP were identified. These articles were carefully reviewed to remove reviews, abstracts, commitments, animal researches, relevant pathophysiological studies or pharmaceutical researches, articles about congenital PAP, and articles about autoimmune PAP. We also removed those diagnosed by autopsy post-mortem and pediatric patients less than 18 years old. In the end, 155 cases of sPAP with underlying diseases were found (Additional file 1 : Figure S1). In Table 2 , we summarized the available characteristics of the 164 cases in total (including our 9 cases).
The patients were diagnosed at an age of 45.0 ± 14.8. The male to female ratio was 1.20:1. Bronchoscopy, including BALF and/or TBLB, was the most frequent method used to diagnose sPAP, with 61.9% of cases diagnosed in this manner. Other procedures used included VATS and open lung biopsy, with 17.7% and 18.9% of cases being diagnosed in those manners respectively. Approximately 70% of cases were secondary to hematological diseases, such as MDS (34.1%), CML (15.2%) and AML (acute myelogenous leukemia) (5.5%).
Underlying infectious diseases, mainly due to TB, non-TB mycobacteria, human immunodeficiency virus, and Aspergillosis , consisted of 7.9% of all cases. Autoimmune diseases including Behcet’s disease, vasculitis, Sjogren’s syndrome, and dermatomyositis accounted for 6.7% of the underlying causes. 10.4% of sPAP patients were secondary to lung or kidney transplantation. The other rare underlying diseases were non-hematological tumors and dust inhalation. As shown in Fig. 1 , the median survival in 92 cases secondary to hematological diseases was only 14.95 months, which was significantly shorter than sPAP secondary to other causes ( p < 0.001).
Survival probability of secondary pulmonary alveolar proteinosis cases. In our 9 cases and 155 cases from literatures, 132 cases had the survival information. In 92 cases secondary to hematological diseases, the median survival was 14.95 months as 66 (71.3%) died within 5 years. In 40 cases secondary to other causes, the median survival was to months and 14 (35%) died within 5 years. Log-rank (Mantel-Cox) test X 2 = 11.78, P < 0.001
Since sPAP is a rare disease, less than 200 cases have been reported throughout the world, with most of them in form of case reports. H. Ishii [ 7 ] summarized the characteristics of 40 Japanese sPAP patients, which was the largest retrospective observational study about sPAP that we found. To the best of our knowledge, our study is the largest series of sPAP from a single center in China. We present some interesting findings. Firstly, TB is also a frequent underlying disease of sPAP in China, and fortunately, after effective anti-TB therapy, sPAP can be improved concurrently. Furthermore, hematologic disorders, especially MDS, are the predominant primary disease of sPAP, and these patients have a terrible prognosis with the majority dying within 2 years after diagnosis. Complications from other infections may accelerate the advancement of the disease.
In our center, 5.73% of PAP cases were determined to be sPAP, which is slightly lower than the percentage of sPAP (8.3% to 10%) in the Japanese case series [ 6 , 7 ]. Both our study and existing literature illustrates that hematologic disorders are the most frequent underlying disease of sPAP. The study including 31 cases of sPAP with MDS found that sPAP is an important risk factor predicting adverse outcome of MDS with a 2-year survival of 46.2% [ 12 ]. In our cases, all of the 4 patients with MDS as the underlying disease died within 2 years. The pathomechanism of sPAP secondary to hematological diseases remains unknown. Mutations in GATA2 may be associated with sPAP [ 13 , 14 ] since an autosomal dominant familial MDS and AML carried a heritable GATA2 mutation [ 15 ] and approximately one third of GATA2 mutation carriers also complicated sPAP [ 16 ]. It is speculated that a GATA2 deficiency leads to the alteration of alveolar cellular immunity because these patients had neither GM-CSF receptor mutations nor anti-GM-CSF autoantibodies. In the mice model, T-bet–over expressing T cells act to initiate the pathogenesis of sPAP [ 17 ]. Treatment of sPAP should focus on curing the underlying diseases. Bone marrow transplantation or cord blood transplantation can cure sPAP in these patients, but at the cost of an increased risk of infection and mortality [ 12 , 18 ].
Some infectious diseases such as TB can cause sPAP, possibly due to microorganisms impairing the function of macrophages. The heavy burden of TB in China makes it a significant underlying disease in our cases. In this circumstance, treating the underlying infection(s) can ameliorate sPAP, as shown in our cases, 3 cases improved and 1 case remained stable after anti-TB therapy. However, autoimmune PAP might cause complications with infections including TB, Nocardia , and Aspergillus . An anti-GM-CSF autoantibody test is effective to distinguish these two situations since it is negative in the former while positive in the later. Besides antibiotics, GM-CSF replacement is ineffective to the former but of benefit to the latter.
In our study, only 33.3% of cases show interlobular septal thickening, which is regarded as a typical feature in the CT scan of autoimmune PAP patients [ 19 ]. Additionally, only 22.2% of them present GGO with patchy geographic pattern, which is more common in autoimmune PAP [ 6 ]. As a result, sPAP should be suspected in PAP patients whose CT scans only present GGO without interlobular septal thickening or in the absence of a geographic pattern. More detailed evaluation about infectious and hematologic diseases should be performed. Moreover, qualification of anti-GM-CSF-Ab is valuable for the differentiation of autoimmune or secondary PAP.
Several limitations must be stressed with regards to this study. First, since PAP is a rare disease, the value of this study is limited by its small sample size. Our study was also limited in scope in that the underlying disease in our sPAP cases only included hematological diseases and infections, without covering dust inhalation, autoimmune disease or tumors. Secondly, as a retrospective study, some patient characteristics and information are not comprehensive, such as arterial blood gas and lung function. Furthermore, because our hospital is the largest rare disease treatment center in China, patients admitted are usually more complex and serious cases, and as such, we cannot avoid selection bias. Meanwhile, occupational diseases were restrictively diagnosed and treated in designated hospitals in China, so PAP secondary to occupational exposure is difficult to diagnose in our hospital. It is also difficult to compare the result of a single Chinese center with those all over the world. Thus, we conducted a comprehensive review of all the available cases published in available literature to minimize selection bias. Finally, in the review, we did not account for other factors that may affect the variation of sPAP, such as the geographical features of the patients.
In summary, this study found that MDS and TB infection were the most frequent underlying causes of sPAP in this single center research in China and cases that secondary to MDS had a poor survival rate. However, after taking all the published cases into consideration, hematological disorders, especially MDS and CML, are the major primary disease in 68.9% of cases. sPAP was often middle age onset, but it had a fairly poor prognosis when the underlying diseases were hematologic disorders. The diagnosis of sPAP should be considered in PAP patients whose CT scan only presents GGO without interlobular septal thickening.
Abbreviations
Acute myelogenous leukemia
Bronchoalveolar lavage fluid
Chronic myelogenous leukemia
Computed tomography
Diffusing capacity for carbon monoxide
Forced expiratory volume in the first 1 second
Forced vital capacity
Ground-glass opacities
Granulocyte macrophage colony stimulating factor autoantibodies
High-resolution computed tomography
Myelodysplastic syndrome
Pulmonary alveolar proteinosis
Periodic Acid-Schiff
Peking Union Medical College Hospital
Secondary pulmonary alveolar proteinosis
Tuberculosis
Transbronchial lung biopsy
Video-assisted thoracic surgery
Whole lung lavage.
Ben-Dov I, Kishinevski Y, Roznman J, Soliman A, Bishara H, Zelligson E, et al. Pulmonary alveolar proteinosis in Israel: ethnic clustering. Isr Med Assoc J. 1999;1:75–8. PMID: 10731299
CAS PubMed Google Scholar
Inoue Y, Trapnell BC, Tazawa R, et al. Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am J Respir Crit Care Med. 2008;177(7):752–62. https://doi.org/10.1164/rccm.200708-1271OC . PMID: 18202348
Article PubMed PubMed Central Google Scholar
Rosen SH, Castleman B, Liebow AA. Pulmonary alveolar proteinosis. N Engl J Med. 1958;258:1123–42. https://doi.org/10.1056/NEJM195806052582301 . PMID:13552931
Article CAS PubMed Google Scholar
Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. N Engl J Med. 2003;349:2527–39. https://doi.org/10.1056/NEJMra023226 . PMID:14695413
Borie R, Danel C, Debray MP, et al. Pulmonary alveolar proteinosis. Eur Respir Rev. 2011;20(120):98–107. https://doi.org/10.1183/09059180.00001311 . PMID:21632797
Ishii H, Trapnell BC, Tazawa R, Inoue Y, Akira M, Kogure Y, et al. Comparative study of high-resolution CT findings between autoimmune and secondary pulmonary alveolar proteinosis. Chest. 2009;136:1348–55. https://doi.org/10.1378/chest.09-0097 . PMID:19892674
Article PubMed Google Scholar
Ishii H, Tazawa R, Kaneko C, Saraya T, Inoue Y, Hamano E, et al. Clinical features of secondary pulmonary alveolar proteinosis: pre-mortem cases in Japan. Eur Respir J. 2011;37:465–8. https://doi.org/10.1183/09031936.00092910 . PMID:21282812
Xu KF, Chen Y, Guo ZJ, Zhu YJ. Autoantibody against granulocyte-macrophage colony-stimulating factor and other serum markers in pulmonary alveolar proteinosis. Zhonghua Jie He He Hu Xi Za Zhi. 2004;27:824–8. PMID: 15730782
PubMed Google Scholar
Uchida K, Nakata K, Trapnell BC, Terakawa T, Hamano E, Mikami A, et al. High-affinity autoantibodies specifically eliminate granulocyte-macrophage colony-stimulating factor activity in the lungs of patients with idiopathic pulmonary alveolar proteinosis. Blood. 2004;103:1089–98. https://doi.org/10.1182/blood-2003-05-1565 . PMID:14512323
Li Y, Tian X, Gui Y, Ma A, Li X, Zeng N, Zhang P, Li G, Xu K. Serum markers in patients with idiopathic pulmonary alveolar proteinosis. Zhonghua Jie He He Hu Xi Za Zhi. 2014;37(7):497–501. PMID: 25262689
Costabel U, Nakata K. Pulmonary alveolar proteinosis associated with dust inhalation: not secondary but autoimmune. Am J Respir Crit Care Med. 2010;181:427–8. https://doi.org/10.1164/rccm.200912-1800ED . PMID:20185750
Ishii H, Seymour JF, Tazawa R, Inoue Y, Uchida N, Nishida A, et al. Secondary pulmonary alveolar proteinosis complicating myelodysplastic syndrome results in worsening of prognosis: a retrospective cohort study in Japan. BMC Pulm Med. 2014;14:37. https://doi.org/10.1186/1471-2466-14-37 . PMID:24597668
Ballerie A, Nimubona S, Meunier C, et al. Association of pulmonary alveolar proteinosis and fibrosis: patient with GATA2 deficiency. Eur Respir J. 2016;48(5):1510–4. https://doi.org/10.1183/13993003.00252-2016 . PMID: 27799394
Griese M, Zarbock R, Costabel U, Hildebrandt J, Theegarten D, Albert M, et al. GATA2 deficiency in children and adults with severe pulmonary alveolar proteinosis and hematologic disorders. BMC Pulm Med. 2015;15:87. https://doi.org/10.1186/s12890-015-0083-2 . PMID: 26264606
Hahn CN, Chong CE, Carmichael CL, Wilkins EJ, Brautigan PJ, Li XC, et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat Genet. 2011;43:1012–7. https://doi.org/10.1038/ng.913 . PMID:21892162
Article CAS PubMed PubMed Central Google Scholar
Chaulagain CP, Pilichowska M, Brinckerhoff L, Tabba M, Erban JK. Secondary pulmonary alveolar proteinosis in hematologic malignancies. Hematol Oncol Stem Cell Ther. 2014;7:127–35. https://doi.org/10.1016/j.hemonc.2014.09.003 . PMID:25300566
Iriguchi S, Kikuchi N, Kaneko S, et al. T-cell-restricted T-bet overexpression induces aberrant hematopoiesis of myeloid cells and impairs function of macrophages in the lung. Blood. 2015;125(2):370–82. https://doi.org/10.1182/blood-2014-05-575225 . PMID: 25349175
Tabata S, Shimoji S, Murase K, Takiuchi Y, Inoue D, Kimura T, et al. Successful allogeneic bone marrow transplantation for myelodysplastic syndrome complicated by severe pulmonary alveolar proteinosis. Int J Hematol. 2009;90:407–12. https://doi.org/10.1007/s12185-009-0404-4 . PMID:19693450
Mehrian P, Homayounfar N, Karimi MA, Jafarzadeh H. Features of idiopathic pulmonary alveolar proteinosis in high resolution computed tomography. Pol J Radiol. 2014;79:65–9. https://doi.org/10.12659/PJR.890218 . PMID:24707326
PubMed PubMed Central Google Scholar
Download references
Acknowledgements
The authors would like to thank Tao Liu, Yongjian Liu, Juhong Shi, Chi Shao, and Xiaoming Huang for their efforts in performing and aiding the clinical diagnosis and following of sPAP patients.
This work was supported by “State Key Project, Clinical Cohort Study of Rare Diseases. Rare Lung Disease Research Group in China” (2016YFC0901502) and The Capital of the Public Health Program: Study on the Evaluation of Diagnosis and Treatment on Seven Treatable Rare Diseases (Z151100003915126)
Availability of data and materials
The data of our patients is available in the department of medical records in PUMCH. This data can be released with the agreement of patients. Identifying patient data cannot be shared.
Author information
Authors and affiliations.
Department of Respiratory Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
Dongmei Zhang, Xinlun Tian, Xiaobei Guo, Yi Xiao & Kai-Feng Xu
Department of Pathology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
Laboratory Department, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
Department of Biochemistry, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Canada
Present address: Department of Respiratory and Critical Care Medicine, Beijing Institute of Respiratory Medicine and Beijing Chaoyang Hospital, Capital Medical University, Beijing, China
Dongmei Zhang
You can also search for this author in PubMed Google Scholar
Contributions
DZ and XT conception, design, obtaining consent, and drafting manuscript; RF reviewed all the pathologicalslides of all the patients, PW detected pathogenic microbes of all the patients. XG collection of data; YS design and drafting manuscript; YX and KFX data analysis and interpretation. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Xinlun Tian .
Ethics declarations
Ethics approval and consent to participate.
This study has been approved by the Ethical Committee of PUMCH with reference number S-K-215, and informed consent forms and contents for publication forms were signed by all participants.
Consent for publication
The patients or their kin have given written informed consent to publication.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1: figure s1..
Trial inclusion flow chart. (TIFF 200 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Zhang, D., Tian, X., Feng, R. et al. Secondary pulmonary alveolar proteinosis: a single-center retrospective study (a case series and literature review). BMC Pulm Med 18 , 15 (2018). https://doi.org/10.1186/s12890-018-0590-z
Download citation
Received : 27 August 2017
Accepted : 15 January 2018
Published : 25 January 2018
DOI : https://doi.org/10.1186/s12890-018-0590-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Secondary pulmonary alveolar proteinosis (sPAP)
- Anti-granulocyte macrophage colony stimulating factor autoantibodies (GM-CSF-Ab)
- Myelodysplastic syndrome (MDS)
- Tuberculosis (TB)
BMC Pulmonary Medicine
ISSN: 1471-2466
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
- What Is a Retrospective Cohort Study? | Definition & Examples
What Is a Retrospective Cohort Study? | Definition & Examples
Published on February 10, 2023 by Tegan George . Revised on June 22, 2023.
A retrospective cohort study is a type of observational study that focuses on individuals who have an exposure to a disease or risk factor in common. Retrospective cohort studies analyze the health outcomes over a period of time to form connections and assess the risk of a given outcome associated with a given exposure.
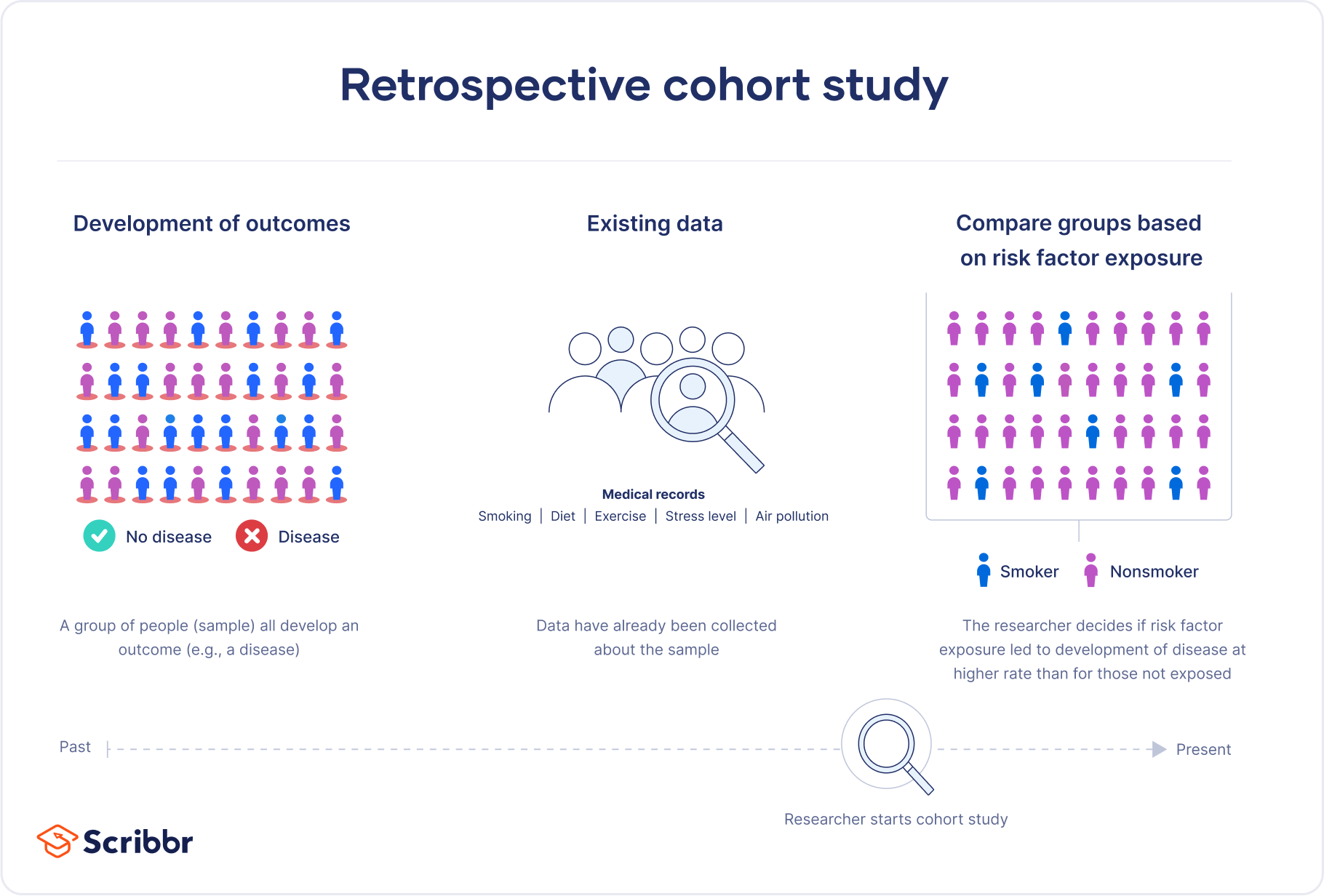
It is crucial to note that in order to be considered a retrospective cohort study, your participants must already possess the disease or health outcome being studied.
Table of contents
When to use a retrospective cohort study, examples of retrospective cohort studies, advantages and disadvantages of retrospective cohort studies, other interesting articles, frequently asked questions.
Retrospective cohort studies are a type of observational study . They are often used in fields related to medicine to study the effect of exposures on health outcomes. While most observational studies are qualitative in nature, retrospective cohort studies are often quantitative , as they use preexisting secondary research data. They can be used to conduct both exploratory research and explanatory research .
Retrospective cohort studies are often used as an intermediate step between a weaker preliminary study and a prospective cohort study , as the results gleaned from a retrospective cohort study strengthen assumptions behind a future prospective cohort study.
A retrospective cohort study could be a good fit for your research if:
- A prospective cohort study is not (yet) feasible for the variables you are investigating.
- You need to quickly examine the effect of an exposure, outbreak, or treatment on an outcome.
- You are seeking to investigate an early-stage or potential association between your variables of interest.
Retrospective cohort studies use secondary research data, such as existing medical records or databases, to identify a group of people with an exposure or risk factor in common. They then look back in time to observe how the health outcomes developed. Case-control studies rely on primary research , comparing a group of participants with a condition of interest to a group lacking that condition in real time.
Prevent plagiarism. Run a free check.
Retrospective cohort studies are common in fields like medicine, epidemiology, and healthcare.
You collect data from participants’ exposure to organophosphates, focusing on variables like the timing and duration of exposure, and analyze the health effects of the exposure. Example: Healthcare retrospective cohort study You are examining the relationship between tanning bed use and the incidence of skin cancer diagnoses.
Retrospective cohort studies can be a good fit for many research projects, but they have their share of advantages and disadvantages.
Advantages of retrospective cohort studies
- Retrospective cohort studies are a great choice if you have any ethical considerations or concerns about your participants that prevent you from pursuing a traditional experimental design .
- Retrospective cohort studies are quite efficient in terms of time and budget. They require fewer subjects than other research methods and use preexisting secondary research data to analyze them.
- Retrospective cohort studies are particularly useful when studying rare or unusual exposures, as well as diseases with a long latency or incubation period where prospective cohort studies cannot yet form conclusions.
Disadvantages of retrospective cohort studies
- Like many observational studies, retrospective cohort studies are at high risk for many research biases . They are particularly at risk for recall bias and observer bias due to their reliance on memory and self-reported data.
- Retrospective cohort studies are not a particularly strong standalone method, as they can never establish causality . This leads to low internal validity and external validity .
- As most patients will have had a range of healthcare professionals involved in their care over their lifetime, there is significant variability in the measurement of risk factors and outcomes. This leads to issues with reliability and credibility of data collected.
If you want to know more about statistics , methodology , or research bias , make sure to check out some of our other articles with explanations and examples.
- Student’s t -distribution
- Normal distribution
- Null and Alternative Hypotheses
- Chi square tests
- Confidence interval
- Quartiles & Quantiles
- Cluster sampling
- Stratified sampling
- Data cleansing
- Reproducibility vs Replicability
- Peer review
- Prospective cohort study
Research bias
- Implicit bias
- Cognitive bias
- Placebo effect
- Hawthorne effect
- Hindsight bias
- Affect heuristic
- Social desirability bias
Receive feedback on language, structure, and formatting
Professional editors proofread and edit your paper by focusing on:
- Academic style
- Vague sentences
- Style consistency
See an example

The primary difference between a retrospective cohort study and a prospective cohort study is the timing of the data collection and the direction of the study.
A retrospective cohort study looks back in time. It uses preexisting secondary research data to examine the relationship between an exposure and an outcome. Data is collected after the outcome you’re studying has already occurred.
Alternatively, a prospective cohort study follows a group of individuals over time. It collects data on both the exposure and the outcome of interest as they are occurring. Data is collected before the outcome of interest has occurred.
Retrospective cohort studies are at high risk for research biases like recall bias . Whenever individuals are asked to recall past events or exposures, recall bias can occur. This is because individuals with a certain disease or health outcome of interest are more likely to remember and/or report past exposures differently to individuals without that outcome. This can result in an overestimation or underestimation of the true relationship between variables and affect your research.
No, retrospective cohort studies cannot establish causality on their own.
Like other types of observational studies , retrospective cohort studies can suggest associations between an exposure and a health outcome. They cannot prove without a doubt, however, that the exposure studied causes the health outcome.
In particular, retrospective cohort studies suffer from challenges arising from the timing of data collection , research biases like recall bias , and how variables are selected. These lead to low internal validity and the inability to determine causality.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
George, T. (2023, June 22). What Is a Retrospective Cohort Study? | Definition & Examples. Scribbr. Retrieved March 25, 2024, from https://www.scribbr.com/methodology/retrospective-cohort-study/
Is this article helpful?
Tegan George
Other students also liked, what is a case-control study | definition & examples, what is an observational study | guide & examples, what is recall bias | definition & examples, what is your plagiarism score.
Artificial neural network to predict post-operative hypocalcemia following total thyroidectomy
- Original Article
- Published: 25 March 2024
Cite this article
- Karthik Nagaraja Rao ORCID: orcid.org/0000-0002-1420-7366 1 ,
- Ripudaman Arora 2 ,
- Renu Rajguru 2 &
- Nitin M Nagarkar 3
The primary objective of this study was to use artificial neural network (ANN) to predict the post operative hypocalcemia and severity of hypocalcemia following total thyroidectomy. The secondary objective was to determine the weightage for the factors predicting the hypocalcemia with the ANN. A single center, retrospective case series included treatment-naive patients undergoing total thyroidectomy for benign or malignant thyroid nodules from January 2020 to December 2022. Artificial neural network (ANN) - Multilayer Perceptron (MLP) used to predict post-operative hypocalcemia in ANN. Multivariate analysis was used construct validity. The data of 196 total thyroidectomy cases was used for training and testing. The mean incorrect prediction during training and testing was 3.18% (± σ = 0.65%) and 3.66% (± σ = 1.88%) for hypocalcemia. The cumulative Root-Mean-Square-Error (RMSE) for MLP model was 0.29 (± σ = 0.02) and 0.32 (± σ = 0.04) for training and testing, respectively. Area under ROC was 0.98 for predicting hypocalcemia 0.942 for predicting the severity of hypocalcemia. Multivariate analysis showed lower levels of post operative parathormone levels to be predictor of hypocalcemia ( p < 0.01). The maximum weightage given to iPTH (100%) > Need for sternotomy (28.55%). Our MLP NN model predicted the post-operative hypocalcemia in 96.8% of training samples and 96.3% of testing samples, and severity in 92.8% of testing sample in 10 generations. however, it must be used with caution and always in conjunction with the expertise of the surgical team. Level of Evidence – 3b.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Data availability
Data is available with the Corresponding Author.
Abiodun OI, Jantan A, Omolara AE, Dada KV, Mohamed NA, Arshad H (2018) State-of-the-art in artificial neural network applications: a survey. Heliyon 4(11):e00938
Article PubMed PubMed Central Google Scholar
Mencio M, Calcatera N, Ogola G, Mahady S, Shiller M, Roe E et al (2019) Factors contributing to unintentional parathyroidectomy during thyroid surgery. Proc Bayl Univ Med Cent 33(1):19–23
Noureldine SI, Genther DJ, Lopez M, Agrawal N, Tufano RP (2014) Early predictors of Hypocalcemia after total thyroidectomy. JAMA Otolaryngol– Head Neck Surg 140(11):1006–1013
Kourou K, Exarchos TP, Exarchos KP, Karamouzis MV, Fotiadis DI (2015) Machine learning applications in cancer prognosis and prediction. Comput Struct Biotechnol J 13:8–17
Article CAS PubMed Google Scholar
McHugh ML (2013) The Chi-square test of independence. Biochem Med 23(2):143–149
Article CAS Google Scholar
Abirami S, Chitra P Chapter Fourteen - Energy-efficient edge based real-time healthcare support system. In: Raj P, Evangeline P, editors. Advances in Computers [Internet]. Elsevier; 2020 [cited 2023 Jan 25]. p. 339–68. (The Digital Twin Paradigm for Smarter Systems and Environments: The Industry Use Cases; vol. 117). Available from: https://www.sciencedirect.com/science/article/pii/S0065245819300506
Mathew G, Agha R (2021) STROCSS 2021 guidelines: what is new? Ann Med Surg 72:103121
Article Google Scholar
Shuchleib-Cung A, Garcia-Gordillo JA, Ferreira-Hermosillo A, Mercado M (2022) Risk factors for hypocalcemia after total thyroidectomy. Cir Cir 90(6):765–769
PubMed Google Scholar
Qin Y, Sun W, Wang Z, Dong W, He L, Zhang T et al (2021) A Meta-analysis of risk factors for transient and permanent Hypocalcemia after total thyroidectomy. Front Oncol 10:614089
Chen Z, Zhao Q, Du J, Wang Y, Han R, Xu C et al (2021) Risk factors for postoperative hypocalcaemia after thyroidectomy: a systematic review and meta-analysis. J Int Med Res 49(3):0300060521996911
Article CAS PubMed PubMed Central Google Scholar
Edafe O, Balasubramanian SP (2017) Incidence, prevalence and risk factors for post-surgical hypocalcaemia and hypoparathyroidism. Gland Surg 6(Suppl 1):S59–68
Bai B, Chen Z, Chen W (2018) Risk factors and outcomes of incidental parathyroidectomy in thyroidectomy: a systematic review and meta-analysis. PLoS ONE 13(11):e0207088
Al Qubaisi M, Haigh PI (2019) Hypocalcemia after Total Thyroidectomy in Graves Disease. Perm J 23:18–188
Nagel K, Hendricks A, Lenschow C, Meir M, Hahner S, Fassnacht M et al (2022) Definition and diagnosis of postsurgical hypoparathyroidism after thyroid surgery: meta-analysis. BJS Open 6(5):zrac102
Noordzij JP, Lee SL, Bernet VJ, Payne RJ, Cohen SM, McLeod IK et al (2007) Early prediction of hypocalcemia after thyroidectomy using parathyroid hormone: an analysis of pooled individual patient data from nine observational studies. J Am Coll Surg 205(6):748–754
Article PubMed Google Scholar
Keijsers NLW (2010) Neural Networks. In: Kompoliti K, Metman LV, editors. Encyclopedia of Movement Disorders [Internet]. Oxford: Academic Press; [cited 2023 May 4]. p. 257–9. Available from: https://www.sciencedirect.com/science/article/pii/B9780123741059004937
Sarker IH, Kayes ASM, Watters P (2019) Effectiveness analysis of machine learning classification models for predicting personalized context-aware smartphone usage. J Big Data 6(1):57
Jiang L, Wu Z, Xu X, Zhan Y, Jin X, Wang L et al (2021) Opportunities and challenges of artificial intelligence in the medical field: current application, emerging problems, and problem-solving strategies. J Int Med Res 49(3):03000605211000157
Chaquet-Ulldemolins J, Gimeno-Blanes FJ, Moral-Rubio S, Muñoz-Romero S, Rojo-Álvarez JL (2022) On the Black-Box Challenge for Fraud Detection Using Machine Learning (I): Linear models and informative feature selection. Appl Sci 12(7):3328
Belenguer L (2022) AI bias: exploring discriminatory algorithmic decision-making models and the application of possible machine-centric solutions adapted from the pharmaceutical industry. AI Ethics 2(4):771–787
Kelly CJ, Karthikesalingam A, Suleyman M, Corrado G, King D (2019) Key challenges for delivering clinical impact with artificial intelligence. BMC Med 17(1):195
Naik N, Hameed BMZ, Shetty DK, Swain D, Shah M, Paul R et al Legal and Ethical Consideration in Artificial Intelligence in Healthcare: Who Takes Responsibility? Front Surg [Internet]. 2022 [cited 2023 Jan 25];9. Available from: https://www.frontiersin.org/articles/ https://doi.org/10.3389/fsurg.2022.862322
Graff AT, Miller FR, Roehm CE, Prihoda TJ (2010) Predicting hypocalcemia after total thyroidectomy: parathyroid hormone level vs. serial calcium levels. Ear Nose Throat J 89(9):462–465
Selberherr A, Scheuba C, Riss P, Niederle B (2015) Postoperative hypoparathyroidism after thyroidectomy: efficient and cost-effective diagnosis and treatment. Surgery 157(2):349–353
Carr AA, Yen TW, Fareau GG, Cayo AK, Misustin SM, Evans DB et al (2014) A single parathyroid hormone level obtained 4 hours after total thyroidectomy predicts the need for postoperative calcium supplementation. J Am Coll Surg 219(4):757–764
Lombardi CP, Raffaelli M, Princi P, Santini S, Boscherini M, De Crea C et al (2004) Early prediction of postthyroidectomy hypocalcemia by one single iPTH measurement. Surgery 136(6):1236–1241
Gupta S, Chaudhary P, Durga CK, Naskar D (2015) Validation of intra-operative parathyroid hormone and its decline as early predictors of hypoparathyroidism after total thyroidectomy: a prospective cohort study. Int J Surg Lond Engl 18:150–153
Reddy AC, Chand G, Sabaretnam M, Mishra A, Agarwal G, Agarwal A et al (2016) Prospective evaluation of intra-operative quick parathyroid hormone assay as an early predictor of post thyroidectomy hypocalcaemia. Int J Surg Lond Engl 34:103–108
Sywak MS, Palazzo FF, Yeh M, Wilkinson M, Snook K, Sidhu SB et al (2007) Parathyroid hormone assay predicts hypocalcaemia after total thyroidectomy. ANZ J Surg 77(8):667–670
Lecerf P, Orry D, Perrodeau E, Lhommet C, Charretier C, Mor C et al (2012) Parathyroid hormone decline 4 hours after total thyroidectomy accurately predicts hypocalcemia. Surgery 152(5):863–868
Lang BHH, Wong KP, Cowling BJ, Fong YK, Chan DKK, Hung GKY (2013) Do low preoperative vitamin D levels reduce the accuracy of quick parathyroid hormone in predicting postthyroidectomy hypocalcemia? Ann Surg Oncol 20(3):739–745
Richards ML, Bingener-Casey J, Pierce D, Strodel WE, Sirinek KR (2003) Intraoperative parathyroid hormone assay: an accurate predictor of symptomatic hypocalcemia following thyroidectomy. Arch Surg Chic Ill 1960 138(6):632–635 discussion 635–636
Google Scholar
Alía P, Moreno P, Rigo R, Francos JM, Navarro MA (2007) Postresection parathyroid hormone and parathyroid hormone decline accurately predict hypocalcemia after thyroidectomy. Am J Clin Pathol 127(4):592–597
Lombardi CP, Raffaelli M, Princi P, Dobrinja C, Carrozza C, Di Stasio E et al (2006) Parathyroid hormone levels 4 hours after surgery do not accurately predict post-thyroidectomy hypocalcemia. Surgery 140(6):1016–1023 discussion 1023–1025
Cayo AK, Yen TWF, Misustin SM, Wall K, Wilson SD, Evans DB et al (2012) Predicting the need for calcium and calcitriol supplementation after total thyroidectomy: results of a prospective, randomized study. Surgery 152(6):1059–1067
Download references
No Funding Received.
Author information
Authors and affiliations.
Principal Consultant, Head and Neck Oncology, Sri Shankara Cancer Hospital and Research Center, Bangalore, India
Karthik Nagaraja Rao
Department of Otolaryngology and Head Neck Surgery, All India Institute of Medical Sciences, Raipur, India
Ripudaman Arora & Renu Rajguru
Dean, SRM Medical College, Chengalpattu, India
Nitin M Nagarkar
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization – KNR
Data collection—KNR, RDA
Statistics and ANN development—KNR
Data analysis and interpretation—KNR
Drafting the article—All Authors
Critical revision of the article—All Authors
Final approval of the version to be published - All Authors
Corresponding author
Correspondence to Karthik Nagaraja Rao .
Ethics declarations
Financial disclosure, ethical review.
Exempted vide letter no. 2848/IEC-AIIMSRPR/2023 dated 6th May 2023 (retrospective study).
Conflict of Interest
Additional information, publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.

Supplementary Material 1
Supplementary material 2, rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Rao, K.N., Arora, R., Rajguru, R. et al. Artificial neural network to predict post-operative hypocalcemia following total thyroidectomy. Indian J Otolaryngol Head Neck Surg (2024). https://doi.org/10.1007/s12070-024-04608-9
Download citation
Received : 23 January 2024
Accepted : 04 March 2024
Published : 25 March 2024
DOI : https://doi.org/10.1007/s12070-024-04608-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Thyroidectomy
- Hypocalcemia
- Parathormone
- Artificial intelligence
- Neural network
- Find a journal
- Publish with us
- Track your research
This paper is in the following e-collection/theme issue:
Published on 21.3.2024 in Vol 12 (2024)
Forecasting Hospital Room and Ward Occupancy Using Static and Dynamic Information Concurrently: Retrospective Single-Center Cohort Study
Authors of this article:

Original Paper
- Hyeram Seo 1 , BS ;
- Imjin Ahn 2 , MS ;
- Hansle Gwon 2 , MS ;
- Heejun Kang 3 , MS ;
- Yunha Kim 2 , MS ;
- Heejung Choi 2 , MS ;
- Minkyoung Kim 1 , BS ;
- Jiye Han 1 , BS ;
- Gaeun Kee 2 , MS ;
- Seohyun Park 2 , BS ;
- Soyoung Ko 2 , BS ;
- HyoJe Jung 2 , BS ;
- Byeolhee Kim 2 , BS ;
- Jungsik Oh 4 , BS ;
- Tae Joon Jun 5 * , PhD ;
- Young-Hak Kim 6 * , MD, PhD
1 Department of Medical Science, Asan Medical Institute of Convergence Science and Technology, Asan Medical Center & University of Ulsan College of Medicine, Seoul, Republic of Korea
2 Department of Information Medicine, Asan Medical Center, Seoul, Republic of Korea
3 Division of Cardiology, Asan Medical Center, Seoul, Republic of Korea
4 Department of Digital Innovation, Asan Medical Center, Seoul, Republic of Korea
5 Big Data Research Center, Asan Institute for Life Sciences, Asan Medical Center, Seoul, Republic of Korea
6 Division of Cardiology, Department of Information Medicine, Asan Medical Center & University of Ulsan College of Medicine, Seoul, Republic of Korea
*these authors contributed equally
Corresponding Author:
Young-Hak Kim, MD, PhD
Division of Cardiology
Department of Information Medicine
Asan Medical Center & University of Ulsan College of Medicine
88, Olympic-ro 43-gil
Seoul, 05505
Republic of Korea
Phone: 82 2 3010 0955
Email: [email protected]
Background: Predicting the bed occupancy rate (BOR) is essential for efficient hospital resource management, long-term budget planning, and patient care planning. Although macro-level BOR prediction for the entire hospital is crucial, predicting occupancy at a detailed level, such as specific wards and rooms, is more practical and useful for hospital scheduling.
Objective: The aim of this study was to develop a web-based support tool that allows hospital administrators to grasp the BOR for each ward and room according to different time periods.
Methods: We trained time-series models based on long short-term memory (LSTM) using individual bed data aggregated hourly each day to predict the BOR for each ward and room in the hospital. Ward training involved 2 models with 7- and 30-day time windows, and room training involved models with 3- and 7-day time windows for shorter-term planning. To further improve prediction performance, we added 2 models trained by concatenating dynamic data with static data representing room-specific details.
Results: We confirmed the results of a total of 12 models using bidirectional long short-term memory (Bi-LSTM) and LSTM, and the model based on Bi-LSTM showed better performance. The ward-level prediction model had a mean absolute error (MAE) of 0.067, mean square error (MSE) of 0.009, root mean square error (RMSE) of 0.094, and R 2 score of 0.544. Among the room-level prediction models, the model that combined static data exhibited superior performance, with a MAE of 0.129, MSE of 0.050, RMSE of 0.227, and R 2 score of 0.600. Model results can be displayed on an electronic dashboard for easy access via the web.
Conclusions: We have proposed predictive BOR models for individual wards and rooms that demonstrate high performance. The results can be visualized through a web-based dashboard, aiding hospital administrators in bed operation planning. This contributes to resource optimization and the reduction of hospital resource use.
Introduction
The global health care market continues to grow, but the burden of health care costs on governments and individuals is reaching its limits. Consequently, there is increasing interest in the efficient use of limited resources in health care systems, and hospitals must develop approaches to maximize medical effectiveness within budgetary constraints [ 1 , 2 ]. One approach to this is optimizing the use of medical resources. Medical resources can be broadly categorized into 3 categories: human resources, physical capital, and consumables. The appropriate and optimized use of these resources is critical for improving health care quality and providing care to a larger number of patients [ 3 , 4 ].
Among the 3 medical resources, hospital beds are considered one of the physical capitals provided by hospitals to patients. These beds are allocated for various purposes, such as rest, hospitalization, postsurgical recovery, etc. They constitute one of the factors that can directly influence the patient’s internal satisfaction within the hospital. However, owing to limited space, hospitals often have a restricted number of beds. Moreover, the number and functionality of beds are often fixed owing to budgetary or environmental constraints, making it difficult to make changes. Nonetheless, if hospital administrators can evaluate bed occupancy rates (BORs) according to different time periods, they can predict the need for health care professionals and resources. On the basis of this information, hospitals can plan resources efficiently, reduce operational costs, and achieve economic objectives [ 5 ]. In addition, excessive BORs can exert a negative effect on the health of staff members and increase the possibility of exposure to infection risks. Hence, emphasizing only maintaining a high BOR may not necessarily lead to favorable outcomes for the hospital [ 6 , 7 ]. Considering these reasons, BOR prediction plays a vital role in hospitals and is recognized as a broadly understood necessity for resource optimization in the competitive medical field.
In the medical field, optimizing resources is crucial in the face of limited bed capacity and intense competition. Therefore, bed planning is a vital consideration aimed at minimizing hospital costs [ 8 ]. To achieve this, hospitals need to plan staffing and vacations weeks or months in advance [ 9 ]. The use of machine learning (ML) technology for BOR prediction is necessary to address fluctuations in patient numbers due to seasonal variations or infectious diseases, ensuring continuous hospital operations. In the Netherlands, hospitals have already implemented ML-based BOR prediction [ 10 ], and Johns Hopkins Hospital uses various metrics to effectively manage bed capacity for optimization. Predicting BORs based on quantitative data contributes to validating the clinical quality and cost-effectiveness of treatments. This, in turn, enhances overall accountability throughout the wards and contributes to improving hospital efficiency [ 11 ].
Hospital BOR prediction has been investigated using various approaches recently. From studies predicting bed demand using mathematical statistics or regression equation models based on given data [ 12 - 15 ], the focus has shifted toward modeling approaches using time-series analysis. This approach observes recorded data over time to predict future values.
A previous study has taken an innovative approach using time-series analysis alongside the commonly used regression analysis for bed demand prediction, and the study demonstrated that using time-series prediction for bed occupancy yielded higher performance results than using a simple trend fitting approach [ 16 ]. Another study used the autoregressive integrated moving average (ARIMA) model for univariate data and a time-series model for multivariate data to predict BORs [ 17 ]. With the advancement of deep learning (DL) models that possess strong long-term memory capabilities, such as recurrent neural network (RNN) and long short-term memory (LSTM), there has been an increase in studies applying these models to time-series data for prediction purposes. For instance, in the study by Kutafina et al [ 9 ], hospital BORs were predicted based on dates and public holiday data from government agencies and schools, without involving the personal information of patients. The study used a nonlinear autoregressive exogenous model to predict a short-term period of 60 days, with an aim to contribute to the planning of hospital staff. The model demonstrated good performance, with an average mean absolute percentage error of 6.24%. In emergency situations, such as the recent global COVID-19 pandemic, the sudden influx of infected patients can disrupt the hospitalization plans for patients with pre-existing conditions [ 18 ]. Studies have been conducted using DL architectures to design models for predicting the BOR of patients with COVID-19 on a country-by-country basis. Some studies incorporated additional inputs, such as vaccination rate and median age, to train the models [ 19 ]. Studies have also been conducted to focus on the short-term prediction of BORs during the COVID-19 period [ 20 , 21 ]. Prior studies are summarized in Table 1 .
Although previous research has contributed to BOR prediction and operational planning at the hospital level, more detailed and systematic predictions are necessary for practical application in real-world operations. To address this issue, studies have developed their own computer simulation hospital systems to not only predict bed occupancy but also execute scheduling for admissions and surgeries to enhance resource utilization [ 22 - 24 ]. Nevertheless, existing studies have the limitation of focusing solely on the overall BOR of the hospital. As an advancement to these studies, we aim to propose a strategy for predicting the BOR at the level of each ward and room using various variables in a time-series manner. Interestingly, to our knowledge, this is the first study to apply DL to predict ward- and room-specific occupancy rates using time-series analysis.
a MLR: multinomial logistic regression.
b LR: linear regression.
c LOS: length of stay.
d ARIMA: autoregressive integrated moving average.
e NARX: nonlinear autoregressive exogenous.
f RNN: recurrent neural network.
g LSTM: long short-term memory.
h GRU: grid recurrent unit.
i SRNN: simple recurrent neural network.
Goal of This Study
The aim of this study was to predict the BORs of hospital wards and rooms using time-series data from individual beds. Although overall bed occupancy prediction is useful for macro-level resource management in hospitals, resource allocation based on the prediction of occupancy rates for each ward and room is required for specific hospital scheduling and practicality. Through this approach, we aim to contribute to the efficient operational cost optimization of the hospital and ensure the availability of resources required for patient care.
We have developed time-series prediction models based on deep neural network (DNN), among which 1 model combines data representing room-specific features (static data) with dynamic data to enhance the prediction performance for room bed occupancy rates (RBORs). Based on bidirectional long short-term memory (Bi-LSTM), the RBOR prediction model demonstrates a lower mean absolute error (MAE) of 0.049, a mean square error (MSE) of 0.042, a root mean square error (RMSE) of 0.007, and a higher R 2 score of 0.291, indicating the highest performance among all RBOR models.
We developed 6 types of BOR prediction models, of which 2 types were used for predicting ward bed occupancy rates (WBORs), and the other 4 types focused on predicting RBORs. These models use LSTM and Bi-LSTM architectures with strong long-term memory capabilities as their basic structure. We created 6 models for each architecture, resulting in a total of 12 models. The WBOR models were used for predicting weekly and monthly occupancy rates, serving long-term hospital administrative planning purposes. Conversely, the RBOR models were designed for immediate and rapid occupancy planning and were trained with 3- and 7-day intervals. Each RBOR model was enhanced by combining static data, which represent room-specific features, to generate more sophisticated prediction models.
Figure 1 shows the potential application of our model as a form of web software in a hospital setting. Through an online dashboard, it can provide timely information regarding bed availability, enabling intelligent management of patient movements related to admission and discharge. It facilitates shared responsibilities within the hospital and simplifies future resource planning [ 25 ].
In the Introduction section, we explored the importance of this research and investigated relevant previous studies, providing a general overview of the direction of our research. In the Methods section, we provide descriptions of the data set used and the structure of the DNN algorithm used, and explain the model architecture and performance. In the Results section, we present the performance and outcomes of this study. Finally, in the Discussion section, we discuss the contributions, limitations, and potential avenues for improvement of the research.

We intended to predict the BORs of individual hospital wards and rooms based on the information accumulated in individual bed–level data on an hourly basis, aggregated on a daily basis. For this purpose, we developed 12 time-series models. As the base models, we applied LSTM and Bi-LSTM, which are suitable for sequence data. These models address the limitation of long-term memory loss in traditional RNNs and were chosen because of their suitability for training bed data represented as sequence data.
Based on the model architecture, there were 2 WBOR prediction model types, which were trained at 7- and 30-day intervals to predict the occupancy rate for the next day. Moreover, there were 2 RBOR prediction model types, similar to the ward models, which were trained at 3- and 7-day intervals. Furthermore, as another approach, each RBOR prediction model was augmented with static data, and 2 DL algorithms were proposed for the final comparison of their performances in predicting RBORs.
Ethical Considerations
The study was approved by the Asan Medical Center (AMC) Institutional Review Board (IRB 2021-0321) and was conducted in accordance with the 2008 Declaration of Helsinki.
Study Setting
This was a retrospective single-center cohort study. Data were collected from AMC, with information on the occupancy status of each bed recorded at hourly intervals between May 27, 2020, and November 21, 2022. The data set comprised a total of 54,632,684 records. This study used ethically preapproved data. Deidentified data used in the study were extracted from ABLE, the AMC clinical research data warehouse.
A total of 57 wards, encompassing specialized wards; 1411 rooms, including private and shared rooms; and 4990 beds were included in this study. Wards and rooms with specific characteristics, such as intensive care unit, newborn room, and nuclear medicine treatment room, were excluded from the analysis as their occupancy prediction using simple and general variables did not align with the direction of this study.
Supporting Data
Supporting data for public holidays were added in our data set. We considered that holidays have both a recurring pattern with specific dates each year and a distinctive characteristic of being nonworking days, which could affect occupancy rates. Based on Korean public holidays, which include Chuseok, Hangeul Proclamation Day, Children’s Day, National Liberation Day, Memorial Day, Buddha’s Birthday, Independence Movement Day, and Constitution Day, there were 27 days that corresponded to public holidays during the period covered by the data set. We denoted these dates with a value of “1” if they were public holidays and “0” if they were not, based on the reference date.
Preprocessing and Description of Variables
Among the variables representing individual beds, the reference date, ward and room information, patient occupancy status, bed cleanliness status, and detailed room information were available. Based on the recorded date of bed status, we derived additional variables, such as the reference year, reference month, reference week (week of the year), reference day, and reference day of the week.
Room data were derived from the input information representing the cleanliness status of beds. This variable had 2 possible states, namely, “admittable” and “discharge.” If neither of these states was indicated, it implied that a patient was currently hospitalized in the bed. As the status of hospitalized patients was indicated by missing values, we replaced them with the number “1” to indicate the presence of a patient in the bed and “0” otherwise. The sum of all “1” values represented the current number of hospitalized patients. The count of beds in each room indicated the capacity of each room. The target variable BOR was calculated by dividing the number of patients in the room by the room capacity, resulting in a room-specific patient occupancy rate variable. The ward data were subjected to a similar process as that of the room data, with the difference being that we generated ward-specific variables, such as ward capacity and WBOR, using the same approach. The static room data consisted of 14 variables, including the title of the room and the detailed information specific to each room.
For the variables in the ward and room data, we disregarded the units of the features and converted them into numerical values for easy comparison, after which we performed normalization. Regarding the variables representing detailed room information, we converted them to numerical values where “yes” was represented as “1” and “no” was represented as “0.”
The final set of variables used in this study was categorized into date, ward, room, and detailed room information. Table 2 provides the detailed descriptions of the variables used in our training, including all the administrative data related to beds that are readily available in the hospital.
The explanation of the classification for generating the data sets for training each model is provided in Table 3 . The static features of the detailed room information were combined with the room data set, which has sequence characteristics, to generate a separate data set termed Room+Static.
c EEG: electroencephalogram.
d ICU: intensive care unit.
Each data set was split into training, validation, and test sets for training and evaluation of the model. The training set consisted of 32,153 rows (67.8%), with data from May 27, 2020, to December 2021. The validation set, used for parameter tuning, included 7085 rows (15.0%), with data from January to June 2022. Finally, the test set comprised 8208 rows (17.2%), with data from July 2022 to November 21, 2022.
DL Algorithms
We used various DL algorithms for in-depth learning. In the following subsections, we will provide explanations for each model algorithm used in our research.
LSTM Network
RNN [ 26 ] is a simple algorithm that passes information from previous steps to the current step, allowing it to iterate and process sequential data. However, it encounters difficulties in handling long-term dependencies, such as those found in time-series data, owing to the vanishing gradient problem. To address this issue, LSTM [ 27 ] was developed. LSTM excels in handling sequence data and is commonly used in natural language processing, machine translation, and time-series data analysis. LSTM consists of an input gate, output gate, and forget gate. The “cell state,” is carefully controlled by each gate to determine whether the memory should be retained or forgotten for the next time step.
Bi-LSTM Network
Although RNN and LSTM possess the ability to remember previous data, they have a limitation in that their results are primarily based on immediate past patterns because the input is processed in a sequential order. This limitation can be overcome through a network architecture known as Bi-LSTM [ 28 ]. Bi-LSTM allows end-to-end learning, minimizing the loss on the output and simultaneously training all parameters. It also has the advantage of performing well even with long data sequences. Because of its suitability for models that require knowledge of dependencies from both the past and future, such as LSTM-based time-series prediction, we additionally selected Bi-LSTM as the base model.
Attention Mechanism
Attention mechanism [ 29 , 30 ] refers to the process of incorporating the encoder’s outputs into the decoder at each time step of predicting the output sequence. Rather than considering the entire input sequence, it focuses more on the relevant components that are related to the predicted output, allowing the model to focus on important areas. This mechanism helps minimize information loss in data sets with long sequences, enabling better learning and improving the model’s performance. It has been widely used in areas such as text translation and speech recognition. Nevertheless, as it is still based on RNN models, it has the drawbacks of slower speed and not being completely free from information loss issues.
Combining Static and Dynamic Features
Data can exhibit different characteristics even at the same time. For instance, in data collected at 1-hour intervals for each hospital bed, we can distinguish between “dynamic data,” which include features that change over time, such as the bed condition, date, and patient occupancy, and “static data,” which consist of information that remains constant, such as the ward and room number.
DL allows us to use all the available information for prediction. Therefore, for predicting the RBOR, we investigated an approach that combines dynamic and static data using an LSTM-based method [ 31 ]. This approach demonstrated better performance than LSTM alone [ 32 ]. Our approach involves adding a layer that incorporates static data as an input to the existing room occupancy prediction model.
Model Architecture
Our objective was to predict the intermediate-term occupancy rates of wards and rooms within the hospital to contribute to hospital operation planning. Bi-LSTM was chosen as the base model owing to its improved predictive performance compared with the traditional LSTM model. However, to quantitatively compare these models, we conducted a comparison of the results for each model (6 for each, with a total of 12 models).
A typical LSTM model processes data sequentially, considering only the information from the past up to the current time step. However, Bi-LSTM, by simultaneously processing data in both forward and backward directions, has a unique feature that allows it to leverage both current and future information for predictions. This bidirectionality helps the model effectively learn temporal dependencies and intricate patterns. However, despite these advantages, Bi-LSTM comes with the trade-off of doubling the number of model parameters, resulting in increased computational costs for training and prediction. While a more complex model can better adapt to the training data, there is an increased risk of overfitting, especially with small data sets. Nevertheless, the reason for choosing Bi-LSTM for tasks like predicting BORs in hospitals, involving time-series data, lies in its ability to harness the power of bidirectional information. Bi-LSTM processes input data from both past and future directions simultaneously, enabling it to effectively incorporate future information into current predictions. This proves beneficial for handling complex patterns in long time-series data [ 28 ].
Moreover, we have enhanced the performance of our models by adding an attention layer to Bi-LSTM. The attention layer assigns higher weights to features that exert a significant impact on the prediction, allowing the model to focus on relevant information and gather necessary input features. This helps improve the accuracy of the prediction. Furthermore, the attention layer reduces the amount of information processed, resulting in improved computational efficiency. Ultimately, this contributes toward enhancing the overall performance of the model.
The window length of the input sequence was divided into 3 different intervals, namely, 3, 7, and 30 days. The WBOR model was trained on sequences with a window length of 7 and 30 days, whereas the RBOR model was trained on sequences with a window length of 3 and 7 days. The first layer of our model consisted of Bi-LSTM, which was followed by the leaky rectified linear unit (LeakyReLU) activation function. LeakyReLU is a linear function that has a small gradient for negative input values, similar to ReLU. It helps the model converge faster. After applying this process once again, the AttentionWithContext layer was applied, which focuses on important components of input sequence data and transforms outputs obtained from the previous layer. After applying the activation function again, a dense layer with 1 neuron was added for generating the final output. The sigmoid function was used to limit the output values between 0 and 1. Finally, our model was compiled using the MSE loss function, Adam optimizer, and MAE metric. The parameters for each layer were selected based on accumulated experience through research. Figure 2 visually represents the above-described structure.

Combining Dynamic and Static Data Using the DL Model
The accumulated bed data, which were collected on a time basis, were divided into dynamic and static data of the rooms, which were then inputted separately. To improve the performance of the BOR prediction model, we designed different DL architectures for the characteristics of these 2 types of data.
We first used a base model based on LSTM and Bi-LSTM to learn the time-series data and then focused the model’s attention using the dense layer to process fixed-size inputs. To prevent overfitting, we applied the dropout function to randomly deactivate neurons in 2 dense layers. The hidden states of the 2 networks were combined, and the resulting output was passed to a single layer, combining the time dynamic and static data.
Finally, the hidden states of the 2 networks were combined, and the combined result was passed to a single layer to effectively integrate the dynamic and static data. This allowed us to use the information from both the dynamic and static data for BOR prediction. This architecture is illustrated in Figure 3 .

Hyperparameter Tuning
One of the fundamental methods to enhance the performance of artificial intelligence (AI) learning models is the use of hyperparameter tuning. Hyperparameters are parameters passed to the model to modify or adjust the learning process. While hyperparameter tuning may rely on the experience of researchers, there are also functionalities that automatically search for hyperparameters, taking into account the diversity of model structures.
Various methods for search optimization have been proposed [ 33 , 34 ], but we implemented our models using the Keras library. By leveraging Keras Tuner, we automatically searched for the optimal combinations of units and learning rates for each model, contributing to the improvement of their performance.
Time Series Cross-Validation
Time-series data exhibit temporal dependencies between data points, making it crucial to consider these characteristics when validating a model. Commonly used K-fold cross-validation is effective for evaluating models on general data sets [ 35 ], providing effectiveness in preventing overfitting and enhancing generalizability by dividing the data into multiple subsets [ 36 , 37 ]. However, for time-series data, shuffling the data randomly is not appropriate owing to the inherent sequential dependency of the observations.
Time series cross-validation is a method that preserves this temporal dependence while dividing the data [ 38 ]. It involves splitting the entire hospital bed data set into 5 periods, conducting training and validation for each period, and repeating this process as the periods shift. This approach is particularly effective when observations in the dynamic data set, such as hospital bed data recorded at 1-hour intervals, play a crucial role in predicting future values based on past observations.
Shuffling data randomly using K-fold may disrupt the temporal continuity, leading to inadequate reflection of past and future observations. Therefore, time series cross-validation sequentially partitions the data, ensuring the temporal flow is maintained, and proves to be more effective in evaluating the model’s performance. This method enables the model to make more accurate predictions of future occupancy based on past trends.
We selected various metrics to evaluate the performance of time-series data predictions. Among them, MAE represents the absolute difference between the model’s predicted values and the actual BOR. We also considered MSE, which is sensitive to outliers. Moreover, to address the limitations of MSE and provide a penalty for large errors, we opted for RMSE. We also used the R 2 score to measure the correlation between the predicted and actual values.
MAE is a commonly used metric to evaluate the performance of time-series prediction models. MAE is intuitive and easy to calculate, making it widely used in practice. Because MAE uses absolute values, it is less sensitive to outliers in the occupancy rate values for specific dates. MAE is calculated using the following formula:
MSE is a metric that evaluates the magnitude of errors by squaring the differences between the predicted and actual values and then taking the average. It is calculated using the following formula:
RMSE is used to address the limitations of MSE where the error scales as a square, providing a more intuitive understanding of the error magnitude between the predicted and actual values. It penalizes large errors, making it less sensitive to outliers. RMSE is calculated using the following formula:
The R 2 score is used to measure the explanatory potential of the prediction model, and it is calculated using the following formula:
Here, SSR represents the sum of squared differences between the predicted and actual values, and SST represents the sum of squared differences between the actual values and the mean value of actual values. Figure 4 shows the prediction method and overall flow in this study.

We used 2 DL models, LSTM and Bi-LSTM, and compared the performance of 12 different prediction models. These models have been denoted as ward 7 days (W7D), ward 30 days (W30D), room 3 days (R3D), room 7 days (R7D), room static 3 days (RS3D), and room static 7 days (RS7D). Using Keras Tuner, we adjusted the hyperparameters of the models and subsequently validated the models through a 5-fold time series cross-validation.
The prediction performances of the models for WBOR and RBOR were compared, which showed that they were more accurate at predicting WBOR, with MAE values of 0.06 to 0.07. The W7D model based on Bi-LSTM, which used 7 days of ward data to predict the next day’s ward occupancy, had a MAE value of 0.067, MSE value of 0.009, and RMSE value of 0.094, showing high accuracy. The R 2 score was also 0.544, which was approximately 0.240 higher than that of the W30D model (0.304), indicating that the variables in that model explained occupancy reasonably well.
We next compared the performances of the 8 models for RBOR prediction, and among them, the RS7D model based on Bi-LSTM, which was trained on a 7-day time step by integrating static and dynamic data, showed the best performance. It achieved a MAE value of 0.129, MSE value of 0.050, RMSE value of 0.227, and R 2 score of 0.260. In particular, the R 2 score outperformed that of the R3D model by 0.014. These data are summarized in Table 4 . Regarding the WBOR prediction model, the model with a shorter training unit, W7D, demonstrated better performance. However, regarding the RBOR prediction model, the model with a longer training unit of 7 days, which incorporated detailed room-specific information, exhibited slightly higher performance than the model with a shorter training unit of 3 days. The model with the added room-specific information still demonstrated superior performance overall.
We visualized the predicted and actual occupancy for Bi-LSTM models and investigated the occupancy trends since July 2022 on our test data set. First, we selected a specific ward in W7D to demonstrate the change in the WBOR over 2 months. The right panel of Figure 5 shows the WBOR change over 5 months from July 2022 in W30D. The blue line represents the actual occupancy value, and the red line represents the predicted occupancy value by the model. This provides an at-a-glance view of the overall predicted occupancy level for each month and allows hospital staff to observe trends to obtain a rough understanding of the WBOR.
Figure 6 shows graphs of occupancy rate values for a randomized specific room, displaying the predicted and actual values for the 4 RBOR prediction models, with 2 graphs for each model. The left graph shows the occupancy rate change over 5 months from July to November 2022, and the right graph shows the occupancy rate for the months of July and August, providing a detailed view of the RBOR. By examining the trends of the predicted and actual values for the 4 models in this period for a specific room, we can observe that the models maintain a similar trend to the actual occupancy rate.
a MAE: mean absolute error.
b MSE: mean square error.
c RMSE: root mean square error.
d LSTM: long short-term memory.
e Bi-LSTM: bidirectional long short-term memory.
f W30D: ward 30 days.
g W7D: ward 7 days.
h R7D: room 7 days.
i R3D: room 3 days.
j RS7D: room static 7 days.
k RS3D: room static 3 days.

Principal Findings
The entire data set of this study consisted of administrative data collected at AMC at an hourly interval for each ward from May 27, 2020, to November 21, 2022. To improve the hospital’s challenges, we developed a model to predict the occupancy rate of wards and rooms. Our aim was to contribute toward administrative and financial planning for bed management within the hospital.
During the specified period, we compared the results of using DL models to predict the overall BOR for each ward and individual rooms. In the case of WBOR prediction, the MAE of the 7-day window model based on Bi-LSTM was approximately 0.067, demonstrating a remarkably close prediction to the occupancy compared with that of the 30-day window model based on LSTM, with a difference of approximately 0.035. Furthermore, the MSE and RMSE were 0.009 and 0.094, respectively, indicating high accuracy in the predictions. Moreover, the R 2 score of 0.544 indicated that the model had better explanatory potential than the average. For the individual RBOR prediction, among the 8 models, the RS7D model based on Bi-LSTM performed the best, exhibiting a MAE of approximately 0.129, which was remarkably lower than that of the other models. Moreover, the MSE and RMSE were significantly lower than those of the RBOR models, with differences of 0.042 and 0.07, respectively. The R 2 score of 0.260 indicated that it had higher explanatory potential than the RS3D models based on LSTM, with the value being higher by 0.291.
Finally, we visualized the predicted and actual values on a graph for a specific period and observed that each model captured the trend of the actual BOR quite well. Although the models were less accurate in predicting low occupancy periods, they followed the general trend closely. Overall, these findings demonstrate that our DL models effectively predicted BORs for both wards and individual rooms, with certain models demonstrating superior performance in different scenarios.
Strengths and Limitations
Although the models in this study demonstrated good performance in following the trends of BORs and achieved good results, there were several limitations in this research. First, there were limitations in the data. Although we used administrative data and detailed room information available from the hospital to enable the models to capture occupancy trends, the relationship between the variables and the model’s explanatory potential showed room for improvement, as indicated by the R 2 score. To achieve higher prediction accuracy, it would be beneficial to incorporate diverse data sources and real-time updated information.
Second, there was variability in external factors. Hospital BORs are heavily influenced by external environmental factors. Sudden events, such as environmental factors and outbreaks of infectious diseases like COVID-19, can render accurate prediction of bed occupancy challenging [ 18 , 32 ]. Furthermore, seasonal effects and accidents can increase the number of patients. Sufficient collection of long-term data on these external factors would be necessary, but such uncertainties can reduce the accuracy of predictions.
Despite these limitations, our study demonstrated a significant level of adherence to trends in the prediction of individual ward and room occupancy. More detailed variables and a longer period of data accumulation would be required to predict the specific number of beds.
We presented models that can predict the occupancy rates of wards and individual hospital rooms using artificial neural networks based on time-series data. The predicted results of these models demonstrated a high level of accuracy in capturing the future trends of the BOR. In particular, we presented 8 RBOR models with structure and window changes to compare their performance and found that the RS7D model showed the best performance. Our results can be implemented as a web application on hospital online dashboards, as depicted in Figure 1 [ 25 ]. In fact, Johns Hopkins University has been applying these methods in their command center to monitor hospital capacity and achieve effectiveness in patient management planning [ 39 ].
Furthermore, predicting BORs supports patient admission and discharge planning, helping to alleviate overcrowding in emergency departments and reduce patient waiting times. Staff members can effectively schedule patient admission and discharge, and minimize waiting times by understanding the BOR, providing urgent treatment to emergency patients. Moreover, providing appropriate information to patients waiting in the emergency department can increase patient satisfaction and facilitate efficient transition to hospital admission [ 40 , 41 ]. By applying AI models that combine BOR prediction, which contributes toward reducing emergency department waiting times with individual patient admission and discharge prediction, hospitals can achieve resource optimization and cost savings, resulting in improved patient satisfaction.
Acknowledgments
This work was supported by a Korea Medical Device Development Fund grant funded by the Korean government (the Ministry of Science and ICT; the Ministry of Trade, Industry and Energy; the Ministry of Health & Welfare, Republic of Korea; the Ministry of Food and Drug Safety) (project number: 1711195603, RS-2020-KD000097, 50%) and by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HR20C0026).
Conflicts of Interest
None declared.
- Reuben DB, Cassel CK. Physician stewardship of health care in an era of finite resources. JAMA. Jul 27, 2011;306(4):430-431. [ CrossRef ] [ Medline ]
- National Health Expenditure Projections 2011-2021. Centers for Medicare and Medicaid Services. URL: https://www.cms.gov/files/document/forecastsummaryandtablespdf [accessed 2024-02-21]
- The world health report 2000 - Health systems: improving performance. World Health Organisation. URL: https://cdn.who.int/media/docs/default-source/health-financing/whr-2000.pdf?sfvrsn=95d8b803_1&download=true [accessed 2024-02-21]
- Kabene SM, Orchard C, Howard JM, Soriano MA, Leduc R. The importance of human resources management in health care: a global context. Hum Resour Health. Jul 27, 2006;4(1):20. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Page K, Barnett AG, Graves N. What is a hospital bed day worth? A contingent valuation study of hospital Chief Executive Officers. BMC Health Serv Res. Feb 14, 2017;17(1):137. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Keegan AD. Hospital bed occupancy: more than queuing for a bed. Med J Aust. Sep 06, 2010;193(5):291-293. [ CrossRef ] [ Medline ]
- Kaier K, Mutters N, Frank U. Bed occupancy rates and hospital-acquired infections--should beds be kept empty? Clin Microbiol Infect. Oct 2012;18(10):941-945. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Anderson D. The impact of resource management on hospital efficiency and quality of care. University of Maryland. 2013. URL: https://api.drum.lib.umd.edu/server/api/core/bitstreams/7ec54849-e2d2-449b-9a9a-f506b429834b/content [accessed 2024-02-21]
- Kutafina E, Bechtold I, Kabino K, Jonas SM. Recursive neural networks in hospital bed occupancy forecasting. BMC Med Inform Decis Mak. Mar 07, 2019;19(1):39. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Baas S, Dijkstra S, Braaksma A, van Rooij P, Snijders FJ, Tiemessen L, et al. Real-time forecasting of COVID-19 bed occupancy in wards and Intensive Care Units. Health Care Manag Sci. Jun 25, 2021;24(2):402-419. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Esteban C, Staeck O, Baier S, Yang Y, Tresp V. Predicting Clinical Events by Combining Static and Dynamic Information Using Recurrent Neural Networks. 2016. Presented at: 2016 IEEE International Conference on Healthcare Informatics (ICHI); October 4-7, 2016;93-101; Chicago, IL. [ CrossRef ]
- Mackay M, Lee M. Using Compartmental Models to Predict Hospital Bed Occupancy. Semantic Scholar. URL: https://www.semanticscholar.org/paper/Using-Compartmental-Models-to-Predict-Hospital-Bed-Mackay-Lee/f2b32e60df7dd80bd48 e8ccd0af920134d1452c5?p2df [accessed 2024-02-21]
- Littig SJ, Isken MW. Short term hospital occupancy prediction. Health Care Manag Sci. Feb 28, 2007;10(1):47-66. [ CrossRef ] [ Medline ]
- Kumar A, Mo J. Models for Bed Occupancy Management of a Hospital in Singapore. In: Proceedings of the 2010 International Conference on Industrial Engineering and Operations Management. 2010. Presented at: 2010 International Conference on Industrial Engineering and Operations Management; January 9-10, 2010; Dhaka, Bangladesh.
- Seematter-Bagnoud L, Fustinoni S, Dung D, Santos-Eggimann B, Koehn V, Bize R, et al. Comparison of different methods to forecast hospital bed needs. European Geriatric Medicine. Jun 2015;6(3):262-266. [ CrossRef ]
- Farmer RD, Emami J. Models for forecasting hospital bed requirements in the acute sector. J Epidemiol Community Health. Dec 01, 1990;44(4):307-312. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kim K, Lee C, O’Leary KJ, Rosenauer S, Mehrotra S. Predicting Patient Volumes in Hospital Medicine: A Comparative Study of Different Time Series Forecasting Methods. Northwestern University. URL: https://www.mcs.anl.gov/~kibaekkim/ForecastingHospitalMedicine.pdf [accessed 2024-02-21]
- Rosenbaum L. Facing Covid-19 in Italy - Ethics, Logistics, and Therapeutics on the Epidemic's Front Line. N Engl J Med. May 14, 2020;382(20):1873-1875. [ CrossRef ] [ Medline ]
- Bouhamed H, Hamdi M, Gargouri R. Covid-19 Patients’ Hospital Occupancy Prediction During the Recent Omicron Wave via some Recurrent Deep Learning Architectures. Int. J. Comput. Commun. Control. Mar 14, 2022;17(3):4697. [ CrossRef ]
- Bekker R, Uit Het Broek M, Koole G. Modeling COVID-19 hospital admissions and occupancy in the Netherlands. Eur J Oper Res. Jan 01, 2023;304(1):207-218. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Farcomeni A, Maruotti A, Divino F, Jona-Lasinio G, Lovison G. An ensemble approach to short-term forecast of COVID-19 intensive care occupancy in Italian regions. Biom J. Mar 30, 2021;63(3):503-513. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Caro JJ, Möller J, Santhirapala V, Gill H, Johnston J, El-Boghdadly K, et al. Predicting Hospital Resource Use During COVID-19 Surges: A Simple but Flexible Discretely Integrated Condition Event Simulation of Individual Patient-Hospital Trajectories. Value Health. Nov 2021;24(11):1570-1577. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Schmidt R, Geisler S, Spreckelsen C. Decision support for hospital bed management using adaptable individual length of stay estimations and shared resources. BMC Med Inform Decis Mak. Jan 07, 2013;13:3. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Hancock WM, Walter PF. The use of computer simulation to develop hospital systems. SIGSIM Simul. Dig. Jul 1979;10(4):28-32. [ CrossRef ]
- Shahpori R, Gibney N, Guebert N, Hatcher C, Zygun D. An on-line dashboard to facilitate monitoring of provincial ICU bed occupancy in Alberta, Canada. JHA. Oct 10, 2013;3(1):47. [ CrossRef ]
- Rumelhart DE, Hinton GE, Williams RJ. Learning representations by back-propagating errors. Nature. Oct 9, 1986;323(6088):533-536. [ CrossRef ]
- Hochreiter S, Schmidhuber J. Long short-term memory. Neural Comput. Nov 15, 1997;9(8):1735-1780. [ CrossRef ] [ Medline ]
- Schuster M, Paliwal K. Bidirectional recurrent neural networks. IEEE Trans. Signal Process. 1997;45(11):2673-2681. [ CrossRef ]
- Bahdanau D, Cho K, Bengio Y. Neural Machine Translation by Jointly Learning to Align and Translate. arXiv. 2014. URL: https://arxiv.org/abs/1409.0473 [accessed 2024-02-21]
- Luong MT, Pham H, Manning CD. Effective Approaches to Attention-based Neural Machine Translation. arXiv. 2015. URL: https://arxiv.org/abs/1508.04025 [accessed 2024-02-21]
- Leontjeva A, Kuzovkin I. Combining Static and Dynamic Features for Multivariate Sequence Classification. 2016. Presented at: 2016 IEEE 3rd International Conference on Data Science and Advanced Analytics (DSAA); October 17-19, 2016;21-30; Montreal, QC. [ CrossRef ]
- Vincent J, Creteur J. Ethical aspects of the COVID-19 crisis: How to deal with an overwhelming shortage of acute beds. Eur Heart J Acute Cardiovasc Care. Apr 29, 2020;9(3):248-252. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Vakharia V, Shah M, Nair P, Borade H, Sahlot P, Wankhede V. Estimation of Lithium-ion Battery Discharge Capacity by Integrating Optimized Explainable-AI and Stacked LSTM Model. Batteries. Feb 09, 2023;9(2):125. [ CrossRef ]
- Joshi S, Owens JA, Shah S, Munasinghe T. Analysis of Preprocessing Techniques, Keras Tuner, and Transfer Learning on Cloud Street image data. 2021. Presented at: IEEE International Conference on Big Data (Big Data); December 15-18, 2021; Orlando, FL. [ CrossRef ]
- Jung Y. Multiple predicting K-fold cross-validation for model selection. Journal of Nonparametric Statistics. Nov 21, 2017;30(1):197-215. [ CrossRef ]
- Nair P, Vakharia V, Borade H, Shah M, Wankhede V. Predicting Li-Ion Battery Remaining Useful Life: An XDFM-Driven Approach with Explainable AI. Energies. Jul 31, 2023;16(15):5725. [ CrossRef ]
- Seo H, Ahn I, Gwon H, Kang HJ, Kim Y, Cho HN, et al. Prediction of hospitalization and waiting time within 24 hours of emergency department patients with unstructured text data. Health Care Manag Sci. Nov 03, 2023.:09660-5. [ CrossRef ] [ Medline ]
- Deng A. Time series cross validation: A theoretical result and finite sample performance. Economics Letters. Dec 2023;233:111369. [ CrossRef ]
- Martinez DA, Kane EM, Jalalpour M, Scheulen J, Rupani H, Toteja R, et al. An Electronic Dashboard to Monitor Patient Flow at the Johns Hopkins Hospital: Communication of Key Performance Indicators Using the Donabedian Model. J Med Syst. Jun 18, 2018;42(8):133. [ CrossRef ] [ Medline ]
- Gartner D, Padman R. Machine learning for healthcare behavioural OR: Addressing waiting time perceptions in emergency care. Journal of the Operational Research Society. Apr 15, 2019;71(7):1087-1101. [ CrossRef ]
- Welch SJ. Twenty years of patient satisfaction research applied to the emergency department: a qualitative review. Am J Med Qual. Dec 04, 2010;25(1):64-72. [ CrossRef ] [ Medline ]
Abbreviations
Edited by C Lovis; submitted 05.10.23; peer-reviewed by V Vakharia, T Leili; comments to author 10.11.23; revised version received 20.12.23; accepted 16.02.24; published 21.03.24.
©Hyeram Seo, Imjin Ahn, Hansle Gwon, Heejun Kang, Yunha Kim, Heejung Choi, Minkyoung Kim, Jiye Han, Gaeun Kee, Seohyun Park, Soyoung Ko, HyoJe Jung, Byeolhee Kim, Jungsik Oh, Tae Joon Jun, Young-Hak Kim. Originally published in JMIR Medical Informatics (https://medinform.jmir.org), 21.03.2024.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in JMIR Medical Informatics, is properly cited. The complete bibliographic information, a link to the original publication on https://medinform.jmir.org/, as well as this copyright and license information must be included.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

A single-center, retrospective study of COVID-19 features in children: a descriptive investigation
1 Imaging Center, Wuhan Children’s Hospital (Wuhan Maternal and Child Healthcare Hospital), Tongji Medical College, Huazhong University of Science & Technology, No.100 Hongkong Road, Wuhan, 430016 China
2 Department of Radiology, School of Medicine, Wayne State University, Detroit, MI 48201 USA
3 Beijing Advanced Innovation Center for Big Data-Based Precision Medicine, School of Medicine and Engineering, Beihang University, Beijing, 100191 China
4 Department of Radiology, Shenzhen Second People’s Hospital, The First Affiliated Hospital of Shenzhen University Health Science Center, 3002 SunGang Xi Road West, Shenzhen, 518035 China
5 Medical department, Wuhan Children’s Hospital (Wuhan Maternal and Child Healthcare Hospital), Tongji Medical College, Huazhong University of Science & Technology, No.100 Hongkong Road, Wuhan, 430016 China
Maxwell Thomas Laws
Luke david wesemann.
6 Department of Radiology, Tongji Hospital, Tongji University School of Medicine, Shanghai, 200065 China
7 Pingshan District People’s Hospital, Pingshan General Hospital of Southern Medical University, Shenzhen, 518118 Guangdong China
Rafael Ramos
Jianbo shao, associated data.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Compared to adults, there are relatively few studies on COVID-19 infection in children, and even less focusing on the unique features of COVID-19 in children in terms of laboratory findings, locations of computerized tomography (CT) lesions, and the role of CT in evaluating clinical recovery. The objective of this study is to report the results from patients at Wuhan Children’s Hospital, located within the initial center of the outbreak.
Clinical, imaging, and laboratory data of 76 children were collected retrospectively and analyzed with the Fisher exact test and Cox regression statistical methods.
Among 50 children with a positive COVID-19 real-time reverse-transcriptase polymerase chain reaction (PCR), five had negative PCR results initially but showed positive results in subsequent tests. Eight (16%) patients had lymphopenia, seven (14%) with thrombocytopenia, four (8%) with lymphocytosis, two (4%) with thrombocytosis, ten (20%) with elevated C-reactive protein, four (8%) with hemoglobin above, and six (12%) with below standard reference values. Seven (14%) of the 50 had no radiologic evidence of disease on chest CT. For the 43 patients who had abnormal CT findings, in addition to previously reported patterns of ground-glass opacity (67%), local patchy shadowing (37%), local bilateral patchy shadowing (21%), and lesion location of lower lobes (65%), other CT features include that an overwhelming number of pediatric patients had lesions in the subpleural area (95%) and 22 of the 28 lower lobe lesions were in the posterior segment (78%). Lesions in most of the 15 patients (67%) who received chest CT at discharge were not completely absorbed, and 26% of these pediatric patients had CT lesions that were either unchanged or worse.
Conclusions
There were a few differences between COVID-19 children and COVID-19 adults in terms of laboratory findings and CT characteristics. CT is a powerful tool to detect and characterize COVID-19 pneumonia but has little utility in evaluating clinical recovery for children. These results oppose current COVID-19 hospital discharge criteria in China, as one requirement is that pulmonary imaging must show significant lesion absorption prior to discharge. These differences between pediatric and adult cases of COVID-19 may necessitate pediatric-specific discharge criteria.
Since initially identified in Wuhan city of China’s Hubei province in December 2019, the coronavirus disease 2019 (COVID-19) has resulted in 466,836 confirmed cases and 21,152 deaths as of March 25, 2020. Two months prior, on January 23, 2020, there were only 581 reported cases. COVID-19 can rapidly spread from human-to-human and is more contagious than other notable members of the coronavirus family, such as severe acute respiratory syndrome (SARS) and Middle Eastern respiratory syndrome (MERS) [ 1 , 2 ]. The World Health Organization recently declared COVID-19 a global pandemic, and the USA has declared a national emergency. Even though the incidence of COVID-19 infection in children is less than it is in adults, the total number of pediatric cases is expected to increase rapidly in the coming weeks.
Compared to adults [ 3 – 7 ], there are a few studies on the COVID-19 in children. Although mortality in children has been reported [ 8 ], studies have demonstrated that COVID-19 is generally less severe compared to adults in terms of both symptoms and computerized tomography (CT) manifestations [ 9 – 18 ]. The common chest CT patterns are ground-glass opacities (GGO) followed by local bilateral shadowing (LPS), in contrast to a large percentage of bilateral patchy shadowing (BPS) pattern in adults [ 19 , 20 ]. However, there are no studies that quantitatively examine the location of lung lesions in COVID-19-positive pediatric patients [ 21 ]. Most of the pediatric patients are at the early stages of the disease when admitted to hospitals. Thus, a detailed localization study is meaningful both clinically and scientifically, as it could help pinpoint lung regions that are particularly susceptible to COVID-19 infection.
Several studies have reported on the laboratory findings of children infected with COVID-19. However, the interpretations of these results vary substantially [ 15 , 22 – 24 ]. The discrepancy in laboratory interpretations could be attributed to the studies each referring to a different set of reference values. Of note, the range of normal lab values changes depending upon the age of the child, i.e., a 1-year-old has a different set of reference values than a 9-year-old. Confounding these results is the fact that the reference values used among the studies lack consistency and appear to be hospital-self-defined values [ 15 , 22 – 24 ]. This inconsistency of reference values makes any systemic review of the published data less meaningful [ 23 ].
There is also no research on the role of CT in monitoring clinical recovery in children. CT has been widely used in the clinical management of adult patients due to its ability to reveal detailed features of pneumonia [ 25 – 28 ]. Because of how many unknowns there were about the disease, particularly at the beginning of the COVID-19 outbreak, CT was frequently used in the clinical management and diagnosis of children in China. Notably, repeated use of CT can be harmful, particularly for children [ 29 , 30 ].
The objective of this study is to report relevant findings from the COVID-19-positive patients treated at Wuhan Children’s Hospital. Specifically, we attempt to answer three questions based on the patient’s clinical, laboratory, diagnostic, and treatment outcome data. The questions are, in hospitalized COVID-19 children, (i) what are the typical laboratory findings, (ii) is there any unique CT feature, and (iii) is CT necessary for evaluating clinical recovery?
Study design and patient selection
For this retrospective, single-center study, patients were recruited from January 21 to February 14, 2020, at Wuhan Children’s Hospital in Wuhan, China. Real-time reverse-transcriptase polymerase chain reaction (PCR) was performed on children 16 years of age and under who had a family or social history of COVID-19 exposure. Subsequently, these patients received a chest CT examination to evaluate lung pathology. Based on the PCR and CT results, these patients were stratified into groups A–C (Fig. 1 ). This study was approved by the Ethics Committee of Wuhan Children’s Hospital (Wuhan Maternal and Child Health Care Hospital # WHCH 2020005). Written informed parental/guardian consent and child assent (where appropriate) were obtained prior to enrollment in the study.

Flow chart for patient selection. Group A: 43 children with COVID-19 exposure history, positive CT, and positive PCR. Group B: seven children with COVID-19 exposure history, negative CT, and positive PCR. Group C: 26 children with COVID-19 exposure history, positive CT, and persistently negative PCR results
We obtained demographic information, clinical symptoms, laboratory results, management, and outcome data from each patient’s electronic medical records. Clinical outcomes were followed up to February 17, 2020.
Chest CT without intravenous contrast was performed on all patients using a Siemens SOMATOM Definition AS128 or GE Optima CT 660 with a 1-mm or 0.625-mm slice thickness, respectively. Children under 5 years old, as well as uncooperative children, received oral chloral hydrate sedation (0.5 ml/kg) prior to CT. Cooperative children above 5 years old were trained with breathing exercises prior to CT.
All CT images were reviewed by at least two radiologists with more than 10 years of experience. Imaging was reviewed independently. When the opinions on the CT features were inconsistent, the two radiologists discussed and decided together. Only final decisions reached by consensus are reported. No negative control cases were examined.
PCR confirmation of COVID-19 was performed at two different institutions: Hubei Center for Disease Control and Prevention and Wuhan Children’s Hospital.
Patient discharge
Criteria for discharging pediatric patients in this hospital were normal body temperature for 3 days, two negative PCR results at 24-h intervals, and resolution of all clinical symptoms.
Statistical analysis
The Fisher exact test method was used to determine whether there is a significant difference in CT image characteristics and lesion locations between group A and group C. The Cox regression analysis was used to determine whether changes in CT images during treatment were associated with clinical outcomes for children with COVID-19 infection. All analyses were performed using EmpowerStats ( http://www.empowerstats.com ) and the statistical package R (version 3.2.3). p value of less than 0.05 was considered to indicate a statistically significant difference.
From January 21 to February 14, 2020, 158 children at Wuhan Children’s Hospital were radiologically examined with chest CT, and respiratory secretions were obtained and subsequently tested for COVID-19 with PCR. A CT scan was considered positive when at least one lesion was identified. Among them, 43 had a positive CT and positive PCR (group A), 7 had a negative CT and positive PCR (group B), and 26 had a positive CT and at least two negative consecutive PCR results (group C, Fig. 1 ).
PCR-positive groups A and B ( n = 50) were chosen to interpret clinical and chest CT features because group C patients were not deemed COVID-19 positive by PCR. Over half of the patients were males (56%, Table 1 ). The most common symptoms at the onset of illness (Table 1 ) were fever (64%) and cough (44%); less common symptoms were rhinorrhea (16%), abdominal pain (4%), diarrhea (6%), fatigue (4%), and pharyngalgia (2%). Six children (12%) were asymptomatic. After treatment, 38 (76%) children were discharged.
Demographics and characteristics of patients
a No clinical symptoms and no abnormal CT findings
Laboratory reference normal ranges were age- and gender-adjusted according to values in Reference Range Values for Pediatric Care 2nd edition , pages 92–98 [ 31 ]. On laboratory assessment, eight (16%) and seven (14%) patients had lymphopenia and thrombocytopenia, respectively. In contrast, four (8%) were noted to have lymphocytosis, and two (4%) had thrombocytosis. Overall, leukopenia was observed in 19 (38%) patients and elevated C-reactive protein in ten (20%) patients. A small set of patients had hemoglobin abnormalities, four (8%) with elevated hemoglobin, and six (12%) with anemia (Table 2 ).
Laboratory examination and CT radiographic characteristics
a Data from the first laboratory examination of the patient admission. The normal range of laboratory examination is the standard of Reference Range Values for Pediatric Care 2nd ed [ 31 ] released by the American Academy of Pediatrics
b How many patients have the following lesion location
Of the 26 patients in group C, all had more than two negative consecutive PCR results. However, they all had a history of exposure to COVID-19 infection (or strongly suspected infection), and their chest CT had similar patterns to confirmed patients in group A (Table 2 ). Fisher exact test results indicated that there was no significant difference in CT characteristics (ground-glass opacity [ p > 0.05], local patchy shadowing [ p > 0.05], bilateral patchy shadowing [ p > 0.05], interstitial abnormalities [ p > 0.05]) and lesion location (parallel pleura [ p > 0.05], visible vascular thickening [ p > 0.05], subpleural [ p > 0.05], lower lobe of the lung [ p > 0.05], middle lobe of the lung [ p > 0.05], upper lobe of the lung [ p > 0.05]) between groups A and C (Table 3 ).
Differences in CT image characteristics between Groups A and C
Using the Fisher exact test method, p < 0.05 was considered to indicate a statistically significant difference
Among the 50 children with positive PCR results, five of them (10%) had negative initial PCR results but showed positive results in subsequent tests. Two of the 50 (4%) had no clinical symptoms and no radiologic findings. Seven of the 50 (14%) were negative for any abnormal CT findings. The spectrum of COVID-19 severity was two (4%) had no symptoms or radiologic signs, five (10%) very mild, 41 (82%) mild, and two (4%) critically ill with one having multiple organ dysfunction syndrome (MODS) and another with renal failure (Table 1 ). There were no patient mortalities in this study. The critically ill patient with renal failure has since fully recovered.
Among the 43 children with positive PCR results and abnormal CT findings, 41 patients had lesions present in the subpleural area (95%) and lower lung lobes (65%), especially in the posterior segment of the lower lung lobes (22 [78%] of 28). Ground-glass opacities (GGO) were the most common radiologic lesion identified on chest CT (67%). Local patchy shadowing (37%) was the second most common radiologic lesion, followed by local bilateral patchy shadowing (21%, Table 2 ). Interstitial lesions were rare (7%). Pleural fluid was observed in one case, and no lymphadenopathy was noted (Table 2 ). Appearances of lesions were irregular shaped, flaky, wedge-shaped, or strip-shaped. The long axis of some lesions (49%) was parallel to the pleura. However, lesions did not follow the segment of the lung lobe, single or multiple, and diffuse consolidation was rare. Bilateral lesions can be seen radiating around the bronchial blood vessels or showing large areas of consolidation, which can be traced by the lung segment into the bronchial tube.
Among the 50 confirmed children (groups A and B), 29 patients (including 23 discharged children) had more than one chest CT. Nineteen of the 29 patients (65%) had improved CT presentations after treatment, and lesions in two of the 19 patients completely disappeared. Two of the 29 patients (7%) showed no change in CT lesions, and 8 of the 29 patients (28%) had more CT lesions after treatment.
Figure 2 illustrates typical radiographic features of COVID-19 pneumonia in children. Figure 3 shows chest CT before and after treatment from three COVID-19 children. Cox regression results (Table 4 ) indicated that there is no association between changes in CT lesions (completely absorbed [ p > 0.05], partially absorbed [ p > 0.05], worse [ p > 0.05]). Table 5 lists changes in CT lesions during treatment. Table 6 lists the normal ranges for children of different ages based on Reference Range Values for Pediatric Care 2nd edition pages 92–98 [ 31 ].

Chest CT images depicting typical radiographic findings of COVID-19 pneumonia in children. 2A A unilateral chest CT from a 14-year-old boy with a cough. Ground-glass opacities under and parallel to the pleura (thick green arrow) in the inferior lobes of the left lungs. Ground-glass opacities distributed along the bronchovascular bundle (thin green arrow). 2B Bilateral ground-glass opacities with vascular thickening (arrowheads) in the subpleural area from a 13-year-old boy with a fever and a cough. 2C Local patchy shadowing (green arrow) image from a 6 month-old girl with a fever and a cough. 2D Lesions in the lower lobe of both lungs (green arrows) on chest CT obtained from a 15-year-old boy with a fever and a cough

Chest CT findings at initial presentation and at discharge. 3A , 3B Chest CT scans obtained from a 1-year-old boy, presenting with fever and diarrhea, at arrival ( 3A ) and after ( 3B ) treatment. The first CT scan shows a large, patchy shadow in the left inferior lobe (green arrow). The second CT scan shows no lesions. The patient was hospitalized for 17 days prior to discharge. 3C , 3D Chest CT scans from a 4-month-old girl, who presented with a fever and a cough at arrival. The first CT scan reveals multiple ground-glass opacities under the pleura in the left superior lobe (green arrows). The second CT scan reveals that the range of original lesions was enlarged and extended to the center. The girl was hospitalized for 13 days and subsequently discharged. 3E , 3F Chest CT scans from a 14-year-old boy, presenting with rhinorrhea and a cough, at arrival and discharge. The first CT scan reveals a patchy shadow in the left middle lobe (arrowhead). There were no obvious changes in the areas of pulmonary consolidation on the second CT scan. The boy was hospitalized for 11 days and then discharged
Association between CT imaging changes and clinical outcome a
a Using the time-vary Cox regression method, p < 0.05 was considered to indicate statistically significant difference
b Adjusted for gender, age, PCR positive and CT positive
c This model failed when analyzing “no change in CT image” due to the small sample
Changes in CT presentation after treatment
a Only for those discharged patients with at least two CT; discharge CT here means CT within 2 days of discharging; total 15 patients out of group A and group B
b Only for those discharged patients with at least two CT but no CT within 2 days of discharging; the nearest CT means that the CT taken closest to the date of discharging; total 8 patients out of group A and group B
c For all patients with at least two CT; total 29 patients out of group A and group B
Laboratory examination reference ranges
From Reference Range Values for Pediatric Care 2nd edition pages 92–98 [ 31 ] released by the American Academy of Pediatrics
The symptoms in children with COVID-19 infection have been well described in the literature [ 9 – 18 ]. Our results are consistent with these previous reports. For example, the clinical symptoms from our study versus the recent study with the most pediatric patients are similar [ 10 ]: fever, 64% versus 41.5%; cough, 44% versus 48.5%; diarrhea, 6% versus 8.8%; and fatigue, 4% versus 7.6%. Results including ours indicate that COVID-19 symptoms in children follow a similar pattern in adults, albeit much less severe.
Our results of abnormal laboratory findings for children infected with COVID-19 contrast with recently published ones [ 15 , 22 – 24 ]. For example, our results for lymphopenia compared to Zheng et al. are 16% versus 40% [ 22 ]. Their normal reference values for lymphocytes were (2.1–5.7) × 10 9 /L (< 3 years), (1.4–4.2) × 10 9 /L (4–6 years), and (1.1–3.2) × 10 9 /L (≥ 6 years). To date, this is the only paper that has explicitly listed the normal ranges for children of different age ranges [ 22 ]. Thus, the differences between ours and those in the literature are most likely due to different normal ranges used for children of different ages or the small number of children who participated in their studies.
Like clinical symptoms, the laboratory findings in COVID-19-positive pediatric patients can vary from adult patients. Guan et al. [ 25 ] noted that 731 (82%) of 890 adult patients had lymphopenia, whereas only eight (16%) children had lymphopenia in this study. Similarly, 481 (61%) of 793 adult patients were found to have an elevated C-reactive protein. In contrast, only ten (20%) children in this study had elevated C-reactive protein. Some laboratory findings were consistent between children and adult groups: leukopenia 38% versus 36% and thrombocytopenia 14% versus 18%. The mechanism behind the observations is unknown and might provide an explanation for the differences between pediatric and adult patients.
The most common pattern of chest CT is ground-glass opacities, followed by local patchy shadowing and then local bilateral patchy shadowing, which is consistent with published data [ 9 – 18 ]. Our study indicates that chest CT manifested with a predominance of lesions in the subpleural area (41 [95%] of 43) and in lower lung lobes (28 [65%] of 43), especially in the posterior segment (22 [78%] of 28), an area with a relatively dense amount of bronchioles, blood vessels, and alveoli. To the best of our knowledge, these are the first quantitative results on the locations of chest CT lesions for COVID-19 children [ 21 ]. COVID-19 is less severe in children than in adults, and the children infected with COVID-19 were at the early stages of the disease when admitted to the hospital. The fact that an overwhelming percentage of pediatric patients had lesions in the subpleural area suggests this site is the first target for the COVID-19 virus.
The current gold standard for the diagnosis of COVID-19 is PCR. However, it has been documented that patients with a negative PCR result cannot be definitively ruled out for COVID-19 infection [ 11 , 26 ]. Our results are consistent with the literature. Among the 50 hospitalized children with positive PCR results, five of them (10%) had negative initial PCR results but showed positive results in subsequent tests. Moreover, 26 patients in group C never had a positive PCR result but had histories of contact with COVID-19 patients. Most of them exhibited clinical symptoms such as fever (81%) and cough (73%). Although they received a negative PCR result at least twice, all 26 patients had similar CT patterns to the PCR-positive COVID-19 patients in group A. Twenty-one (81%) had ground-glass opacities (GGO). Seven (27%) had local patchy shadowing. Five (19%) had bilateral patchy shadowing. Furthermore, our Fisher exact analysis indicated that there was no significant difference in CT image characteristics and lesion location between groups A and C. Although a positive CT alone cannot rule out the possibility of other causes of virus-induced pneumonia [ 11 , 26 ], all 26 children were hospitalized and given immediate antiviral and supportive therapy. Whether or not a child presents with pneumonia is one of the key considerations for clinical management, and it is crucial to start treatment as early as possible, considering that many deaths in the adult population are due to complications resulting from severe pneumonia [ 3 – 7 ].
It has been well documented that chest CT is a powerful tool to identify and characterize pneumonia for COVID-19 adult patients [ 25 – 28 ]. However, there is no publication to study its usefulness in evaluating clinical recovery for children with COVID-19 infection. To determine whether CT is necessary, we investigated the data of 23 patients who had been discharged after effective treatment and had at least two CT scans. All patients had normal body temperatures for more than 3 days at the time of discharge, clinical symptoms disappeared, and PCR tests all returned negative twice at 24-h intervals. Of the 23 children, eight patients did not receive CT scans within the 2 days before their discharge. However, in their most recent CT scan performed in the hospital, most children either still had lesions (50%), or more developed lesions since the previous scan (37%). The remaining 15 discharged children had a CT obtained within 2 days of discharge. Again, ten patients had lesions that were not completely absorbed (67%), two were the same (13%), and lesions in another two became worse (13%). These results indicate that CT may not be better than symptoms in evaluating recovery. Our Cox regression analysis further showed that there was no association between changes in CT lesions and clinical outcomes. The results are consistent with the knowledge that clinical improvement predates radiographic improvement by weeks for children with community-acquired pneumonia.
When deciding whether to use CT on children, the harmful effects that radiation may have on a growing body must be considered. Hong et al., in a study of 12,068,821 children aged 0 to 19 years, found a statistically significant increase in cancer in children exposed at least once to diagnostic low-dose ionizing radiation after adjusting for age and sex [ 29 , 30 ]. Based on our data, we do not recommend using CT for determining clinical recovery unless it is necessary to evaluate the status of pneumonia. For comparison, the current criteria for discharging adult patients infected with COVID-19 in China are (1) normal body temperature for 3 days, (2) two negative PCR tests at 24-h intervals, (3) resolution of clinical symptoms (these three are the current criteria for discharging pediatric patients in this hospital), plus (4) a chest imaging requirement: pulmonary imaging must show significant absorption of lesions. To date, there are no child-specific discharge criteria for COVID-19 in China.
Our study had a few limitations. First, this study has a small sample size and was conducted at a single-center in Wuhan, China, located at the center of the outbreak. The clinical severity of pediatric patients outside Wuhan may be less severe. Indeed, it is reported that there is a lower death rate of adult patients outside Wuhan areas. Second, long-term follow-up was not done because of the short time for data collection.
The severity of COVID-19 infection in children is less than it is in adults in terms of symptoms, lung consolidation as visualized by CT, and laboratory abnormalities. COVID-19 has a preference for subpleural areas of the lung in pediatric patients. Chest CT is an excellent tool to detect and characterize COVID-19 pneumonia but not to evaluate the resolution of illness for children.
Acknowledgements
We would like to thank Pin He for her help in image processing.
Abbreviations
Authors’ contributions.
HM, JH, JT, JX, and JS had roles in the study design, data analysis, data interpretation, literature search, and writing of the manuscript. XZ, HL, MTL, LDW, BZ, WC, and RR had roles in the data analysis, data interpretation, literature search, and writing of the manuscript. JS, HM, HL, and BZ had roles in clinical management, patient recruitment, and data collection and had full access to all of the data in the study and take responsibility for the integrity of the data. HM, JH, and JT contributed equally. The authors read and approved the final manuscript.
The authors received no specific funding for this work.
Availability of data and materials
Ethics approval and consent to participate.
This study was approved by the Ethics Committee of Wuhan Children’s Hospital (Wuhan Maternal and Child Health Care Hospital # WHCH 2020005). Written informed parental/guardian consent and child assent (where appropriate) were obtained prior to enrollment in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Huijing Ma, Jiani Hu, and Jie Tian are joint first authors.
Contributor Information
Jun Xia, Email: nc.ude.uzs.liame@nujaix .
Jianbo Shao, Email: moc.anis@bjoahsrd .
Use and Effects of Augmentation of Labor with Oxytocin: A Single-Center, Retrospective, Case-Control Study of 4350 Women in Warsaw, Poland, 2015-2020
Affiliations.
- 1 Department of Obstetrics and Gynecology Didactics, Medical University of Warsaw, Warsaw, Poland.
- 2 St. Sophia's Specialist Hospital, Żelazna Medical Center, Warsaw, Poland.
- 3 Eastern Center of Burns Treatment and Reconstructive Surgery, Department of Plastic Surgery, Medical University of Lublin, Lublin, Poland.
- 4 Gynecology and Obstetrics Ward, Medical Center Ujastek, Cracow, Poland.
- 5 Department of Emergency Medical Services, Faculty of Health Sciences, Medical University of Warsaw, Warsaw, Poland.
- PMID: 35982582
- PMCID: PMC9397144
- DOI: 10.12659/MSM.937557
BACKGROUND Although there have been some recent clinical trials on the effects of augmentation of labor with oxytocin, or augmentation of labor, there are no clinical guidelines to explain the variations in obstetric practice between countries and within countries. This retrospective case-control study from a single center in Warsaw, Poland aimed to evaluate the use and effects of augmentation of labor with oxytocin in 4350 women between 2015 and 2020. MATERIAL AND METHODS This was a single-center, retrospective, case-control study in which 29 455 cases were qualified for analysis. The study included the analysis of 2 groups: the study group consisted of 4382 patients who underwent stimulation of childbirth, and the control group consisted of 25 073 patients who did not undergo this obstetric procedure. RESULTS Multivariate logistic regression analysis showed that the factors increasing the frequency of augmentation of labor were higher BMI (P<0.05), preinduction (P<0.05), epidural anesthesia (P<0.05), and family present at birth (P<0.05). Factors influencing reduction in the frequency of augmentation of labor were higher number of deliveries (P<0.05), vaginal birth after cesarean (P<0.05), and pre-pregnancy hypertension (P<0.05). CONCLUSIONS This study from a single center in Poland showed that BMI, preinduction, epidural anesthesia, and family present at birth significantly increased the frequency of labor stimulation with oxytocin. However, a history of previous pregnancies, previous cesarean sections, and pre-pregnancy hypertension significantly reduced the frequency of augmentation of labor with oxytocin.
- Case-Control Studies
- Cesarean Section
- Hypertension* / drug therapy
- Infant, Newborn
- Labor, Obstetric*
- Oxytocin / pharmacology
- Retrospective Studies

COMMENTS
Balloon occluded TACE (B-TACE) vs DEM-TACE for HCC: a single center retrospective case control study BMC Gastroenterol. 2021 Feb 3;21(1):51. doi: 10.1186/s12876-021-01631-w. ... Retrospective Studies Treatment Outcome ...
This is a case-control, retrospective, single center study.The data of 159 consecutive patients with 248 LI-RADS-5 HCC tumors managed in our tertiary center for liver cancer treatment between January 2015 and March-2019 were reviewed.
Moreover, this study is a retrospective case-control design of a single center and a rather small sample size may limit our selection of potential risk factors in the multiple regression ...
The purpose of our work was to retrospectively analyse in a case-control, retrospective, single center study the results obtained in two groups of HCC patients who underwent catheter based treatment with drug eluted microsphere with a standard micro-catheter and with the use of a balloon micro-catheter (DEM-TACE versus b-TACE ...
The present retrospective study included a total of 60 cases who were diagnosed as COVID-19 patients and hospitalized in the Wuxi Fifth People's Hospital during February 2020 to March 2020. Among all patients 14 cases were asymptomatic, 25 cases were with mild stage, 13 cases were with moderate stage, and 8 cases were with severe stage.
A retrospective cohort study of patients discharged home after IS showed that primary care follow up shortly after discharge was associated with fewer 30-day readmissions. 8 Despite recommending outpatient follow up, 1 study showed that only 14% of patients with 30-day readmission had 1 scheduled before discharge and inadequate outpatient care ...
High Risk Features Contributing to 30-Day Readmission After Acute Ischemic Stroke: A Single Center Retrospective Case-Control Study. / Loebel, Emma M.; Rojas, Mary; Wheelwright, Danielle et al. In: The Neurohospitalist, Vol. 12, No. 1, 01.2022, p. 24-30. Research output: Contribution to journal › Article › peer-review
Methods This is a retrospective, single-center, case-control study. We reviewed 473 newly diagnosed PTCL patients in the First Affiliated Hospital, Zhejiang University School of Medicine(Zhejiang, China) from January 2014 to December 2021.
The study was approved by the PLA General Hospital Medical Ethics Committee (S2020-447-01). All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived.
Improved clinical outcome using transarterial chemoembolization combined with radiofrequency ablation for patients in Barcelona clinic liver cancer stage A or B hepatocellular carcinoma regardless of tumor size: results of a single-center retrospective case control study
The current study suggests that there is a small subset of patients (~2%) with advanced metastatic disease whose tumors still demonstrate only single pathway alterations on NGS, and that such ...
For this retrospective, single-center study, patients were recruited from January 21 to February 14, 2020, at Wuhan Children's Hospital in Wuhan, China. Real-time reverse-transcriptase polymerase chain reaction (PCR) was performed on children 16 years of age and under who had a family or social history of COVID-19 exposure.
If this is the case, the high ratio of agonal bacteremia observed in patients with kidney damage in the present study could be understood as a result of increased bacterial translocation via the intestine. ... Hayasaka, Y. et al. Kidney damage relates to agonal bacteremia: a single-center retrospective study. Clin Exp Nephrol (2024). https ...
Incidence Rate, Pathogens and Economic Burden of Catheter-Related Bloodstream Infection: A Single-Center, Retrospective Case-Control Study Infect Drug Resist. 2023 Jun 5 ... Patients and methods: A case-control study was retrospectively carried out in six ICUs of one hospital between July 2013 and June 2018. The Department of Infection Control ...
This study was a single-center, retrospective case-control study that included adult patients with severe influenza who were hospitalized in the Department of Infectious Diseases and Clinical Microbiology and Department of Respiratory and Critical Care Medicine at Beijing Chao-Yang Hospital of the Capital Medical University across six ...
Pulmonary alveolar proteinosis (PAP), a rare disease with an estimated prevalence of 3.7 to 6.2 cases per million persons [1, 2], is characterized by the accumulation of lipoprotein material in alveoli and terminal respiratory airways [].Current knowledge about PAP is based on case studies and individual case reports, and as such, the prevalence of PAP may vary due to differences in medical ...
Revised on June 22, 2023. A retrospective cohort study is a type of observational study that focuses on individuals who have an exposure to a disease or risk factor in common. Retrospective cohort studies analyze the health outcomes over a period of time to form connections and assess the risk of a given outcome associated with a given exposure.
Acute kidney injury in 3182 patients admitted with COVID-19: a single-center, retrospective, case-control study Clin Kidney J. 2021 Jan 28;14(6) :1557 ... Methods: This was an observational retrospective case-control study based on patients hospitalized between 1 March and 15 April 2020 with SARS-CoV-2 infection and AKI. Confirmed AKI cases ...
This retrospective, single-center, case-control study evaluated the safety and efficacy of Computed tomography (CT)-guided microwave ablation (MWA) for pulmonary nodules located in the right middle lobe (RML), a challenging location associated with a high frequency of complications.
The study was a single-center retrospective case-control study. We reviewed the charts of patients with the discharge diagnosis of "COVID-19, virus identified" (ICD code U07.1) from March 1 to July 31, 2020 in a tertiary care center in North Carolina, United States of America (USA). We identified 796 charts that had confirmed SARS-CoV-2 ...
The primary objective of this study was to use artificial neural network (ANN) to predict the post operative hypocalcemia and severity of hypocalcemia following total thyroidectomy. The secondary objective was to determine the weightage for the factors predicting the hypocalcemia with the ANN. A single center, retrospective case series included treatment-naive patients undergoing total ...
This single-center retrospective study explored the prevalence, clinical associations, and impact on survival of subcutaneous calcinosis in 86 patients with immune-mediated rheumatic diseases (IMRD). Calcinosis predominantly appeared in individuals with longstanding disease, particularly systemic sclerosis (SSc), constituting 74% of cases ...
The Use of Clear Aligners in Multi-Segmental Maxillary Surgery: A Case-Control Study in Cleft Lip and Palate and Skeletal Class III Patients. Journals. Active Journals Find a Journal Proceedings Series. ... A Single-Center Retrospective Study. Journal of Clinical Medicine. 2024; 13(7):1939. https: ...
Forecasting Hospital Room and Ward Occupancy Using Static and Dynamic Information Concurrently: Retrospective Single-Center Cohort Study JMIR Med Inform 2024;12:e53400 doi: 10.2196/53400 PMID: 38513229. Copy Citation to Clipboard Export Metadata ...
Study Design. This was a single-center, retrospective, cohort study. To ensure that the results were reported properly, the Strobe guidelines for case-control studies were used [].Electronic patient records from Saint Sophia's Hospital in Warsaw, Poland, which is a tertiary hospital that has the most deliveries per year both in the city of Warsaw and Mazowieckie Voivodeship (the largest and ...
This single-center case-control study of 236 multiple and 567 singleton preterm infants born <33 weeks' gestation compared neonatal mortality and major neonatal morbidities between preterm infants of singleton and multiple pregnancies. ... this was a retrospective study, and the data for both mothers and infants were obtained from charts and ...
Background: Clostridium difficile infection (CDI) has become a worldwide health problem in view of its significant incidence and medical and economic impact on the health system. Prior studies have been undergone about risk factors and disease characteristics. We wanted to study the characteristics, prognostic factors associated with CDI at our institute, as well as a new prognostic factor.
For this retrospective, single-center study, patients were recruited from January 21 to February 14, 2020, at Wuhan Children's Hospital in Wuhan, China. ... Yang Z, Xu L, Qu Z, et al. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. 2020. 10.1093/cid/ciaa198. [PMC free ...
Background: This study aimed to evaluate the association between initial fibrinogen levels and massive transfusion (MT) in emergency department (ED) patients with primary postpartum hemorrhage (PPH). Methods: This retrospective study was conducted in the ED of a university-affiliated, tertiary referral center from January 2004 to August 2023. Patients were divided into two groups: the MT group ...
This retrospective case-control study from a single center in Warsaw, Poland aimed to evaluate the use and effects of augmentation of labor with oxytocin in 4350 women between 2015 and 2020. MATERIAL AND METHODS This was a single-center, retrospective, case-control study in which 29 455 cases were qualified for analysis.